-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn Lewis, Mingyan Dai, Kristen K Patton, Yong-Mei Cha, Travis Pollema, Gregory K Feld, Ulrika Birgersdotter-Green, Victor Pretorius, Lead extraction for reduction of chronic pain related to cardiovascular implantable electronic device, EP Europace, Volume 21, Issue 5, May 2019, Pages 781–786, https://doi.org/10.1093/europace/euy320
Close - Share Icon Share
Abstract
Chronic pain at the cardiovascular implantable electronic device (CIED) generator or lead insertion site that is not otherwise manageable carries a IIA indication for extraction. However, limited data exist evaluating causes of pain and outcomes of extraction in eliminating pain. A multi-centre retrospective observational study was conducted to evaluate outcomes of patients undergoing device extraction for treatment of chronic device pain.
Twenty-seven out of 2188 lead extraction candidates (1.3%) met the chronic pain IIA indication for extraction [50 ± 16 years; 14 (51%) women]. Onset, severity, triggers, and pain management were measured before and after extraction. Device type, procedure done (with/without reimplantation), and positive tissue cultures were noted. Pain was reported as constant (n = 14; 50%), intermittent (n = 13; 46%), and movement-triggered (n = 14; 50%). Average severity of pain was seven out of 10 (10 being the worst). Post-extraction, 18 (66%) received freedom from pain, including all patients with poorly formed pockets (n = 2) and subclinical infections (n = 2). Of the 18, 11 underwent reimplantation (61%) without recurrent pain. Nine still had pain (44 ± 17 years; seven women) after extraction. Eight of the nine underwent reimplantation, three on the contralateral chest wall and five ipsilaterally. Pain severity decreased (n = 5), increased (n = 1), or was unchanged (n = 3).
Chronic pain at the CIED generator site can present as chronic or movement-triggered pain, and can be due to subclinical infection or a poorly formed device pocket. Extraction relieved constant and intermittent pain in two-thirds of patients. Extraction appears less successful in eliminating pain in women who undergo subsequent reimplantation.
Less than 1.3% of patients who undergo device extraction have chronic pain reported as the indication.
Subclinical infections and poorly formed device pockets are common causes of chronic pain at the cardiovascular implantable electronic device site.
When medical management fails, device extraction is a reasonable treatment option for the elimination of chronic pain.
Extraction is less successful in eliminating pain in patients who undergo subsequent device reimplantation.
Younger, female patients may benefit less from extraction for the elimination of chronic pain.
Introduction
It is currently estimated that 1.2–1.4 million cardiovascular implantable electronic device (CIED) implantations occur annually worldwide.1,2 Inevitably, a fraction of this population will require lead extraction due to various causes such as bacterial infection or lead malfunction. Although less frequent, chronic pain possibly caused by the presence of the CIED that is otherwise not manageable, carries a IIA indication for CIED and lead extraction.1 Cardiovascular implantable electronic device implantation has previously been associated with a spectrum of post-operative discomfort, pain, and limited mobility. Cases of complex regional pain syndrome secondary to pacemaker insertion have been reported, resulting in burning pain, erythema, and stiffness of the ipsilateral hand.3 Furthermore, a 5-year longitudinal study following device intervention revealed that 23.6% of patients reported pain and discomfort with movement at the generator site.4 However, limited data exist that elucidates the risk factors and causes of chronic pain that result in extraction. Suspected aetiologies include chronic musculoskeletal derangement, subclinical infection, and psychosomatic issues. Additionally, the optimal treatment and success rates of extraction for alleviating pain have not been determined. We performed a multi-centre, retrospective, observational study to evaluate the underlying causes of chronic pain and the success of lead extraction in eliminating pain, in patients with prior CIED implantation.
Methods
Patient selection
A total 2188 standard-of-care CIED lead extractions conducted from January 2000 to June 2017 at University of California, San Diego Health System (UCSD), Mayo Clinic, and University of Washington Medical Center were screened for the Class IIA indication of CIED and lead extraction for chronic pain. Patients with a history of generator replacements were included in this study. Only subcutaneous pockets were investigated. There was no attempted conversion to sub-pectoral pockets prior to extraction. Thirty-one (1.4%) patients met this indication, but those lost to follow-up post-extraction, and those with subcutaneous implantable cardioverter-defibrillators (ICDs) were excluded from the study. Thus, 27 patients were included (1.2%) in the study across all three participating sites.
