-
PDF
- Split View
-
Views
-
Cite
Cite
Rainer Zbinden, Christian Wollmann, Johannes Brachmann, Jochen Michaelsen, Clemens Steinwender, Pramesh Kovoor, Sebastian Kelle, Andrew D McGavigan, Chi Keong Ching, Gemma A Figtree, Jan Schmidt, Tobias Timmel, Joachim Lotz, Clinical safety of the ProMRI implantable cardioverter-defibrillator systems during head and lower lumbar magnetic resonance imaging at 3 T: results of the ProMRI 3T ENHANCED Master study, EP Europace, Volume 21, Issue 11, November 2019, Pages 1678–1685, https://doi.org/10.1093/europace/euz189
Close - Share Icon Share
Abstract
There have been no published studies on the safety of magnetic resonance imaging (MRI) at 3 Tesla (3 T) in patients with MRI-conditional implantable cardioverter-defibrillators (ICDs). The aim of this study was to assess clinical safety of the Biotronik ProMRI ICD system during non-diagnostic head and lower lumbar scans under 3 T MRI conditions.
The study enrolled 129 patients at 12 sites in Australia, Singapore, and Europe. Predefined head and lower lumbar MR scans (total duration ≈30 min) were performed in 112 patients. Three primary endpoints were evaluated from the pre-MRI to the 1-month post-MRI visit: (i) freedom from serious adverse device effects (SADEs) related to MRI (hypothesized to be >90%); (ii) pacing threshold invariance for all leads (geometric mean of the patient-wise ratios for 1 month vs. pre-MRI was hypothesized to be <1.07); and (iii) sensing amplitude invariance (geometric mean of the ratios was hypothesized to be >0.993). No MRI-related SADE occurred (SADE-free rate 100%, 95% confidence interval 95.98–100%). Pacing threshold and sensing amplitudes fulfilled the invariance hypotheses with high statistical significance (P < 0.0013). No threshold increase >0.5 V or sensing amplitude decrease by >50% was observed (secondary endpoints). Lead impedances, battery capacity, and detection and treatment of arrhythmias by ICDs were not affected by MRI scans.
The head and lower lumbar scans under specific 3 T MRI conditions were safe in the investigated MR-conditional ICD systems. There was no evidence of harm to the patients or any negative influence of the MRI scan on the implanted systems.
To improve resolution of magnetic resonance (MR) images and shorten procedure times, the newer 3 T MR imaging (MRI) technology applies a higher magnetic field strength (magnetic induction of 3 T) when compared with the traditional 1.5 T MRI technology.
Stronger magnetic fields may induce more electromagnetic interference within implanted metallic and electronic devices. There is a paucity of clinical studies demonstrating safety of MRI-conditional implantable cardioverter-defibrillator (ICD) systems in the 3 T MRI surrounding.
We assessed clinical safety of predefined non-diagnostic head and lower lumbar MRI scans at 3 T in 112 patients treated with Biotronik ProMRI ICD systems. There was no evidence of harm to the patients or any negative influence of the MRI scans on the implanted systems.
Introduction
Magnetic resonance imaging (MRI) uses magnetic fields and radiofrequency waves to produce detailed anatomical images valuable in the diagnosis of a wide variety of conditions.1,2 The decreasing costs and better availability of MRI have resulted in ever increasing use of MRI in clinical practice.2 Since many recipients of implantable cardioverter-defibrillators (ICDs) and pacemakers will have a lifetime indication for a magnetic resonance (MR) scan, implantable systems should be designed to be as safe as possible during MRI scans.3–5 An array of MR-conditional pacemaker and ICD systems has recently been developed with hardware and software tested and approved for MRI scanning at 1.5 Tesla (1.5 T) under predefined conditions.3–13 To improve resolution of MR images and potentially shorten procedure times, the newer, 3 T MRI technology applies a higher magnetic field strength,2,3 which may induce more electromagnetic interference within implanted metallic and electronic devices. As yet, no published clinical trial demonstrated the safety of MR-conditional ICD systems in the 3 T MR surrounding.
Methods
The purpose of the prospective, non-randomized, multicentre ProMRI 3 T ENHANCED Master Study was to assess clinical safety of several ProMRI ICD systems manufactured by Biotronik (Biotronik SE & Co. KG, Berlin, Germany) during non-diagnostic head and lower lumbar scans under specific 3 T MR conditions. Alterations of electrical lead parameters and adverse events after MRI were evaluated during a follow-up period of 3 months. The study was performed in compliance with good clinical practice guidelines and the Declaration of Helsinki. The study protocol was approved by the appropriate national and local ethics committees. All patients gave written informed consent before enrolment (ClinicalTrials.gov Identifier: NCT02506569).
Patient selection
Patients were enrolled if they were ≥18 years old and ≥140 cm tall, had an ICD implanted for standard indications in pectoral position ≥5 weeks prior to enrolment, and were able and willing to attend follow-up examinations and complete all testing required by the clinical protocol, including a non-diagnostic MRI scan. The implanted ICD system had to be one of the options listed in the next section.
Patients were not enrolled if they had a contraindication for MRI scanning, a lead impedance <200 or >1500 Ohm, an atrial or ventricular pacing threshold >2.0 V at 0.4 ms pulse width or non-measurable threshold, a Twiddler’s syndrome, ICD battery status ‘elective replacement indicator’ or ‘end of service’, or an MRI scan performed or ICD/lead repositioned or explanted ≤5 weeks before enrolment. Other patient exclusion criteria were short life expectancy (<6 months), planned cardiac surgery within next 6 months, pregnancy, breastfeeding, or participation in another interventional clinical investigation.
