-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan P Piccini, Kurt Stromberg, Kevin P Jackson, Robert C Kowal, Gabor Z Duray, Mikhael F El-Chami, George H Crossley, John D Hummel, Calambur Narasimhan, Razali Omar, Philippe Ritter, Paul R Roberts, Kyoko Soejima, Dwight Reynolds, Shu Zhang, Clemens Steinwender, Larry Chinitz, Micra Transcatheter Pacing Study Group, Patient selection, pacing indications, and subsequent outcomes with de novo leadless single-chamber VVI pacing, EP Europace, Volume 21, Issue 11, November 2019, Pages 1686–1693, https://doi.org/10.1093/europace/euz230
Close - Share Icon Share
Abstract
Patient selection is a key component of securing optimal patient outcomes with leadless pacing. We sought to describe and compare patient characteristics and outcomes of Micra patients with and without a primary pacing indication associated with atrial fibrillation (AF) in the Micra IDE trial.
The primary outcome (risk of cardiac failure, pacemaker syndrome, or syncope related to the Micra system or procedure) was compared between successfully implanted patients from the Micra IDE trial with a primary pacing indication associated with AF or history of AF (AF group) and those without (non-AF group). Among 720 patients successfully implanted with Micra, 228 (31.7%) were in the non-AF group. Reasons for selecting VVI pacing in non-AF patients included an expectation for infrequent pacing (66.2%) and advanced age (27.2%). More patients in the non-AF group had a condition that precluded the use of a transvenous pacemaker (9.6% vs. 4.7%, P = 0.013). Atrial fibrillation patients programmed to VVI received significantly more ventricular pacing compared to non-AF patients (median 67.8% vs. 12.6%; P < 0.001). The overall occurrence of the composite outcome at 24 months was 1.8% with no difference between the AF and non-AF groups (hazard ratio 1.36, 95% confidence interval 0.45–4.2; P = 0.59).
Nearly one-third of patients selected to receive Micra VVI therapy were for indications not associated with AF. Non-AF VVI patients required less frequent pacing compared to patients with AF. Risks associated with VVI therapy were low and did not differ in those with and without AF.
Nearly one-third of patients selected to receive Micra VVI therapy were for indications not associated with atrial fibrillation (AF).
Non-AF VVI patients received significantly less ventricular pacing compared to AF patients.
The risk of cardiac failure, pacemaker syndrome, or syncope related to the Micra system or procedure was low (1.8%) and did not differ in those with vs. without a pacing indication associated with AF.
Introduction
The majority of patients who receive single-chamber pacemakers have persistent or permanent atrial fibrillation (AF). In patients with intact atrial transport function, synchronous ventricular pacing leads to improved stroke volume, cardiac output, and quality of life,1 whereas asynchronous ventricular pacing can lead to pacemaker syndrome and the need for upgrade to a dual-chamber device. Despite this, randomized clinical trials have failed to identify significant improvements in all-cause mortality or reduction in major adverse cardiovascular events, although single-chamber pacing is associated with a modest increase in the risk of incident AF compared with synchronous atrioventricular (AV) pacing.2,3 While dual-chamber AV synchronous pacing is generally preferred in patients with intact atrial transport function, single-chamber VVI pacing may be satisfactory in patients who are expected to have a low pacing burden, sedentary patients, those with significant comorbidities that are likely to influence survival and clinical outcomes, and those with vascular access limitations.4,5 The availability of leadless pacemakers may alter the risk-benefit profile of single-chamber pacing in patients with intact atrial transport function. More specifically, the reduction in all-cause complications and infection observed with the Micra leadless pacemaker may be particularly attractive in patients with low pacing burden. Accordingly, we sought to describe the characteristics of patients without persistent or permanent AF undergoing single-chamber leadless pacemaker implantation in the Micra investigation device exemption trial as well as their indications for implantation and to compare the safety, effectiveness, and incidence of major adverse events historically associated with asynchronous ventricular pacing, including pacemaker syndrome in those with and without persistent or permanent AF.
Methods
Study design and oversight
The rationale, design, and primary results of the Micra Transcatheter Pacing Study have been previously described.6 In brief, enrolled patients met Class I or II guideline recommendations for de novo ventricular pacing. Of note, patients with major comorbidities were not excluded. The protocol was approved by the ethics committee at each of the centres, and all patients provided written informed consent. Adverse events were adjudicated by a Clinical Events Committee comprised of independent physicians.
Objectives
The study enrolled 745 patients, with 720 of 726 patients (99.2%) successfully implanted at 56 centres in 19 countries between December 2013 and May 2015. For the purposes of this analysis, implanted patients were divided into two groups, based upon de novo indication for pacing, those with a primary pacing indication associated with permanent/persistent atrial arrhythmias or a history of permanent or persistent AF (AF group) or those without (non-AF group). The composite outcome of interest included occurrence of cardiac failure, pacemaker syndrome, or syncope related to the Micra system or procedure regardless of severity.
Outcomes assessments
All patients were queried for adverse events, including symptoms of pacemaker syndrome, heart failure, and syncope at follow-up visits. Scheduled follow-up visits occurred at discharge, 1 month, 3 months, 6 months, and every 6 months thereafter including at any unscheduled visits.
Statistical analysis
Baseline characteristics and pacemaker programming following implant between groups were compared using t-tests (continuous variables) or Fisher’s exact test (categorical variables). The percentage of ventricular pacing obtained from the device memory at each patient’s last follow-up visit was compared using the Wilcoxon rank-sum test. The Fine–Gray competing risk model was used to compare the rate of safety outcomes with a competing risk of death for any cause between groups through 24 months of follow-up. All analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC, USA) or the R statistical package (R Project for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Of the 720 patients successfully implanted with Micra, 492 (68.3%) had an indication for pacing associated with permanent/persistent AF or a history of AF. The baseline characteristics according to AF status are shown in Table 1. Patients without an AF indication or history of AF were younger (mean age 73 vs. 77 years, P < 0.001) and had fewer comorbidities, including heart failure (7% vs. 23%, P < 0.001), renal dysfunction (14% vs. 23%, P = 0.004), and hypertension (68% vs. 83%, P < 0.001). However, left bundle branch block was more common in those without AF (20% vs. 11%, P = 0.002).