Lead extraction, cardiovascular implantable electronic device explant, and reimplant
Lead extraction, CIED explant, and reimplantation were performed following standard procedures at all three institutions. The decision for ipsilateral or contralateral device reimplantation was made on a case-by-case basis.
Data collection
Age, gender, and previous medical conditions, including psychiatric diagnoses and chronic musculoskeletal problems, were documented from review of the patient’s electronic medical records. Onset, location, duration, type (e.g. dull), triggers, and management of pain were assessed before and after lead extraction. Severity of pain was measured on a scale from 1 to 10 (10 being the worst). Device type and number of leads implanted were recorded. Operative reports were reviewed for procedural details (with or without CIED and lead reimplantation), the location of reimplanted generator, and positive tissue cultures were obtained from the medical chart. Evaluation of poorly formed pockets and tissue quality was performed at the time of extraction. Poorly, or malformed, pockets are defined in this study as those that do not lie in the plane between the subcutaneous fat and pectoral fascia, pockets that allow for device migration, and those that appear to induce pressure on the dermis from within the pocket. Reports of post-extraction pain were evaluated at least 6 months following the procedure. Institutional Review Board (IRB) approval was obtained from all three institutions involved prior to data collection.
Statistical analysis
Study demographics, clinical features of pain, and outcomes of extraction were analysed using means with standard deviations. The χ2 tests and two-tailed t-tests were performed to evaluate for significance of the different between patients without pain following extraction and those with continued pain. An alpha value of less than 0.05 was considered significant. GraphPad Prism and Excel were used for analysis.
Results
Baseline characteristics
Twenty-seven patients were included in the study with a mean age 50 ± 16 years, 14 (51%) were women. Six (22.2%) patients reported chronic musculoskeletal conditions unrelated to their CIED chronic pain and an additional three (11.1%) patients reported a diagnosis of depression prior to lead extraction. Devices extracted included 12 permanent pacemakers (44%), nine ICDs (33.3%), four cardiac resynchronization therapy (CRT)-pacemakers (14.8%), and two CRT-defibrillators (7.4%) (Table 1). Prior to lead extraction, no patients underwent procedures for pocket revisions. Six (22.2%) patients included in this study had undergone prior generator replacements. The remaining 21 (77.7%) patients had not undergone prior procedures aside from implantation. At the time of extraction, two CIED pockets (7.4%) cultured positive for Propionibacterium. Gross examination of one pocket revealed caseous necrotic tissue. The patient was subsequently treated with Vancomycin and Cefepime. Examination of the second pocket did not reveal purulent discharge or necrotic material. There was no antibiotic management for this case. Two CIED pockets (7.4%) were deemed poorly formed at the time of device extraction (Table 2). One generator migrated into the axilla from its original placement and another was implanted deep into the subpectoral space via the axilla (Figure 1).
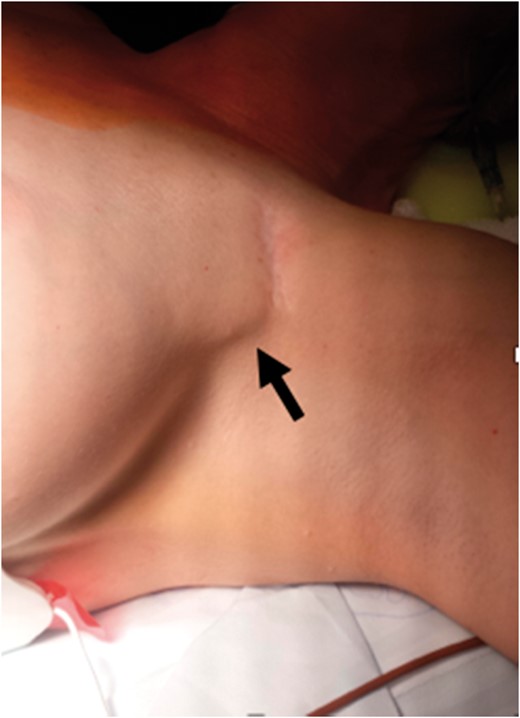
Generator migration (arrow) into the apex of the axilla that resulted in movement-triggered pain in one study patient.
