-
PDF
- Split View
-
Views
-
Cite
Cite
Hiro Kawata, Victor Pretorius, Huy Phan, Siva Mulpuru, Varuna Gadiyaram, Jigar Patel, Dawna Steltzner, David Krummen, Gregory Feld, Ulrika Birgersdotter-Green, Utility and safety of temporary pacing using active fixation leads and externalized re-usable permanent pacemakers after lead extraction, EP Europace, Volume 15, Issue 9, September 2013, Pages 1287–1291, https://doi.org/10.1093/europace/eut045
Close - Share Icon Share
Abstract
After extraction of an infected cardiac implantable electronic device (CIED) in a pacemaker-dependent patient, a temporary pacemaker wire may be required for long periods during antibiotic treatment. Loss of capture and under sensing are commonly observed over time with temporary pacemaker wires, and patient mobility is restricted. The use of an externalized permanent active-fixation pacemaker lead connected to a permanent pacemaker generator for temporary pacing may be beneficial because of improved lead stability, and greater patient mobility and comfort. The aim of this study was to investigate the efficacy and safety of a temporary permanent pacemaker (TPPM) system in patients undergoing transvenous lead extraction due to CIED infection.
Of 47 patients who underwent lead extraction due to CIED infection over a 2-year period at our centre, 23 were pacemaker dependent and underwent TPPM implantation. A permanent pacemaker lead was implanted in the right ventricle via the internal jugular vein and connected to a TPPM generator, which was secured externally at the base of the neck. The TPPM was used for a mean of 19.4 ± 11.9 days (median 18 days, range 3–45 days), without loss of capture or sensing failure in any patient. Twelve of 23 patients were discharged home or to a nursing facility with the TPPM until completion of antibiotic treatment and re-implantation of a new permanent pacemaker.
External TPPMs are safe and effective in patients requiring long-term pacing after infected CIED removal.
The use of an active fixation transvenous pacing lead for temporary pacing after lead extraction is safe.
The use of an active fixation transvenous pacing lead for temporary pacing after lead extraction provides an effective and stable pacing mechanism.
Temporary permanent pacing allows for increased patient mobility during long-term antibiotic therapy.
Re-sterilized pacemaker can safely be used for pacing support.
Introduction
Infections associated with cardiac implantable electronic devices (CIEDs) are increasingly frequent and their treatment is associated with prolonged hospital stays and high financial cost.1 The duration of antimicrobial therapy for CIED infection ranges typically from 2 to 6 weeks.2 In addition, in patients with evidence of valvular infection, it is recommended that new transvenous lead placement be delayed for at least 14 days after CIED system removal. During this waiting period, pacemaker-dependent patients require temporary pacing. Traditional temporary transvenous pacing systems are bulky and associated with frequent loss of capture and under-sensing. They also require continuous monitoring and patient confinement to the telemetry ward or intensive care unit.3–5 To circumvent the limitations posed by a conventional temporary pacing wire, the use of a temporary pacemaker with active fixation lead provides a more stable and safer approach for pacemaker-dependent patients. Temporary pacing with active fixation leads and an externalized re-usable permanent pacemaker has been reported to be cost effective and safe.6–10 However, the safety and efficacy of this approach have not been thoroughly investigated in patients with infected CIEDs undergoing lead extraction. In this study, we investigated the efficacy and safety of externalized active fixation leads connected to re-usable permanent pacemaker, after transvenous lead extraction.
Methods
A total of 105 patients underwent laser lead extraction at UCSD Medical Center from August 2010 to August 2012. Forty-seven of these 105 patients underwent lead extraction for suspected or confirmed diagnosis of CIED infection. A diagnosis of CIED infection was made based on current guidelines.2 All patients had at least two sets of blood cultures drawn at the initial evaluation before initiation of antimicrobial therapy for CIED infection. Antimicrobial therapy was started as soon as CIED infection was diagnosed or suspected. The antimicrobial treatment was based on recommendations from an infectious disease specialist.11 Before lead extraction, transoesophageal echocardiogram (TEE) was performed in all patients with bacteraemia due to Staphylococcus aureus or other organism known to be a common cause of infective endocarditis. For patients with normal transthoracic echocardiogram, TEE was recommended if there was a high clinical suspicion of infective endocarditis. In addition, TEE was performed intraoperatively in all patients during lead extraction to monitor for possible complications and identify any intra-cardiac masses suggesting infective endocarditis.