| Patient characteristics . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 77.2 ± 8.5 | 72.8 ± 14.6 | <0.001 |
| Male, n (%) | 293 (59.6) | 132 (57.9) | 0.68 |
| LVEF (%), mean ± SD | 58.4 ± 8.8 | 59.8 ± 8.4 | 0.07 |
| Comorbidities (%), n (%) | |||
| Atrial fibrillation (persistent/permanent) | 405 (82.3) | 0 (0.0) | <0.001 |
| Diabetes | 153 (31.1) | 52 (22.8) | 0.026 |
| Congestive heart failure | 114 (23.2) | 15 (6.6) | <0.001 |
| Hypertension | 409 (83.1) | 156 (68.4) | <0.001 |
| COPD | 70 (14.2) | 21 (9.2) | 0.07 |
| Renal dysfunction | 115 (23.4) | 32 (14.0) | 0.004 |
| Left bundle branch block | 54 (11.0) | 45 (19.7) | 0.002 |
| Vascular disease | 43 (8.7) | 12 (5.3) | 0.13 |
| Primary pacing indication (%), n (%) | <0.001 | ||
| Bradyarrhythmia with persistent/permanent AF | 460 (93.5) | 0 (0) | |
| Sinus node dysfunction | 21 (4.3) | 104 (45.6) | |
| AV block | 2 (0.4) | 106 (46.5) | |
| Syncope | 8 (1.6) | 8 (3.5) | |
| Other | 1 (0.2) | 10 (4.4) | |
| Reason for selecting VVI, n (%)a | |||
| Frequent pacing not expected | 63 (12.8) | 151 (66.2) | <0.001 |
| Significant comorbidity | 10 (2.0) | 16 (7.0) | 0.002 |
| Indication associated with atrial tachyarrhythmia | 467 (94.9) | 0 (0.0) | <0.001 |
| Previous/planned AV node ablation | 64 (13.0) | 3 (1.3) | <0.001 |
| Advanced age | 67 (13.6) | 62 (27.2) | <0.001 |
| Patient expected to be sedentary | 15 (3.0) | 10 (4.4) | 0.38 |
| Patient anatomy precludes atrial lead | 2 (0.4) | 7 (3.1) | 0.006 |
| Dual chamber patient risk too high | 1 (0.2) | 8 (3.5) | 0.001 |
| Patient preference | 44 (8.9) | 44 (19.3) | <0.001 |
| Patient condition | 6 (1.2) | 7 (3.1) | 0.13 |
| Other reason | 0 (0.0) | 5 (2.2) | 0.003 |
| Condition precluding transvenous pacemaker implant, n (%) | |||
| Any condition | 23 (4.7) | 22 (9.6) | 0.013 |
| Need to preserve veins | 9 (1.8) | 9 (3.9) | |
| Thrombosis | 5 (1.0) | 7 (3.1) | |
| Infection history | 4 (0.8) | 0 (0.0) | |
| Need for in dwelling catheter | 4 (0.8) | 2 (0.9) | |
| Other condition | 1 (0.2) | 5 (2.2) | |
| Pacing mode, n (%)b | |||
| VVIR | 104 (21.1) | 24 (10.5) | 0.001 |
| VVI | 387 (78.7) | 204 (89.5) | |
| Lower pacing rate (b.p.m.)b | |||
| Mean ± SD | 59.0 ± 9.4 | 54.6 ± 9.7 | <0.001 |
| Lower pacing rate (b.p.m.), n (%)b | |||
| ≤40 | 28 (5.7) | 33 (14.5) | <0.001 |
| >40–50 | 104 (21.1) | 79 (34.6) | |
| >50–60 | 283 (57.5) | 91 (39.9) | |
| >60–70 | 35 (7.1) | 20 (8.8) | |
| >70 | 35 (7.1) | 20 (8.8) |
| Patient characteristics . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 77.2 ± 8.5 | 72.8 ± 14.6 | <0.001 |
| Male, n (%) | 293 (59.6) | 132 (57.9) | 0.68 |
| LVEF (%), mean ± SD | 58.4 ± 8.8 | 59.8 ± 8.4 | 0.07 |
| Comorbidities (%), n (%) | |||
| Atrial fibrillation (persistent/permanent) | 405 (82.3) | 0 (0.0) | <0.001 |
| Diabetes | 153 (31.1) | 52 (22.8) | 0.026 |
| Congestive heart failure | 114 (23.2) | 15 (6.6) | <0.001 |
| Hypertension | 409 (83.1) | 156 (68.4) | <0.001 |
| COPD | 70 (14.2) | 21 (9.2) | 0.07 |
| Renal dysfunction | 115 (23.4) | 32 (14.0) | 0.004 |
| Left bundle branch block | 54 (11.0) | 45 (19.7) | 0.002 |
| Vascular disease | 43 (8.7) | 12 (5.3) | 0.13 |
| Primary pacing indication (%), n (%) | <0.001 | ||
| Bradyarrhythmia with persistent/permanent AF | 460 (93.5) | 0 (0) | |
| Sinus node dysfunction | 21 (4.3) | 104 (45.6) | |
| AV block | 2 (0.4) | 106 (46.5) | |
| Syncope | 8 (1.6) | 8 (3.5) | |
| Other | 1 (0.2) | 10 (4.4) | |
| Reason for selecting VVI, n (%)a | |||
| Frequent pacing not expected | 63 (12.8) | 151 (66.2) | <0.001 |
| Significant comorbidity | 10 (2.0) | 16 (7.0) | 0.002 |
| Indication associated with atrial tachyarrhythmia | 467 (94.9) | 0 (0.0) | <0.001 |
| Previous/planned AV node ablation | 64 (13.0) | 3 (1.3) | <0.001 |
| Advanced age | 67 (13.6) | 62 (27.2) | <0.001 |
| Patient expected to be sedentary | 15 (3.0) | 10 (4.4) | 0.38 |
| Patient anatomy precludes atrial lead | 2 (0.4) | 7 (3.1) | 0.006 |
| Dual chamber patient risk too high | 1 (0.2) | 8 (3.5) | 0.001 |
| Patient preference | 44 (8.9) | 44 (19.3) | <0.001 |
| Patient condition | 6 (1.2) | 7 (3.1) | 0.13 |
| Other reason | 0 (0.0) | 5 (2.2) | 0.003 |
| Condition precluding transvenous pacemaker implant, n (%) | |||
| Any condition | 23 (4.7) | 22 (9.6) | 0.013 |
| Need to preserve veins | 9 (1.8) | 9 (3.9) | |
| Thrombosis | 5 (1.0) | 7 (3.1) | |
| Infection history | 4 (0.8) | 0 (0.0) | |
| Need for in dwelling catheter | 4 (0.8) | 2 (0.9) | |
| Other condition | 1 (0.2) | 5 (2.2) | |
| Pacing mode, n (%)b | |||
| VVIR | 104 (21.1) | 24 (10.5) | 0.001 |
| VVI | 387 (78.7) | 204 (89.5) | |
| Lower pacing rate (b.p.m.)b | |||
| Mean ± SD | 59.0 ± 9.4 | 54.6 ± 9.7 | <0.001 |
| Lower pacing rate (b.p.m.), n (%)b | |||
| ≤40 | 28 (5.7) | 33 (14.5) | <0.001 |
| >40–50 | 104 (21.1) | 79 (34.6) | |
| >50–60 | 283 (57.5) | 91 (39.9) | |
| >60–70 | 35 (7.1) | 20 (8.8) | |
| >70 | 35 (7.1) | 20 (8.8) |
AF, atrial fibrillation; AV, atrioventricular; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SD, standard deviation.