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Age (years) | 50 ± 16.2 | 53 ± 16.5 | 44 ± 17.1 | 0.252 |
| Gender | 14 (51.8%) | 7 (50.0%) | 7 (50.0%) | 0.056 |
| History of depression | 3 (11.1%) | 3 (100%) | 0 (0%) | 0.193 |
| History of chronic musculoskeletal pain | 6 (33.3%) | 3 (50.0%) | 3 (50.0%) | 0.326 |
| Device type | ||||
| CRT-D | 2 (7.4%) | 1 (50.0%) | 1 (50.0%) | 0.945 |
| CRT-P | 4 (14.8%) | 3 (75.0%) | 1 (25.0%) | |
| ICD | 9 (33.3%) | 6 (66.6%) | 3 (33.3%) | |
| PPM | 12 (44.4%) | 8 (66.6%) | 4 (33.3%) | |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Age (years) | 50 ± 16.2 | 53 ± 16.5 | 44 ± 17.1 | 0.252 |
| Gender | 14 (51.8%) | 7 (50.0%) | 7 (50.0%) | 0.056 |
| History of depression | 3 (11.1%) | 3 (100%) | 0 (0%) | 0.193 |
| History of chronic musculoskeletal pain | 6 (33.3%) | 3 (50.0%) | 3 (50.0%) | 0.326 |
| Device type | ||||
| CRT-D | 2 (7.4%) | 1 (50.0%) | 1 (50.0%) | 0.945 |
| CRT-P | 4 (14.8%) | 3 (75.0%) | 1 (25.0%) | |
| ICD | 9 (33.3%) | 6 (66.6%) | 3 (33.3%) | |
| PPM | 12 (44.4%) | 8 (66.6%) | 4 (33.3%) | |
CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter-defibrillator; PPM, permanent pacemaker.
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Age (years) | 50 ± 16.2 | 53 ± 16.5 | 44 ± 17.1 | 0.252 |
| Gender | 14 (51.8%) | 7 (50.0%) | 7 (50.0%) | 0.056 |
| History of depression | 3 (11.1%) | 3 (100%) | 0 (0%) | 0.193 |
| History of chronic musculoskeletal pain | 6 (33.3%) | 3 (50.0%) | 3 (50.0%) | 0.326 |
| Device type | ||||
| CRT-D | 2 (7.4%) | 1 (50.0%) | 1 (50.0%) | 0.945 |
| CRT-P | 4 (14.8%) | 3 (75.0%) | 1 (25.0%) | |
| ICD | 9 (33.3%) | 6 (66.6%) | 3 (33.3%) | |
| PPM | 12 (44.4%) | 8 (66.6%) | 4 (33.3%) | |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Age (years) | 50 ± 16.2 | 53 ± 16.5 | 44 ± 17.1 | 0.252 |
| Gender | 14 (51.8%) | 7 (50.0%) | 7 (50.0%) | 0.056 |
| History of depression | 3 (11.1%) | 3 (100%) | 0 (0%) | 0.193 |
| History of chronic musculoskeletal pain | 6 (33.3%) | 3 (50.0%) | 3 (50.0%) | 0.326 |
| Device type | ||||
| CRT-D | 2 (7.4%) | 1 (50.0%) | 1 (50.0%) | 0.945 |
| CRT-P | 4 (14.8%) | 3 (75.0%) | 1 (25.0%) | |
| ICD | 9 (33.3%) | 6 (66.6%) | 3 (33.3%) | |
| PPM | 12 (44.4%) | 8 (66.6%) | 4 (33.3%) | |
CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter-defibrillator; PPM, permanent pacemaker.