Prior to lead extraction, all devices were interrogated to determine whether or not the patient was pacemaker dependent. If the patient was deemed to be pacemaker dependent, temporary pacing support was provided during and after lead extraction, until the patient had a new permanent CIED re-implanted. During lead extraction, a temporary transvenous pacemaker wire was placed via a femoral vein and used for pacing support until the CIED was completely extracted. If the patient needed several weeks of antibiotic therapy prior to re-implantation of a new CIED, a temporary permanent pacemaker (TPPM) system was used for pacing support. In these cases, a TPPM lead was placed via the internal jugular vein, contralateral to the side of the infected CIED that was extracted. Internal jugular vein access was obtained percutaneously under ultrasound guidance, in a sterile fashion using standard Seldinger technique, and a peel-away introducer sheath was inserted over a guidewire. A TPPM lead was then inserted through the sheath, and actively fixed at the right ventricular apex. The active-fixation lead was then connected to a re-used, sterilized, permanent pacemaker pulse generator programmed in the VVI mode. If the patient needed atrio-ventricular (AV) sequential pacing (DDD mode), a second active-fixation lead was be placed in the right atrial appendage in a similar fashion. The sensing amplitude, impedance, and pacing threshold for each lead were measured. After suturing one or both TPPM leads to the patient's neck, the externalized lead(s) were then connected to the TPPM generator. Finally, the TPPM generator and lead(s) were securely taped to the patient's neck with a Tegaderm™ dressing (Figure 1).
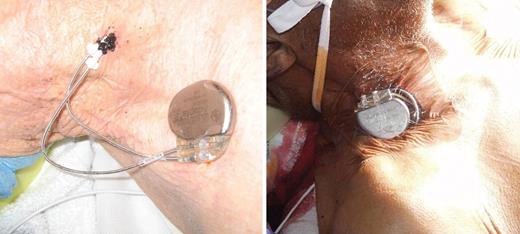
The temporary permanent pacemaker system securely taped to the patient's neck with a Tegaderm™ dressing.
Pacing lead threshold at implantation was considered acceptable if <1.0 V at a pulse width of 0.5 ms, as were sensing amplitudes of ≥5 mV for the R-wave and ≥1 mV for the P-wave. A wound check, chest X-ray, device interrogation, and blood test were performed during the first 24 h after implantation (Figure 2). After confirming that blood cultures were negative for at least 72 h, some patients were discharged home or to a nursing care facility to complete their course of parenteral antibiotic therapy.
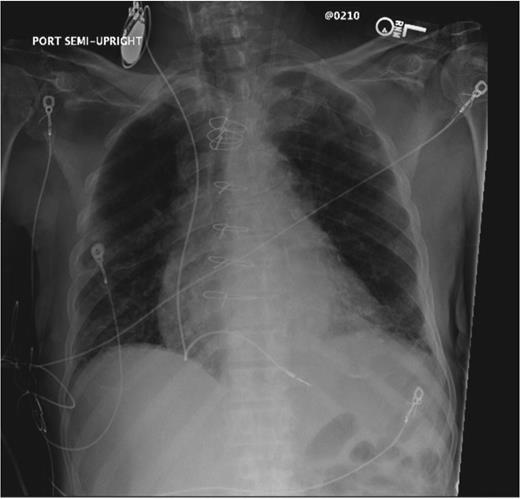
Chest X-ray after an implantation of the temporary permanent pacemaker system.
As is standard practice, re-implanted permanent CIEDs were placed on the contralateral side from the previously infected device. If blood cultures were positive at the time of CIED extraction, repeat blood cultures were performed before re-implantation of a new device, which was delayed until repeat cultures were negative for at least ≥72 h. Re-implantation of a new permanent CIED was also postponed for at least 14 days after device system removal if there was evidence of valvular infection or lead vegetation. Patients with infected implantable cardioverter defibrillators (ICDs) or biventricular ICDs were discharged with both TPPM and a wearable external defibrillator (LifeVest®; Zoll Medical Corp.).