The reason for selecting VVI in the view of the implanting physician. Thus, this categorization represents the subjective view of the implanting physician.
As recorded on device interrogation file immediately following implant.
| Patient characteristics . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 77.2 ± 8.5 | 72.8 ± 14.6 | <0.001 |
| Male, n (%) | 293 (59.6) | 132 (57.9) | 0.68 |
| LVEF (%), mean ± SD | 58.4 ± 8.8 | 59.8 ± 8.4 | 0.07 |
| Comorbidities (%), n (%) | |||
| Atrial fibrillation (persistent/permanent) | 405 (82.3) | 0 (0.0) | <0.001 |
| Diabetes | 153 (31.1) | 52 (22.8) | 0.026 |
| Congestive heart failure | 114 (23.2) | 15 (6.6) | <0.001 |
| Hypertension | 409 (83.1) | 156 (68.4) | <0.001 |
| COPD | 70 (14.2) | 21 (9.2) | 0.07 |
| Renal dysfunction | 115 (23.4) | 32 (14.0) | 0.004 |
| Left bundle branch block | 54 (11.0) | 45 (19.7) | 0.002 |
| Vascular disease | 43 (8.7) | 12 (5.3) | 0.13 |
| Primary pacing indication (%), n (%) | <0.001 | ||
| Bradyarrhythmia with persistent/permanent AF | 460 (93.5) | 0 (0) | |
| Sinus node dysfunction | 21 (4.3) | 104 (45.6) | |
| AV block | 2 (0.4) | 106 (46.5) | |
| Syncope | 8 (1.6) | 8 (3.5) | |
| Other | 1 (0.2) | 10 (4.4) | |
| Reason for selecting VVI, n (%)a | |||
| Frequent pacing not expected | 63 (12.8) | 151 (66.2) | <0.001 |
| Significant comorbidity | 10 (2.0) | 16 (7.0) | 0.002 |
| Indication associated with atrial tachyarrhythmia | 467 (94.9) | 0 (0.0) | <0.001 |
| Previous/planned AV node ablation | 64 (13.0) | 3 (1.3) | <0.001 |
| Advanced age | 67 (13.6) | 62 (27.2) | <0.001 |
| Patient expected to be sedentary | 15 (3.0) | 10 (4.4) | 0.38 |
| Patient anatomy precludes atrial lead | 2 (0.4) | 7 (3.1) | 0.006 |
| Dual chamber patient risk too high | 1 (0.2) | 8 (3.5) | 0.001 |
| Patient preference | 44 (8.9) | 44 (19.3) | <0.001 |
| Patient condition | 6 (1.2) | 7 (3.1) | 0.13 |
| Other reason | 0 (0.0) | 5 (2.2) | 0.003 |
| Condition precluding transvenous pacemaker implant, n (%) | |||
| Any condition | 23 (4.7) | 22 (9.6) | 0.013 |
| Need to preserve veins | 9 (1.8) | 9 (3.9) | |
| Thrombosis | 5 (1.0) | 7 (3.1) | |
| Infection history | 4 (0.8) | 0 (0.0) | |
| Need for in dwelling catheter | 4 (0.8) | 2 (0.9) | |
| Other condition | 1 (0.2) | 5 (2.2) | |
| Pacing mode, n (%)b | |||
| VVIR | 104 (21.1) | 24 (10.5) | 0.001 |
| VVI | 387 (78.7) | 204 (89.5) | |
| Lower pacing rate (b.p.m.)b | |||
| Mean ± SD | 59.0 ± 9.4 | 54.6 ± 9.7 | <0.001 |
| Lower pacing rate (b.p.m.), n (%)b | |||
| ≤40 | 28 (5.7) | 33 (14.5) | <0.001 |
| >40–50 | 104 (21.1) | 79 (34.6) | |
| >50–60 | 283 (57.5) | 91 (39.9) | |
| >60–70 | 35 (7.1) | 20 (8.8) | |
| >70 | 35 (7.1) | 20 (8.8) |
| Patient characteristics . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Age (years), mean ± SD | 77.2 ± 8.5 | 72.8 ± 14.6 | <0.001 |
| Male, n (%) | 293 (59.6) | 132 (57.9) | 0.68 |
| LVEF (%), mean ± SD | 58.4 ± 8.8 | 59.8 ± 8.4 | 0.07 |
| Comorbidities (%), n (%) | |||
| Atrial fibrillation (persistent/permanent) | 405 (82.3) | 0 (0.0) | <0.001 |
| Diabetes | 153 (31.1) | 52 (22.8) | 0.026 |
| Congestive heart failure | 114 (23.2) | 15 (6.6) | <0.001 |
| Hypertension | 409 (83.1) | 156 (68.4) | <0.001 |
| COPD | 70 (14.2) | 21 (9.2) | 0.07 |
| Renal dysfunction | 115 (23.4) | 32 (14.0) | 0.004 |
| Left bundle branch block | 54 (11.0) | 45 (19.7) | 0.002 |
| Vascular disease | 43 (8.7) | 12 (5.3) | 0.13 |
| Primary pacing indication (%), n (%) | <0.001 | ||
| Bradyarrhythmia with persistent/permanent AF | 460 (93.5) | 0 (0) | |
| Sinus node dysfunction | 21 (4.3) | 104 (45.6) | |
| AV block | 2 (0.4) | 106 (46.5) | |
| Syncope | 8 (1.6) | 8 (3.5) | |
| Other | 1 (0.2) | 10 (4.4) | |
| Reason for selecting VVI, n (%)a | |||
| Frequent pacing not expected | 63 (12.8) | 151 (66.2) | <0.001 |
| Significant comorbidity | 10 (2.0) | 16 (7.0) | 0.002 |
| Indication associated with atrial tachyarrhythmia | 467 (94.9) | 0 (0.0) | <0.001 |
| Previous/planned AV node ablation | 64 (13.0) | 3 (1.3) | <0.001 |
| Advanced age | 67 (13.6) | 62 (27.2) | <0.001 |
| Patient expected to be sedentary | 15 (3.0) | 10 (4.4) | 0.38 |
| Patient anatomy precludes atrial lead | 2 (0.4) | 7 (3.1) | 0.006 |
| Dual chamber patient risk too high | 1 (0.2) | 8 (3.5) | 0.001 |
| Patient preference | 44 (8.9) | 44 (19.3) | <0.001 |
| Patient condition | 6 (1.2) | 7 (3.1) | 0.13 |
| Other reason | 0 (0.0) | 5 (2.2) | 0.003 |
| Condition precluding transvenous pacemaker implant, n (%) | |||