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implant to lead extraction (years) | 4.08 ± 4.2 | 4.5 ± 5.2 | 5.3 ± 5.3 | 0.661 |
| Malformed pocket | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Positive culture (Propionibacterium) | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Procedure type | ||||
| Extraction without reimplantation | 8 (29.6%) | 7 (87.5%) | 1 (12.5%) | 0.136 |
| Extraction with reimplantation | 19 (70.3%) | 11 (57.8%) | 8 (42.1%) | |
| Ipsilateral placement | 8 (42.1%) | 3 (37.5%) | 5 (62.5%) | 0.124 |
| Contralateral placement | 11 (57.8%) | 8 (72.7%) | 3 (27.2%) | |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implant to lead extraction (years) | 4.08 ± 4.2 | 4.5 ± 5.2 | 5.3 ± 5.3 | 0.661 |
| Malformed pocket | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Positive culture (Propionibacterium) | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Procedure type | ||||
| Extraction without reimplantation | 8 (29.6%) | 7 (87.5%) | 1 (12.5%) | 0.136 |
| Extraction with reimplantation | 19 (70.3%) | 11 (57.8%) | 8 (42.1%) | |
| Ipsilateral placement | 8 (42.1%) | 3 (37.5%) | 5 (62.5%) | 0.124 |
| Contralateral placement | 11 (57.8%) | 8 (72.7%) | 3 (27.2%) | |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implant to lead extraction (years) | 4.08 ± 4.2 | 4.5 ± 5.2 | 5.3 ± 5.3 | 0.661 |
| Malformed pocket | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Positive culture (Propionibacterium) | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Procedure type | ||||
| Extraction without reimplantation | 8 (29.6%) | 7 (87.5%) | 1 (12.5%) | 0.136 |
| Extraction with reimplantation | 19 (70.3%) | 11 (57.8%) | 8 (42.1%) | |
| Ipsilateral placement | 8 (42.1%) | 3 (37.5%) | 5 (62.5%) | 0.124 |
| Contralateral placement | 11 (57.8%) | 8 (72.7%) | 3 (27.2%) | |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implant to lead extraction (years) | 4.08 ± 4.2 | 4.5 ± 5.2 | 5.3 ± 5.3 | 0.661 |
| Malformed pocket | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Positive culture (Propionibacterium) | 2 (7.4%) | 2 (100%) | 0 (0%) | 0.298 |
| Procedure type | ||||
| Extraction without reimplantation | 8 (29.6%) | 7 (87.5%) | 1 (12.5%) | 0.136 |
| Extraction with reimplantation | 19 (70.3%) | 11 (57.8%) | 8 (42.1%) | |
| Ipsilateral placement | 8 (42.1%) | 3 (37.5%) | 5 (62.5%) | 0.124 |
| Contralateral placement | 11 (57.8%) | 8 (72.7%) | 3 (27.2%) | |
Characteristics of pain
The average onset of pain was 5 months ± 1.3 years (185.8 ± 503.5 days) following implantation. The average time from implantation to lead extraction was 4.08 ± 4.28 years. There was no significant difference in the onset of pain and time to extraction, respectively, between those with continued, post-extraction pain and those without (P = 0.656, P = 0.661). Prior to lead extraction, the average severity of pain was rated as 6.1 ± 2.3 out of 10 (10 being the worst) (P = 0.308). Pain was described as sharp by 17 (58.6%) patients, dull and achy by four (14.8%), and tender by one (3.4%). Five (20.6%) patients could not describe the pain they experienced using available terminology. There was no difference in the type of pain experienced between patients with post-extraction pain and those without (P = 0.380). Fourteen (51.8%) patients reported pain triggered by movement and positional changes. Radiation of pain from the pectoral region to the ipsilateral axilla, and shoulder was documented in 13 (48.1%) patients. There was no difference in the presence of movement-triggered and radiating pain, respectively between the patient groups following extraction (P = 0.275, P = 0.785). Fourteen (51.8%) patients experienced constant pain, while 13 (48.1%) reported intermittent pain. There was no difference between the patients with post-extraction pain and those without regarding intermittent vs. constant pain (P = 0.785). Prior to initial CIED implantation, six (22.2%) patients were noted to have been prescribed narcotics for pain management, whereas 13 (48.1%) patients used over-the-counter medications including non-steroidal anti-inflammatories (NSAIDs) and/or acetaminophen. There was no significant difference in the use of narcotics prior to surgery between those with resolution of pain and those with continued pain (P = 0.326) (Table 3).