The duration of the implant procedure, length of admission, pacing parameters, and associated complications were recorded and analysed.
Results
Among 47 patients who underwent lead extraction due to confirmed or suspected CIED infection, 23 patients were pacemaker dependent (Table 1). All 23 patients underwent implantation of an external TPPM system via the contralateral internal jugular vein. Mean age was 72 ± 12 years. Blood cultures were positive in 15 patients, and TEE identified either valvular or lead vegetations in 8 patients. The indication for pacing was sick sinus syndrome in 4 patients and complete or high-grade AV nodal block in 19 patients (Table 2).
| Patients | 23 |
| Male | 16 |
| Age (years) | 72 ± 12 |
| Type of infection | |
| Pocket erosion or infection | 8 |
| Bacteraemia | 15 |
| TEE | |
| Vegetation negative | 15 |
| Vegetation positive | 8 |
| Extracted device | |
| Pacemaker | 15 |
| ICD | 6 |
| Biventricular ICD | 2 |
| Indication for temporary pacemaker | |
| Sick sinus syndrome | 4 |
| Complete or high-grade AV block | 19 |
| Patients | 23 |
| Male | 16 |
| Age (years) | 72 ± 12 |
| Type of infection | |
| Pocket erosion or infection | 8 |
| Bacteraemia | 15 |
| TEE | |
| Vegetation negative | 15 |
| Vegetation positive | 8 |
| Extracted device | |
| Pacemaker | 15 |
| ICD | 6 |
| Biventricular ICD | 2 |
| Indication for temporary pacemaker | |
| Sick sinus syndrome | 4 |
| Complete or high-grade AV block | 19 |
| Patients | 23 |
| Male | 16 |
| Age (years) | 72 ± 12 |
| Type of infection | |
| Pocket erosion or infection | 8 |
| Bacteraemia | 15 |
| TEE | |
| Vegetation negative | 15 |
| Vegetation positive | 8 |
| Extracted device | |
| Pacemaker | 15 |
| ICD | 6 |
| Biventricular ICD | 2 |
| Indication for temporary pacemaker | |
| Sick sinus syndrome | 4 |
| Complete or high-grade AV block | 19 |
| Patients | 23 |
| Male | 16 |
| Age (years) | 72 ± 12 |
| Type of infection | |
| Pocket erosion or infection | 8 |
| Bacteraemia | 15 |
| TEE | |
| Vegetation negative | 15 |
| Vegetation positive | 8 |
| Extracted device | |
| Pacemaker | 15 |
| ICD | 6 |
| Biventricular ICD | 2 |
| Indication for temporary pacemaker | |
| Sick sinus syndrome | 4 |
| Complete or high-grade AV block | 19 |
| Right ventricular lead (N = 23) | |
| Amplitude (mV) | 9.7 ± 5.5 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.76 ± 0.37 |
| Impedance | 671 ± 192 |
| Right atrial lead (N = 3) | |
| Amplitude (mV) | 2.5 ± 0.4 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.73 ± 0.17 |
| Impedance (Ω) | 487 ± 66 |
| No. of days | |
| Temporary permanent pacemaker (median) | 19.4 ± 11.8 (18.0) |
| Lead extraction to discharge (median) | 11.6 ± 12.8 (9.0) |
| Antimicrobial therapy days (median) | 31.4 ± 14.1 (42.0) |
| Complication | 1a |
| Right ventricular lead (N = 23) | |
| Amplitude (mV) | 9.7 ± 5.5 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.76 ± 0.37 |
| Impedance | 671 ± 192 |
| Right atrial lead (N = 3) | |
| Amplitude (mV) | 2.5 ± 0.4 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.73 ± 0.17 |
| Impedance (Ω) | 487 ± 66 |
| No. of days | |
| Temporary permanent pacemaker (median) | 19.4 ± 11.8 (18.0) |
| Lead extraction to discharge (median) | 11.6 ± 12.8 (9.0) |
| Antimicrobial therapy days (median) | 31.4 ± 14.1 (42.0) |
| Complication | 1a |
aRecurrent vegetation on the temporary pacemaker lead.