| Any condition | 23 (4.7) | 22 (9.6) | 0.013 |
| Need to preserve veins | 9 (1.8) | 9 (3.9) | |
| Thrombosis | 5 (1.0) | 7 (3.1) | |
| Infection history | 4 (0.8) | 0 (0.0) | |
| Need for in dwelling catheter | 4 (0.8) | 2 (0.9) | |
| Other condition | 1 (0.2) | 5 (2.2) | |
| Pacing mode, n (%)b | |||
| VVIR | 104 (21.1) | 24 (10.5) | 0.001 |
| VVI | 387 (78.7) | 204 (89.5) | |
| Lower pacing rate (b.p.m.)b | |||
| Mean ± SD | 59.0 ± 9.4 | 54.6 ± 9.7 | <0.001 |
| Lower pacing rate (b.p.m.), n (%)b | |||
| ≤40 | 28 (5.7) | 33 (14.5) | <0.001 |
| >40–50 | 104 (21.1) | 79 (34.6) | |
| >50–60 | 283 (57.5) | 91 (39.9) | |
| >60–70 | 35 (7.1) | 20 (8.8) | |
| >70 | 35 (7.1) | 20 (8.8) |
AF, atrial fibrillation; AV, atrioventricular; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SD, standard deviation.
The reason for selecting VVI in the view of the implanting physician. Thus, this categorization represents the subjective view of the implanting physician.
As recorded on device interrogation file immediately following implant.
Pacing indications
Among the 228 (32%) patients in the non-AF group, the primary indications for pacing included: AV block (47%), sinus node dysfunction (46%), syncope (4%), and other indication (4%). As expected, the primary pacing indications were different between the AF and non-AF indication/history group (Table 1). Of the 352 patients with a pacing indication for AV block, 53%, 31%, and 16% were indicated for 2nd degree AV block, persistent 3rd degree AV block, and intermittent 3rd degree AV block, respectively. In addition, 5% of AV block patients were considered pacemaker dependent. In contrast to the primary pacing indications, the reasons cited by the implanting physician for selecting VVI were markedly different in the AF and non-AF group. Patients in the non-AF group were more often implanted due to an expectation for infrequent pacing (66% vs. 13%, P < 0.001), significant comorbidity in the view of the implanting physician (7% vs. 2%, P = 0.002), advanced age (27% vs. 14%, P < 0.001), and patient preference (19% vs. 9%, P < 0.001). Compared with patients in the AF group, a significantly higher proportion of patients in the non-AF group had a condition that precluded the use of a transvenous pacemaker (9.6% vs. 4.7%, P = 0.013).
Implant procedure and pacing characteristics
The characteristics of the leadless pacemaker implant procedure according to AF status are shown in Table 2. The implant duration was slightly shorter in the non-AF group (31 ± 23 vs. 37 ± 24 min, P = 0.005) and fewer patients required >2 deployments compared with the AF group (16% vs. 24%, P = 0.015). More patients in the AF group underwent a concomitant procedure during the Micra implant (9% vs. 3%, P = 0.002) with 42 patients in the AF group undergoing a concomitant AV nodal ablation procedure. Other concomitant procedures included implantable loop recorder explant (three patients in the AF group and four patients in the non-AF group), electrophysiology study (one patient in non-AF group), and temporary pacemaker placement (one patient in the non-AF group). Electrical parameters at implant did not differ significantly between the groups. R-wave amplitude and pacing thresholds were similar at implant. A threshold greater than 2 V (with pulse width 0.24 ms) was uncommon in those with AF (2%) and those without (1%).
| Variables . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Implant duration (min)a | 36.5 ± 24.2 | 31.1 ± 23.4 | 0.005 |
| Fluoroscopy (min)b | 8.4 ± 9.3 | 8.4 ± 7.0 | 1.00 |
| Concomitant procedure, n (%) | 43 (8.7) | 6 (2.6) | 0.002 |
| >2 deployments required, n (%) | 117 (23.8) | 36 (15.8) | 0.015 |
| R-wave amplitude (mV) | 11.2 ± 5.1 | 11.3 ± 4.8 | 0.85 |
| Threshold at 0.24 ms (V) | 0.65 ± 0.49 | 0.58 ± 0.36 | 0.053 |
| Threshold at 0.24 ms >2.0 V, n (%) | 10 (2.0) | 2 (0.9) | 0.36 |
| Variables . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Implant duration (min)a | 36.5 ± 24.2 | 31.1 ± 23.4 | 0.005 |
| Fluoroscopy (min)b | 8.4 ± 9.3 | 8.4 ± 7.0 | 1.00 |
| Concomitant procedure, n (%) | 43 (8.7) | 6 (2.6) | 0.002 |
| >2 deployments required, n (%) | 117 (23.8) | 36 (15.8) | 0.015 |
| R-wave amplitude (mV) | 11.2 ± 5.1 | 11.3 ± 4.8 | 0.85 |
| Threshold at 0.24 ms (V) | 0.65 ± 0.49 | 0.58 ± 0.36 | 0.053 |
| Threshold at 0.24 ms >2.0 V, n (%) | 10 (2.0) | 2 (0.9) | 0.36 |
AF, atrial fibrillation; AV, atrioventricular.
Defined as the time from the Micra introducer inserted to it was removed from the body.