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implantation to onset of pain (days) | 185.7 ± 503.5 | 217.1 ± 609.1 | 123.2 ± 169.4 | 0.656 |
| Description of pain | ||||
| Sharp | 17 (58.6%) | 10 (58.8%) | 7 (41.1%) | 0.380 |
| Non-descriptive discomfort | 5 (20.6%) | 3 (60.0%) | 2 (40.0%) | |
| Dull and achy | 4 (14.8%) | 4 (100%) | 0 (0%) | |
| Tenderness | 1 (3.4%) | 1 (100%) | 0 (0%) | |
| Movement-triggered pain | 14 (51.8%) | 8 (57.1%) | 6 (42.8%) | 0.275 |
| Radiation of paina | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Intermittent pain | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Constant pain | 14 (51.8%) | 9 (64.2%) | 5 (35.7%) | |
| Scaling of pain at worst (1–10) | 6.1 ± 2.3 | 6.7 ± 1.7 | 5.7 ± 2.6 | 0.308 |
| Narcotic prescription for pain prior to surgery | 6 (22.2%) | 3 (100%) | 3 (100%) | 0.326 |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implantation to onset of pain (days) | 185.7 ± 503.5 | 217.1 ± 609.1 | 123.2 ± 169.4 | 0.656 |
| Description of pain | ||||
| Sharp | 17 (58.6%) | 10 (58.8%) | 7 (41.1%) | 0.380 |
| Non-descriptive discomfort | 5 (20.6%) | 3 (60.0%) | 2 (40.0%) | |
| Dull and achy | 4 (14.8%) | 4 (100%) | 0 (0%) | |
| Tenderness | 1 (3.4%) | 1 (100%) | 0 (0%) | |
| Movement-triggered pain | 14 (51.8%) | 8 (57.1%) | 6 (42.8%) | 0.275 |
| Radiation of paina | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Intermittent pain | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Constant pain | 14 (51.8%) | 9 (64.2%) | 5 (35.7%) | |
| Scaling of pain at worst (1–10) | 6.1 ± 2.3 | 6.7 ± 1.7 | 5.7 ± 2.6 | 0.308 |
| Narcotic prescription for pain prior to surgery | 6 (22.2%) | 3 (100%) | 3 (100%) | 0.326 |
From the pectoral region to the ipsilateral axilla and shoulder.
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implantation to onset of pain (days) | 185.7 ± 503.5 | 217.1 ± 609.1 | 123.2 ± 169.4 | 0.656 |
| Description of pain | ||||
| Sharp | 17 (58.6%) | 10 (58.8%) | 7 (41.1%) | 0.380 |
| Non-descriptive discomfort | 5 (20.6%) | 3 (60.0%) | 2 (40.0%) | |
| Dull and achy | 4 (14.8%) | 4 (100%) | 0 (0%) | |
| Tenderness | 1 (3.4%) | 1 (100%) | 0 (0%) | |
| Movement-triggered pain | 14 (51.8%) | 8 (57.1%) | 6 (42.8%) | 0.275 |
| Radiation of paina | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Intermittent pain | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Constant pain | 14 (51.8%) | 9 (64.2%) | 5 (35.7%) | |
| Scaling of pain at worst (1–10) | 6.1 ± 2.3 | 6.7 ± 1.7 | 5.7 ± 2.6 | 0.308 |
| Narcotic prescription for pain prior to surgery | 6 (22.2%) | 3 (100%) | 3 (100%) | 0.326 |
| Characteristics . | All patients (n = 27) . | No post-operative pain (n = 18) . | Post-operative pain (n = 9) . | P-value . |
|---|---|---|---|---|
| Implantation to onset of pain (days) | 185.7 ± 503.5 | 217.1 ± 609.1 | 123.2 ± 169.4 | 0.656 |
| Description of pain | ||||
| Sharp | 17 (58.6%) | 10 (58.8%) | 7 (41.1%) | 0.380 |
| Non-descriptive discomfort | 5 (20.6%) | 3 (60.0%) | 2 (40.0%) | |
| Dull and achy | 4 (14.8%) | 4 (100%) | 0 (0%) | |
| Tenderness | 1 (3.4%) | 1 (100%) | 0 (0%) | |
| Movement-triggered pain | 14 (51.8%) | 8 (57.1%) | 6 (42.8%) | 0.275 |
| Radiation of paina | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Intermittent pain | 13 (48.1%) | 9 (69.2%) | 4 (30.7%) | 0.785 |
| Constant pain | 14 (51.8%) | 9 (64.2%) | 5 (35.7%) | |
| Scaling of pain at worst (1–10) | 6.1 ± 2.3 | 6.7 ± 1.7 | 5.7 ± 2.6 | 0.308 |
| Narcotic prescription for pain prior to surgery | 6 (22.2%) | 3 (100%) | 3 (100%) | 0.326 |
From the pectoral region to the ipsilateral axilla and shoulder.
Eight patients underwent extraction without reimplantation (29.6%) and 19 patients underwent extraction with reimplantation (70.3%). Of the 19 patients that underwent reimplantation, 8 (42.1%) underwent ipsilateral generator placement and 11 (57.8%) underwent contralateral generator reimplantation.