| Right ventricular lead (N = 23) | |
| Amplitude (mV) | 9.7 ± 5.5 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.76 ± 0.37 |
| Impedance | 671 ± 192 |
| Right atrial lead (N = 3) | |
| Amplitude (mV) | 2.5 ± 0.4 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.73 ± 0.17 |
| Impedance (Ω) | 487 ± 66 |
| No. of days | |
| Temporary permanent pacemaker (median) | 19.4 ± 11.8 (18.0) |
| Lead extraction to discharge (median) | 11.6 ± 12.8 (9.0) |
| Antimicrobial therapy days (median) | 31.4 ± 14.1 (42.0) |
| Complication | 1a |
| Right ventricular lead (N = 23) | |
| Amplitude (mV) | 9.7 ± 5.5 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.76 ± 0.37 |
| Impedance | 671 ± 192 |
| Right atrial lead (N = 3) | |
| Amplitude (mV) | 2.5 ± 0.4 |
| Pacing threshold (V, pulse width 0.5 ms) | 0.73 ± 0.17 |
| Impedance (Ω) | 487 ± 66 |
| No. of days | |
| Temporary permanent pacemaker (median) | 19.4 ± 11.8 (18.0) |
| Lead extraction to discharge (median) | 11.6 ± 12.8 (9.0) |
| Antimicrobial therapy days (median) | 31.4 ± 14.1 (42.0) |
| Complication | 1a |
aRecurrent vegetation on the temporary pacemaker lead.
Duration of temporary permanent pacemaker, hospitalization after lead extraction, and antimicrobial therapy
A new permanent CIED was re-implanted at 19.4 ± 11.9 days (median 18, range 3–45) after lead extraction and implantation of the TPPM. The duration of TPPM was 12.1 ± 6.9 days (median 10, range 3–21) in 8 patients with negative blood culture, 23.3 ± 12.3 days (median 20, range 5–45) in 15 patients with positive blood culture, and 28.4 ± 12.9 days (median 22, range 17–45) in 8 patients with positive blood culture. The average time from lead extraction to discharge was 11.6 ± 12.8 days (median 9.0, range 1–61). The average duration of antimicrobial therapy was 31.7 ± 13.6 days (median 42, range 14–56).
Follow-up
One patient remained in the intensive care unit for 2 weeks with a functioning TPPM, but died from overwhelming sepsis due to Enterococcus faecalis bacteraemia and fungaemia, 9 days after lead extraction. One TPPM system was revised to the contralateral side due to persistent positive blood cultures and development of a new vegetation on the tip of the TPPM wire, 2 weeks after initial lead extraction. This patient was found to have an infected femoral pseudoaneurysm which was surgically treated, and the patient subsequently underwent placement of a new permanent CIED without further complications.
The remaining 21 patients did not develop any complications after initial CIED system extraction, and underwent subsequent successful re-implantation of a permanent CIED and removal of the TPPM system. During an average follow-up of 7.1 ± 5.9 months after re-implantation of the new permanent CIED, only one patient developed recurrent CIED infection (3 months after CIED replacement). This patient had a TPPM system for 13 days before CIED re-implantation, which was done on the same side as the original implantation, due to venous occlusion on the contralateral side. The procedure was complicated by bleeding requiring surgical intervention. He presented 3 months after CIED replacement with recurrent S. aureus pocket infection, after sustaining an injury to the site during rafting.
Discussion
Complete removal of the entire CIED system is recommended for patients with established CIED infection. Moreover, re-implantation of a new permanent CIED should be delayed for at least 3 days after CIED system removal, or at least 14 days when there is evidence of positive blood cultures or valvular infection. Temporary pacing is indicated for pacemaker-dependent patients with CIED infection after lead extraction. In previous studies, complication rates of conventional, temporary passive-fixation leads have been reported as high as 37%.3,4 Rastan et al.6 first reported utilization of active-fixation leads attached to pacemaker generators as a bridging therapy. Thus far, only a few studies have described the outcome of the use of TPPMs with active-fixation leads in patients with systemic infection.6–10 In these studies, TPPMs were only used for a relatively short period (median 2 and 8 days, mean 13 days), and no analysis was performed with regard to its usage after transvenous extraction of infected CIED systems. As mentioned above, bridge-pacing therapy using TPPM in patients with systemic CIED infection may require a longer duration than just a few days. In our study, the average duration of TPPM system use was 19.4 days (median 18 days). During this period, no lead dislodgement or sensing abnormality was observed. In contrast, the use of temporary pacing with passive-fixation leads was reported to have higher rates of complications.3,4 Our study suggests that if a long duration of temporary pacing therapy is required, it is better to use active-fixation leads instead of passive fixation leads.