Excludes one outlier of 387 min that occurred during a concomitant AV nodal ablation.
| Variables . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Implant duration (min)a | 36.5 ± 24.2 | 31.1 ± 23.4 | 0.005 |
| Fluoroscopy (min)b | 8.4 ± 9.3 | 8.4 ± 7.0 | 1.00 |
| Concomitant procedure, n (%) | 43 (8.7) | 6 (2.6) | 0.002 |
| >2 deployments required, n (%) | 117 (23.8) | 36 (15.8) | 0.015 |
| R-wave amplitude (mV) | 11.2 ± 5.1 | 11.3 ± 4.8 | 0.85 |
| Threshold at 0.24 ms (V) | 0.65 ± 0.49 | 0.58 ± 0.36 | 0.053 |
| Threshold at 0.24 ms >2.0 V, n (%) | 10 (2.0) | 2 (0.9) | 0.36 |
| Variables . | AF indication/history (N = 492) . | No AF indication/history (N = 228) . | P-value . |
|---|---|---|---|
| Implant duration (min)a | 36.5 ± 24.2 | 31.1 ± 23.4 | 0.005 |
| Fluoroscopy (min)b | 8.4 ± 9.3 | 8.4 ± 7.0 | 1.00 |
| Concomitant procedure, n (%) | 43 (8.7) | 6 (2.6) | 0.002 |
| >2 deployments required, n (%) | 117 (23.8) | 36 (15.8) | 0.015 |
| R-wave amplitude (mV) | 11.2 ± 5.1 | 11.3 ± 4.8 | 0.85 |
| Threshold at 0.24 ms (V) | 0.65 ± 0.49 | 0.58 ± 0.36 | 0.053 |
| Threshold at 0.24 ms >2.0 V, n (%) | 10 (2.0) | 2 (0.9) | 0.36 |
AF, atrial fibrillation; AV, atrioventricular.
Defined as the time from the Micra introducer inserted to it was removed from the body.
Excludes one outlier of 387 min that occurred during a concomitant AV nodal ablation.
As shown in Figure 1, among the 127 patients programmed to VVIR pacing (103 with AF pacing indications vs. 24 without AF pacing indications), the percentage of ventricular pacing was not different [median 81.3% (interquartile range, IQR 39.2–95.9%) vs. median 68.8% (IQR 1–59.84%) P = 0.40]. However, among the 587 patients programmed to VVI pacing, the median percentage of ventricular pacing was 67.8% (IQR 26.6–95.1%) for the 383 patients with AF indications/history compared to 12.6% (IQR 35.8–93.5%) for the 204 patients without an AF indication or history (P < 0.001).
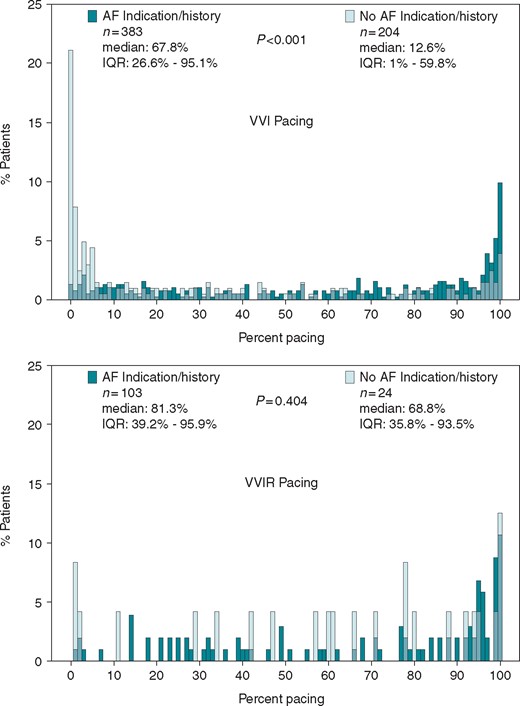
Pacing percentage distribution between groups over mean follow-up duration 16.4 ± 4.9 months. The percentage of ventricular pacing obtained from the device memory at each patient’s last follow-up visit and was compared using the Wilcoxon rank-sum test. Green bars represent AF indication/history, light green bars represent no AF indication/history. The top panel shows those patients programmed to VVI and the bottom panel shows those patients programmed to VVIR (light green bars for no AF indication/history are transparent so that pacing distributions for both groups may be visualized). AF, atrial fibrillation; IQR, interquartile range.
Adverse cardiovascular events in follow-up
Table 3 shows the patient-level adverse cardiovascular events of interest in this analysis, including cardiac failure, pacemaker syndrome, and syncope. When considering all events, there was no significant difference in the composite outcome between the AF and non-AF groups (8 events vs. 5 events, respectively; hazard ratio 1.36, 95% confidence interval 0.45–4.2; P = 0.590; Figure 2). The risk for the composite outcome did not differ between those patients paced less than 40% and those 40% or more of the time (P interaction = 0.740). There were no (0.0%) AF events considered related to the Micra system or procedure.
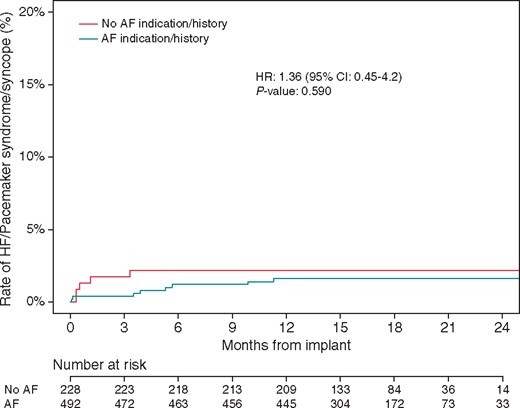
Rate of HF/pacemaker syndrome/syncope events in patients with and without AF indication or history. Subdistributional HR from the Fine–Gray competing risk model derived from data through 24 months post-implant for each cohort by comparing the cumulative incidence of safety outcomes in the presence of competing risk of death for any reason. AF, atrial fibrillation; HF, heart failure; HR, hazard ratio.