Outcomes of extraction
Following CIED extraction, 18 (66.6%) patients were free of pain, while nine (33.3%) patients still reported pain at the initial implantation site. Those with poorly formed CIED pockets and subclinical infections identified at the time of CIED extraction, were subsequently free of pain. Additionally, pain was effectively relieved in those patients in this population who had either intermittent or constant pain. Patients underwent reimplantation following extraction (n = 19, 70.3%) or extraction alone (n = 8, 29.6%). Eleven of 19 (57.8%) device reimplantations were done immediately following extraction. The remaining eight (42.1%) were performed at a later time following extraction. Of the 19 (70.3%) patients that underwent CIED reimplantation, 11 were done at the contralateral pectoral region and the other eight at the ipsilateral pectoral region. Of the 11 that underwent contralateral reimplantation, eight reported resolution of their pain, while five of eight that underwent ipsilateral reimplantation reported pain unresolved. Following extraction and relief of pain, three patients reported discontinuation of the use of their prescription narcotics.
Of the nine (33.3%) patients that still reported pain following extraction, the average age was 44 ± 17.1 years (P = 0.252) and seven were women (P = 0.056). There was no significant difference in age or gender between those patients without pain and those with post-extraction pain. Post-extraction, five (55.5%) patients required continued pain management with narcotics, three of whom did so prior to extraction as well. The severity of pain decreased in five, increased in one, or remained the same three patients in this group, compared with the pain experienced before extraction. Two of the nine reported constant pain post-extraction, compared with five that reported constant pain prior to extraction. Eight of the nine underwent device reimplantation, five (55.5%) of whom were implanted on the ipsilateral side. One patient had post-extraction pain despite extraction without reimplantation. There was no significant difference in the resolution of pain between patients who underwent extraction alone vs. extraction and reimplantation (n = 27, P = 0.136). Concerning those that underwent reimplantation (n = 19), there was no significant difference in the resolution of pain between those with ipsilateral vs. contralateral reimplantation (P = 0.124).
Additional relationships: allergies, previous medical conditions, and device type
No patients had signs of allergy at the device site, including rash, warmth, or pruritis. Patients reported allergies to medications and foods, but not to silicone or metals, such as nickel and copper. Three patients had a history of diagnosed depression, and all denied the persistence of pain following extraction without reimplantation of their CIED (P = 0.193). Six (22.2%) patients reported a history of chronic musculoskeletal pain and three (50%) reported relief of site pain following extraction (P = 0.326). There did not appear to be a relationship between the type of device extracted and the persistence of pain following extraction (P = 0.945).
Discussion
Chronic pain at the CIED implantation site is a complex and multifactorial condition that pervasively impacts the quality-of-life of those affected. It is currently estimated that less than 3% of patients undergo lead extraction for chronic pain after medical management fails.1 Although chronic pain is a Class IIA indication for extraction, there is limited investigation on CIED extraction for chronic pain. Previous studies have described certain characteristics of the CIED system in patients with associated pain. For example, Celikyurt et al.5 observed that 52% of patients with CIEDs who reported chronic shoulder pain in their study had at least three leads implanted. It is proposed that the larger size of three-lead devices and the coiling of the leads posterior to the device contributed to a bulkier pocket and increased pain. Contact dermatitis resulting from allergic reactions to silicone and titanium in the generator has also been documented to present with intermittent pain and pruritus.6,7 These reactions reportedly resolved with extraction and reimplantation of silicone-free materials and gold-plated generators.6 Despite these case studies, the causes of chronic pain and the success of extraction remain uncertain.
Our study suggests that possible causes of pain include subclinical infection and incorrect surgical technique at initial implantation resulting in a poorly formed CIED pocket. In our study, 66% of patients were free of pain following extraction, including those with different types of pain (e.g. sharp) and pain duration (e.g. constant or intermittent). Of the remaining 33% who experienced post-extraction pain, there was still an overall reduction in severity of pain. Therefore, we found lead extraction to be a reasonably effective strategy for either the elimination or improvement of chronic pain.