In our study, one patient developed a vegetation on the tip of the TPPM lead. In this patient, blood cultures were positive for methicillin-sensitive S. aureus and repeat TEE confirmed a newly developed lead vegetation. After replacement of the infected TPPM lead, a complete course of vancomycin was successful in eradicating the infection. This case raises several important issues. All patients should stay in the hospital until confirmation of negative blood cultures, preferably for 3 days, after extraction. In the case of persistently positive blood cultures, additional hardware (e.g. dialysis catheters) may need to be removed or replaced since it may be infected as well. Vegetations on the TPPM lead may occur if a patient's blood cultures remain positive after CIED extraction and implantation of a TPPM.
During the follow-up period, one patient developed recurrent infection due to traumatic injury to the site of new ICD. We could not help implanting a new ICD on the ipsilateral side due to occluded contralateral venous system. Although this patient developed recurrent infection due to traumatic injury, contralateral implantation of a new device system might increase the chance of re-infection.
Traditionally, the use of passive-fixation leads would require pacemaker-dependent patients with infected CIED who undergo extraction to stay in the hospital until re-implantation of a new CIED. In our study, 12 of 23 patients were able to be discharged with TPPM to home or nursing facility to complete their course of antibiotic therapy without any complications. Those patients who remained in hospital, did so as a result of other comorbidities. Thus, utilization of a TPPM may reduce the duration of hospital stay and healthcare cost. In addition, patients do not have to stay bed-bound for 2–3 weeks because of temporary pacing. Chihrin et al. evaluated the TPPM with passive fixation leads in a cardiac care unit and found that after 18 hours the TPPM was cost-effective.
In this study, pacemakers extracted from other patients were re-used after cleaning and re-sterilization. Although CIEDs are made for ‘single use’, re-sterilized generators that are not implanted internally and are functioning normally can be re-used as an external TPPM generator during the time period required for temporary pacing. Klug et al.12 reported that use of a peri-procedural temporary pacemaker, prior to permanent CIED implantation, was a risk factor for infection. We could not conclude whether or not this is true in our small cohort study since there was only one recurrent infection at 3 months following CIED re-implantation. A larger cohort study would be required to address the risk of recurrent infection after lead extraction. In addition, it is currently unknown if active-fixation leads would pose an increased risk of infection compared with passive-fixation leads.
Conclusion
The use of active-fixation leads connected to an externally placed permanent pacemaker for temporary pacing in patients requiring long-term antibiotic therapy provides a safe and effective method for bridge pacing. Active fixation leads improve lead stability while allowing greater patient mobility and comfort, including early hospital discharge.
Conflict of interest: G.F.: Medtronic, Fellowship support; Boston Scientific, Fellowship support; St. Jude, Fellowship support; Biotronic, Fellowship support; U.B.-G.: Grant Support/Research Contract, Medtronic, moderate; St. Jude, moderate; Boston Scientific, moderate; H.K.: Japanese Heart Rhythm Society, grant support.
Funding
Gregory Feld:Medtronic, Fellowship support; Boston Scientific, Fellowship support; St. Jude, Fellowship support; Biotronic, Fellowship support. Ulrika Birgersdotter-Green: Grant Support/Research Contract, Medtronic, moderate; St. Jude, moderate; Boston Scientific, moderate. Hiro Kawata: Japanese Heart Rhythm Society, grant support.
References
- antibiotics
- artificial cardiac pacemaker
- neck
- pacemaker leads
- cardiac pacemaker failure to capture
- temporary cardiac pacemaker procedure
- right ventricle
- bone wires
- nursing homes
- surgical replantation
- safety
- infections
- heart
- pacemaker, permanent
- pacemaker, temporary
- internal jugular vein
- medical devices
- mobility
- cardiovascular implantable electronic device
- generators