| Event no. . | Event type . | Patient age (years) . | Days post- implant . | Gender . | Implant indication . | History of AF . | History of HF . | LVEF . | %Vp prior to event . | Outcomes . | Major complication . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cardiac failure | 80 | 300 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 59 | 87.4% | Hospitalized, IV diuretics, resolved | Yes |
| 2 | Cardiac failure | 62 | 172 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 45 | 98.9% | Upgraded to CRT-P device | Yes |
| 3 | Cardiac failure | 81 | 343 | Male | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 60 | 99.1% | Hospitalized, IV diuretics, resolved | Yes |
| 4 | Cardiac failure | 78 | 107 | Female | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | Yes | 45 | 97.9% | Hospitalized, IV diuretics, resolved | Yes |
| 5 | Cardiac failure | 82 | 118 | Female | Other indications with persistent/permanent atrial arrhythmias | Yes | No | 60 | 41.3% | Hospitalized, IV diuretics, resolved | Yes |
| 6 | Cardiac failure | 82 | 161 | Male | AV block with persistent/permanent atrial arrhythmias | Yes | No | 60 | 96.4% | Hospitalized, IV diuretics, device reprogrammed | Yes |
| 7 | Pacemaker syndrome | 81 | 4 | Male | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | No | 65 | 81.2% | Device reprogrammed to LR 60 b.p.m. | No |
| 8 | Pacemaker syndrome | 63 | 2 | Female | AV block with persistent/permanent atrial arrhythmias | Yes | No | 55 | 0.13% | Device reprogrammed | No |
| 9 | Cardiac failure | 86 | 101 | Male | SND with AV block but without persistent/permanent atrial arrhythmias | No | No | 70 | 98.7% | Hospitalized, oral diuretics | No |
| 10 | Pacemaker syndrome | 43 | 9 | Female | Syncope | No | No | 64 | 0.02% | Micra turned OOO, implanted with dual chamber PM | Yes |
| 11 | Pacemaker syndrome | 88 | 34 | Male | 2rd degree block without atrial arrhythmias | No | No | 55 | 85.4% | Micra turned OOO, implanted with CRT-P | Yes |
| 12 | Pacemaker syndrome | 80 | 9 | Male | 2nd degree block without atrial arrhythmias | No | No | Not provided | 2.8% | Device reprogrammed | No |
| 13 | Syncope | 85 | 16 | Male | 2nd degree block without atrial arrhythmias | No | No | 60 | 31.5% | Hospitalized, IV fluids | Yes |
| Event no. . | Event type . | Patient age (years) . | Days post- implant . | Gender . | Implant indication . | History of AF . | History of HF . | LVEF . | %Vp prior to event . | Outcomes . | Major complication . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cardiac failure | 80 | 300 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 59 | 87.4% | Hospitalized, IV diuretics, resolved | Yes |
| 2 | Cardiac failure | 62 | 172 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 45 | 98.9% | Upgraded to CRT-P device | Yes |
| 3 | Cardiac failure | 81 | 343 | Male | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 60 | 99.1% | Hospitalized, IV diuretics, resolved | Yes |
| 4 | Cardiac failure | 78 | 107 | Female | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | Yes | 45 | 97.9% | Hospitalized, IV diuretics, resolved | Yes |
| 5 | Cardiac failure | 82 | 118 | Female | Other indications with persistent/permanent atrial arrhythmias | Yes | No | 60 | 41.3% | Hospitalized, IV diuretics, resolved | Yes |
| 6 | Cardiac failure | 82 | 161 | Male | AV block with persistent/permanent atrial arrhythmias | Yes | No | 60 | 96.4% | Hospitalized, IV diuretics, device reprogrammed | Yes |
| 7 | Pacemaker syndrome | 81 | 4 | Male | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | No | 65 | 81.2% | Device reprogrammed to LR 60 b.p.m. | No |
| 8 | Pacemaker syndrome | 63 | 2 | Female | AV block with persistent/permanent atrial arrhythmias | Yes | No | 55 | 0.13% | Device reprogrammed | No |
| 9 | Cardiac failure | 86 | 101 | Male | SND with AV block but without persistent/permanent atrial arrhythmias | No | No | 70 | 98.7% | Hospitalized, oral diuretics | No |
| 10 | Pacemaker syndrome | 43 | 9 | Female | Syncope | No | No | 64 | 0.02% | Micra turned OOO, implanted with dual chamber PM | Yes |
| 11 | Pacemaker syndrome | 88 | 34 | Male | 2rd degree block without atrial arrhythmias | No | No | 55 | 85.4% | Micra turned OOO, implanted with CRT-P | Yes |
| 12 | Pacemaker syndrome | 80 | 9 | Male | 2nd degree block without atrial arrhythmias | No | No | Not provided | 2.8% | Device reprogrammed | No |
| 13 | Syncope | 85 | 16 | Male | 2nd degree block without atrial arrhythmias | No | No | 60 | 31.5% | Hospitalized, IV fluids | Yes |
AF, atrial fibrillation; AV, atrioventricular; HF, heart failure; LR, lower rate; LVEF, left ventricular ejection fraction; PM, pacemaker; SND, sinus node dysfunction.