Bacteria have been known to colonize CIEDs without obvious clinical signs of infection. At the time of CIED extraction, Rohacek et al.8 found that 38% without previous signs of infection (n = 115) were colonized with bacteria. In our study, two (7.4%) patients presented with chronic pain and after CIED extraction cultures were found to be positive for Propionibacterium. Both underwent reimplantation and chronic pain resolved. Although colonization may not represent infection, pain has been determined to be a common symptom of early CIED infections within the first 12 months of implantation. Welch et al.9 evaluated early vs. latent presentations of infection in CIED generators. Pain was shown to be associated with early infection within 12 months of implantation compared to those with latent infections 1–17.4 years after intervention (71.2% vs. 53.8%, P = 0.007). With the combination of early onset pain and positive cultures, it is possible that subclinical, limited infections are a cause of chronic pain and irritation at the CIED site.
In our study, 51.8% of patients with pain at the site of prior CIED implantation reported that it was triggered by movement, including flexion, adduction, and abduction of the ipsilateral arm. Nerve impingement at the pectoral region may contribute to the pain experienced. In one study of chronic shoulder pain following CIED implantation, it was observed that performing exercises to combat impingement resulted in less pain following implantation.10 Individualized factors are also likely to contribute to movement-triggered pain, including the amount of subcutaneous tissue present, the position of the CIED in relation to the pectoral muscles, and the activity level of the patient.
Chronic pain following general surgical procedures is increasingly common, with greater than 20% of patients citing surgery as the source of their pain.11 There are multiple contributing factors, including perception of pain, anatomical variation, and surgical technique of the physician. In our study, two (7.4%) pockets with the generator having migrated to the axilla, were deemed poorly formed at the time of extraction. Pain subsequently resolved for both patients following contralateral reimplantation. Generator implantation can be complicated by various factors that may be responsible for pain. Traction induced on the surrounding tissues and nerve fibres is likely to contribute to pain. This may include suturing technique, which varies between specialists. Other clinical variables that may play an important role in the generation of pocket pain include anchoring sleeves and end caps. Either type of hardware may contribute to increased pressure and tension in the pocket, therefore, adding to pain symptoms. Insufficient or loose subcutaneous fat, as seen in the elderly, may also increase the likelihood of generator rotation and migration that can contribute to pain. Improper dissection of the pectoral fascia and subcutaneous fat will result in a generator placed subcutaneously (Figure 2). This type of technical error has been reported to cause chronic pain to patients that limits mobility and ultimately requires revision.12,13
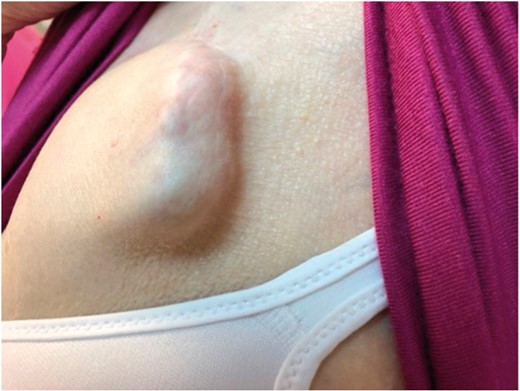
Example of poor surgical technique that resulted in subcutaneous generator placement.
This study has both demonstrated and discussed causes of chronic pain at the CIED site. These numerous causes can be further separated into three major groups: surgical, patient, and lead/CIED factors (Table 4). Patient factors, such as perception of pain and wound healing capacity, and hardware factors such as the presence of suture sleeves and bulk of the generator, are just a few of many causes. However, surgical factors, including skill and technique of pocket formation remain an important cause of pain.
| Surgical factors . | Patient factors . | Lead/CIED factors . |
|---|---|---|
| Subcutaneous vs. subpectoral approach | Perception of pain | Bulk of device generator |
| Device implantation directly under the skin | Level of exercise and use of arm on the affected side | Lead number and diameter |
| Proximity of device to the clavicle | Poor wound healing capacity | Presence of lead end caps |
| Lack of fixation sutures used vs. too tight of sutures | Thin skin vs. hypertrophic scarring | Presence of suture sleeve |
| Poorly determined location for implantation | Subclinical infection | |
| Dermatitis |
| Surgical factors . | Patient factors . | Lead/CIED factors . |
|---|---|---|
| Subcutaneous vs. subpectoral approach | Perception of pain | Bulk of device generator |
| Device implantation directly under the skin | Level of exercise and use of arm on the affected side | Lead number and diameter |
| Proximity of device to the clavicle | Poor wound healing capacity | Presence of lead end caps |
| Lack of fixation sutures used vs. too tight of sutures | Thin skin vs. hypertrophic scarring | Presence of suture sleeve |
| Poorly determined location for implantation | Subclinical infection | |
| Dermatitis |
CIED, cardiovascular implantable electronic device.