| Event no. . | Event type . | Patient age (years) . | Days post- implant . | Gender . | Implant indication . | History of AF . | History of HF . | LVEF . | %Vp prior to event . | Outcomes . | Major complication . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cardiac failure | 80 | 300 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 59 | 87.4% | Hospitalized, IV diuretics, resolved | Yes |
| 2 | Cardiac failure | 62 | 172 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 45 | 98.9% | Upgraded to CRT-P device | Yes |
| 3 | Cardiac failure | 81 | 343 | Male | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 60 | 99.1% | Hospitalized, IV diuretics, resolved | Yes |
| 4 | Cardiac failure | 78 | 107 | Female | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | Yes | 45 | 97.9% | Hospitalized, IV diuretics, resolved | Yes |
| 5 | Cardiac failure | 82 | 118 | Female | Other indications with persistent/permanent atrial arrhythmias | Yes | No | 60 | 41.3% | Hospitalized, IV diuretics, resolved | Yes |
| 6 | Cardiac failure | 82 | 161 | Male | AV block with persistent/permanent atrial arrhythmias | Yes | No | 60 | 96.4% | Hospitalized, IV diuretics, device reprogrammed | Yes |
| 7 | Pacemaker syndrome | 81 | 4 | Male | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | No | 65 | 81.2% | Device reprogrammed to LR 60 b.p.m. | No |
| 8 | Pacemaker syndrome | 63 | 2 | Female | AV block with persistent/permanent atrial arrhythmias | Yes | No | 55 | 0.13% | Device reprogrammed | No |
| 9 | Cardiac failure | 86 | 101 | Male | SND with AV block but without persistent/permanent atrial arrhythmias | No | No | 70 | 98.7% | Hospitalized, oral diuretics | No |
| 10 | Pacemaker syndrome | 43 | 9 | Female | Syncope | No | No | 64 | 0.02% | Micra turned OOO, implanted with dual chamber PM | Yes |
| 11 | Pacemaker syndrome | 88 | 34 | Male | 2rd degree block without atrial arrhythmias | No | No | 55 | 85.4% | Micra turned OOO, implanted with CRT-P | Yes |
| 12 | Pacemaker syndrome | 80 | 9 | Male | 2nd degree block without atrial arrhythmias | No | No | Not provided | 2.8% | Device reprogrammed | No |
| 13 | Syncope | 85 | 16 | Male | 2nd degree block without atrial arrhythmias | No | No | 60 | 31.5% | Hospitalized, IV fluids | Yes |
| Event no. . | Event type . | Patient age (years) . | Days post- implant . | Gender . | Implant indication . | History of AF . | History of HF . | LVEF . | %Vp prior to event . | Outcomes . | Major complication . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cardiac failure | 80 | 300 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 59 | 87.4% | Hospitalized, IV diuretics, resolved | Yes |
| 2 | Cardiac failure | 62 | 172 | Female | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 45 | 98.9% | Upgraded to CRT-P device | Yes |
| 3 | Cardiac failure | 81 | 343 | Male | Complete AV block anticipated after AV nodal ablation with atrial arrhythmias | Yes | No | 60 | 99.1% | Hospitalized, IV diuretics, resolved | Yes |
| 4 | Cardiac failure | 78 | 107 | Female | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | Yes | 45 | 97.9% | Hospitalized, IV diuretics, resolved | Yes |
| 5 | Cardiac failure | 82 | 118 | Female | Other indications with persistent/permanent atrial arrhythmias | Yes | No | 60 | 41.3% | Hospitalized, IV diuretics, resolved | Yes |
| 6 | Cardiac failure | 82 | 161 | Male | AV block with persistent/permanent atrial arrhythmias | Yes | No | 60 | 96.4% | Hospitalized, IV diuretics, device reprogrammed | Yes |
| 7 | Pacemaker syndrome | 81 | 4 | Male | SND with AV block and with persistent/permanent atrial arrhythmias | Yes | No | 65 | 81.2% | Device reprogrammed to LR 60 b.p.m. | No |
| 8 | Pacemaker syndrome | 63 | 2 | Female | AV block with persistent/permanent atrial arrhythmias | Yes | No | 55 | 0.13% | Device reprogrammed | No |
| 9 | Cardiac failure | 86 | 101 | Male | SND with AV block but without persistent/permanent atrial arrhythmias | No | No | 70 | 98.7% | Hospitalized, oral diuretics | No |
| 10 | Pacemaker syndrome | 43 | 9 | Female | Syncope | No | No | 64 | 0.02% | Micra turned OOO, implanted with dual chamber PM | Yes |
| 11 | Pacemaker syndrome | 88 | 34 | Male | 2rd degree block without atrial arrhythmias | No | No | 55 | 85.4% | Micra turned OOO, implanted with CRT-P | Yes |
| 12 | Pacemaker syndrome | 80 | 9 | Male | 2nd degree block without atrial arrhythmias | No | No | Not provided | 2.8% | Device reprogrammed | No |
| 13 | Syncope | 85 | 16 | Male | 2nd degree block without atrial arrhythmias | No | No | 60 | 31.5% | Hospitalized, IV fluids | Yes |
AF, atrial fibrillation; AV, atrioventricular; HF, heart failure; LR, lower rate; LVEF, left ventricular ejection fraction; PM, pacemaker; SND, sinus node dysfunction.
There were five cases of pacemaker syndrome, three events occurred in the non-AF group and two events occurred in the AF group. Thus, the absolute risk of pacemaker syndrome through 24 months in the AF group was 0.4% and 1.3% in the non-AF group (P = 0.176). Most of the pacemaker syndrome events occurred in elderly, male patients (three of five patients male ≥80 years). Ventricular pacing prior to pacemaker syndrome events ranged from 0.02% to 85.4%. In the non-AF cases, two of the three patients had 2nd or 3rd degree AV block without atrial arrhythmias. Two of the three of the non-AF patients with pacemaker syndrome required intervention: Micra reprogrammed OOO and dual-chamber implant and Micra reprogrammed OOO and CRT-P implant. In the remaining patient, the Micra device was reprogrammed. In the two patients with AF and pacemaker syndrome, the Micra device was reprogrammed to disable rate response and decrease the lower rate in both cases. There was one syncope event considered related to the Micra system or procedure. This event occurred in an 85-year-old man with 2nd degree AV block with no atrial arrhythmia, who had 31.5% ventricular pacing prior to the event. The event resolved after hospitalization with IV fluids and met the criteria for a major adverse event; however, the event did not require reprogramming or device revision/upgrade.
The majority of cardiac failure events (six of seven) were in the AF group and occurred in elderly patients (six of seven patients >75 years) with a high percentage of ventricular pacing prior to the event (five of seven >95% ventricular pacing). Most of the cardiac failure events resolved via hospitalization and administration of diuretics, only one event required an upgrade to a CRT-P device.
Overall, the rate of device upgrade was 0.7% in those with AF and 1.4% in those without AF throughout follow-up (P = 0.338).
Discussion
We investigated the patient characteristics, indications for pacing, and outcomes associated with persistent or permanent AF in the pivotal Micra leadless pacing IDE trial. In this analysis of patient selection for leadless pacing, there are several important findings. First, while the majority of patients implanted in the IDE trial had persistent or permanent AF, nearly one-third of the patients had a pacing indication without persistent or permanent AF. Non-AF indicated patients received Micra for reasons supported by consensus guidelines, and they tended to be younger and had fewer comorbidities. Non-AF patients required less frequent ventricular pacing throughout follow-up. Finally, risks of syncope, congestive heart failure, or pacemaker syndrome events were very low, including in patients with intact atrial transport function (no persistent or permanent AF).
The decision to use Micra must weigh the benefits and risks of leadless VVIR pacing relative to transvenous pacemaker systems. Per design, Micra has no transvenous lead and requires no subcutaneous pocket, the sources for common complications and lifestyle restrictions associated with transvenous pacemakers. Patients with Micra from the IDE study experienced 48% fewer major complications at 1 year follow-up compared to patients with transvenous systems.7 This reduction was largely attributed to a lack of dislodgement and lower rate of system revision with Micra.8 Further long-term benefits are anticipated due to reduced risks of infections, venous obstruction, lead fractures and insulation breaches, tricuspid valve injury, and Twiddler’s syndrome that are attributable to transvenous pacemakers. Micra may also serve as an alternative to epicardial pacemakers, as 6.3% of patients had an indication that precluded the use of a transvenous system.
Despite its advantages, there are potential risks with Micra. Micra’s procedure is novel as it is placed with a steerable catheter via a 23-Fr introducer; yet, implant success was 99.2% in the IDE study and 99.6% in a post-market registry, and complications at the groin puncture site are low (0.7% IDE, 0.8% registry).6,9 There are concerns over cardiac perforations and pericardial effusions as these occurred in 1.8% in the IDE study patients and tended to require intervention.6 However, these events appear to be occurring less frequently in the registry at a rate of 0.6%—possibly due to implanting less often at the apex.9
The risks of VVI pacing relative to atrial-based pacing modes are well-known. VVI pacing is the preferred mode for patients with persistent/permanent AF, and not surprisingly the majority of patients who received Micra had AF. Unexpectedly, nearly one-third of patients did not have AF and yet were treated with VVI therapy for sinus node dysfunction (SND), AV block, or syncope. A key question is whether implantation in this group of patients, those with intact sinus node function, leads to safe and effective pacing without increased risk of long-term complications. This is the crux of our investigation.
Physiologic assessments have shown that AV synchronous pacing is associated with better stroke volume, cardiac output, and patient well-being.1 However, randomized trials have failed to demonstrate reduced all-cause mortality or major cardiovascular events with dual-chamber pacing for either sinus node dysfunction or AV block indications.2,3,10,11 Toff et al.2 reported that pacing mode via VVI pacing or DDD pacing does not influence mortality or cardiovascular events in elderly patients with AV block. For patients with SND treated with VVI pacing, the CTOPP and MOST studies showed no overall difference in mortality or heart failure.3,12 However, there were increased risks of AF with VVI pacing, and patients who received higher percentages of VP experienced increased risk of heart failure, regardless of pacing mode.10 In our study, only 2.2% of the non-AF patients experienced heart failure, pacemaker syndrome, or syncope and none reported a complication due to procedure or system related AF. The reduction in complications observed with leadless VVI pacing may alter the risk-benefit profile even further such that leadless pacing may be a very reasonable and perhaps preferable alternative to traditional transvenous dual-chamber pacing.
The reasons the physicians and patients selected VVI pacing provide some insight as to why Micra was chosen in a non-AF patient. Among these patients without AF, clinicians favoured leadless pacing for the majority (66%) patients due to an expectation for infrequent pacing, and the follow-up data show that these patients experienced less pacing than the AF patients with a median value of 17%. Advanced age (27%) and patient preference for new technology (19%) were also common selections. The latter requires further exploration to understand the impact to lifestyle restrictions and cosmetic concerns that may be related to transvenous systems. Further, an additional 10% of patients had conditions that precluded the use of transvenous systems. These reasons are consistent with guideline recommendations as an acceptable alternative to dual-chamber pacing.5 Taken together, these data suggest that Micra leadless pacemaker implantation and subsequent VVI pacing may be a reasonable alternative in patients with no AF.
Limitations
There are several limitations that should be kept in mind when considering these data. First, this analysis is observational in nature and thus is subject to confounding. While the Micra trial did not exclude patients with major comorbidities, patients undergoing concomitant transcatheter aortic valve replacement and those with prior cardiac implantable electronic devices were excluded. Moreover, while patients were followed for a mean of 16.4 ± 4.9 months, our data cannot inform longer-term outcomes. The low event rate made multivariable analyses difficult. Finally, the causes of pacemaker syndrome are different in patients with AF and those without. In sinus rhythm, pacemaker syndrome can be due to AV dyssynchrony, retrograde conduction, and/or to the altered ventricular mechanical sequence under pacing conditions. However, in AF patients, while pacemaker syndrome can be due to the paced ventricular activation, most cases are due to inappropriate programming of rate-responsiveness. Thus, the frequency of pacemaker syndrome in the AF population might be overestimated.
Conclusions
More than one-third of patients in the Micra IDE trial did not have a pacing indication associated with persistent or permanent AF. These patients with intact sinus function and/or atrial transport function were younger, had fewer comorbidities, more efficient implant procedures, and much lower rates of pacing after implant. Overall rates of syncope, cardiac failure, and pacemaker syndrome were low, including in patients with no persistent or permanent AF. These data suggest that Micra leadless pacing is a reasonable treatment strategy in patients without persistent or permanent AF. Further investigations are required to validate these findings.
Acknowledgements
We thank Dedra Fagan and Harrison Hudnall of Medtronic for assistance in the preparation of this manuscript.
Funding
The Micra Transcatheter Pacing Study was funded by Medtronic, plc (ClinicalTrials.gov identifier: NCT02004873).
Conflict of interest: J.P.P. receives grants for clinical research from Abbott, ARCA biopharma, Boston Scientific, Gilead, and Janssen Pharmaceuticals, and serves as a consultant to Allergan, ARCA Biopharma, Bayer, Biotronik, GSK, Motif Bio, Sanofi, Johnson & Johnson, Medtronic, and Philips. K.Stromberg and R.C.K. are employees and shareholders of Medtronic. K.P.J. serves as a consultant to Medtronic and Abbott. G.Z.D. reports research grants from Boston Scientific, Biotronik, and Medtronic and lecture/consulting fees from: Abbott, Bayer, Biotronik, Boehringer Ingelheim, Medtronic. M.F.E.C. has received compensation for services from Boston Scientific and Medtronic. G.H.C. reports compensation for services from Boston Scientific, Medtronic, Spectranetics Corporation, speaker’s bureau for Spectranetics Corporation, and fellowship support from Biosense Webster, Biotronik, Boston Scientific, and Medtronic. J.D.H. serves as a consultant to Abbott, Biosense Webster, and Medtronic and reports research grants from Abbott and Medtronic. C.N. reports no disclosures. R.O. reports no disclosures. P.R. has received compensation for services from Medtronic. P.R.R. has received compensation for services from Medtronic; speakers’ bureau from Medtronic; and research grant from Medtronic. K.Soejima reports no disclosures. D.R. reports lecture/consulting fees from Medtronic. S.Z. reports no disclosures. C.S. reports compensation for services from Biotronik and Medtronic and speaker’s bureau for Abbott, Biotronik, Boston Scientific, and Medtronic. L.A.C. reports compensation for services from Abbott, Biosense Webster, Pfizer, Biotronik, and Medtronic and fellowship support from Biotronik, Boston Scientific, and Medtronic.