| Surgical factors . | Patient factors . | Lead/CIED factors . |
|---|---|---|
| Subcutaneous vs. subpectoral approach | Perception of pain | Bulk of device generator |
| Device implantation directly under the skin | Level of exercise and use of arm on the affected side | Lead number and diameter |
| Proximity of device to the clavicle | Poor wound healing capacity | Presence of lead end caps |
| Lack of fixation sutures used vs. too tight of sutures | Thin skin vs. hypertrophic scarring | Presence of suture sleeve |
| Poorly determined location for implantation | Subclinical infection | |
| Dermatitis |
| Surgical factors . | Patient factors . | Lead/CIED factors . |
|---|---|---|
| Subcutaneous vs. subpectoral approach | Perception of pain | Bulk of device generator |
| Device implantation directly under the skin | Level of exercise and use of arm on the affected side | Lead number and diameter |
| Proximity of device to the clavicle | Poor wound healing capacity | Presence of lead end caps |
| Lack of fixation sutures used vs. too tight of sutures | Thin skin vs. hypertrophic scarring | Presence of suture sleeve |
| Poorly determined location for implantation | Subclinical infection | |
| Dermatitis |
CIED, cardiovascular implantable electronic device.
In addition to exploring the causes of pain, this study demonstrated the effect of CIED generator and lead extraction for the reduction of chronic pain, regardless of the aetiology of pain being identified. Ninety percent of patients who underwent extraction alone were rendered free of pain, indicating that scar tissue is an unlikely source of pain in this group. Five of six patients that underwent previous generator replacement procedures, which can result in more tissue scarring, had resolution of pain following extraction. The location of device reimplantation also appears to play a role in the alleviation of chronic pain. Of 11 patients who underwent CIED reimplantation and were free of pain subsequently, 8 (72.7%) underwent contralateral generator placement, whereas five (55%) patients with persistent post-extraction pain underwent ipsilateral generator placement. Thus, it appears that CIED reimplantation on the contralateral chest wall may be a preferable option for the elimination of pain. Notably, CIED and lead extraction was found to be less successful in alleviating pain in younger women.
Furthermore, regardless of the length of duration of pain prior to extraction, it appears to be a reasonably effective mean for alleviating pain. On average, there was a 3-year delay between the onset of pain and time of lead extraction in our patient population. This delay likely reflects the desire to attempt medical management prior to invasive surgical intervention. Thirteen (48.1%) patients reported the sole use of NSAIDs and/or acetaminophen in attempts to control their pain, whereas six were prescribed narcotics (22.2%). Despite the three year delay, lead extraction remained a successful strategy to bring resolution to chronic pain in the majority of the study population.
Chronic pain is a multifactorial condition, making it difficult to isolate exact causes of pain. Perception of pain is believed to be influenced by mood, memories, and expectations.11 One study found that patients with more preoperative anxiety were more likely to experience post-operative pain.11 Additional studies have demonstrated that patients with proper education of what they may experience post-procedure had less pain and distress compared to those who did not.11 Therefore, resolving chronic pain in the CIED patients requires consideration of psychosocial factors that contribute to pain as well as other suspected causes.
Limitations
Given the small sample size in this study, it may be difficult to extrapolate the results to the general extraction population at large. There is limited data currently in the literature surrounding this population, and therefore, little comparative data to support findings in this study. A larger study would need to be conducted to support and elaborate on the findings presented here. Another limitation of this study includes an inability to assess the variation in implanter experience, as this study included three referral centres for lead extraction. The difference in experience is likely to contribute to chronic pain; however, it could not be measured in this study. The assessment of the pocket was done by very experienced implanters with greater than 10 years of experience at all three centres. This may have had a positive impact on the percentage of patients without pain following extraction (66%).
Conclusions
This study indicates that causes of chronic pain at the CIED site include subclinical infection, movement-triggered pain, and surgical technique at the time of implantation. Constant and intermittent pain can be relieved by extraction, for various types of pain. Cardiovascular implantable electronic device reimplantation on the contralateral chest wall and extraction without reimplantation may eliminate chronic CIED-associated pain. Extraction appears less successful in eliminating pain in younger women.
Conflict of interest: none declared.
References
Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy L et al.