Implantable cardioverter-defibrillator systems studied
In 2011, the first Biotronik ICD family labelled ProMRI was conditionally approved for MRI scanning at 1.5 T.11 Thereafter, several ICD families with their respective leads have been designed to be also conditionally eligible for 3 T MR scans. Three generations of proMRI 3 T-conditional ICDs were included in the present study (Table 1). These ICD systems can be defined as technically identical from an MR-conditionality point of view because all devices must fulfil identical MR acceptance criteria and identical MR conditions. All ICDs had a programmable MRI mode to provide pacing without the risk of inappropriate inhibition by electromagnetic fields and to eliminate functional interference during the MRI procedure by temporary deactivation of anti-tachycardia therapy and by several other features. All ICDs offered daily, automatic Biotronik® Home Monitoring function.16
| Device type . | Connectorb . | Device family . | Model options . |
|---|---|---|---|
| ICD device (ProMRI 3T-conditional) | DF-1 or DF4 | Generation 1: Ilestoc, Iforiac, Idovad | eVR-T, DR-T, VR-T DX (for all three generations) |
| Generation 2: Iperiac, Itreviac, Inventrad | |||
| Generation 3: Inticac, Iliviad | |||
| ICD leadf (in the right ventricle) | DF-1 | Linoxsmart (ProMRI)g S | h65, 75 |
| Linoxsmart (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart (ProMRI)g S DX | j65/15, 65/17 | ||
| Protego DF-1 (ProMRI)g S | h65, 75 | ||
| Protego DF-1 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Protego DF-1 (ProMRI)g S DX | j65/15, 65/17 | ||
| ICD leadf (in the right ventricle) | DF4 | Protego (ProMRI)g S | h65, 75 |
| Protego (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart DF4 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Plexa ProMRI S | h65, 75 | ||
| Plexa ProMRI SD | i65/16, 65/18, 75/18 | ||
| Pacing/sensing leadf (in the right atrium) | IS-1 | Solia S, Siello S | h45, 53, 60 |
| Setrox S, Safio S | h53 |
| Device type . | Connectorb . | Device family . | Model options . |
|---|---|---|---|
| ICD device (ProMRI 3T-conditional) | DF-1 or DF4 | Generation 1: Ilestoc, Iforiac, Idovad | eVR-T, DR-T, VR-T DX (for all three generations) |
| Generation 2: Iperiac, Itreviac, Inventrad | |||
| Generation 3: Inticac, Iliviad | |||
| ICD leadf (in the right ventricle) | DF-1 | Linoxsmart (ProMRI)g S | h65, 75 |
| Linoxsmart (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart (ProMRI)g S DX | j65/15, 65/17 | ||
| Protego DF-1 (ProMRI)g S | h65, 75 | ||
| Protego DF-1 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Protego DF-1 (ProMRI)g S DX | j65/15, 65/17 | ||
| ICD leadf (in the right ventricle) | DF4 | Protego (ProMRI)g S | h65, 75 |
| Protego (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart DF4 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Plexa ProMRI S | h65, 75 | ||
| Plexa ProMRI SD | i65/16, 65/18, 75/18 | ||
| Pacing/sensing leadf (in the right atrium) | IS-1 | Solia S, Siello S | h45, 53, 60 |
| Setrox S, Safio S | h53 |
DX, single-chamber ICD with atrial diagnostics (atrial sensing via floating dipole)14; ICD, implantable cardioverter-defibrillator; MRI, magnetic resonance imaging; S models of ICD leads have one shock coil; SD models have two shock coils; S DX models have one shock coil and atrial sensing dipole.
All devices were CE (European Conformity) approved for MRI scans at 3 T under conditions defined by the Biotronik ProMRI manual. Investigators were free to choose among listed ICD and lead options.
DF4 connector (four-pole in-line) gradually replaces the bulkier DF-1 connector (bi- or trifurcated).15 IS-1 is the low-profile international lead connector standard 1.
Models 5 or 7.
Model 7.
Suffix ‘-T’ indicates Biotronik® Home Monitoring function. ‘VR’ denotes single-chamber device; ‘DR’, dual-chamber device; ‘VR-T DX’, DX device.
Endocardial, bipolar, with a steroid-eluting screw-in tip electrode made of fractally-coated iridium.
Optional possibility.
Length of the lead in cm.
Length of the lead in cm/distance of the proximal shock coil to the lead tip in cm.
Length of the lead/distance of the centre of the atrial floating sensing dipole to the lead tip in cm.
| Device type . | Connectorb . | Device family . | Model options . |
|---|---|---|---|
| ICD device (ProMRI 3T-conditional) | DF-1 or DF4 | Generation 1: Ilestoc, Iforiac, Idovad | eVR-T, DR-T, VR-T DX (for all three generations) |
| Generation 2: Iperiac, Itreviac, Inventrad | |||
| Generation 3: Inticac, Iliviad | |||
| ICD leadf (in the right ventricle) | DF-1 | Linoxsmart (ProMRI)g S | h65, 75 |
| Linoxsmart (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart (ProMRI)g S DX | j65/15, 65/17 | ||
| Protego DF-1 (ProMRI)g S | h65, 75 | ||
| Protego DF-1 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Protego DF-1 (ProMRI)g S DX | j65/15, 65/17 | ||
| ICD leadf (in the right ventricle) | DF4 | Protego (ProMRI)g S | h65, 75 |
| Protego (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart DF4 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Plexa ProMRI S | h65, 75 | ||
| Plexa ProMRI SD | i65/16, 65/18, 75/18 | ||
| Pacing/sensing leadf (in the right atrium) | IS-1 | Solia S, Siello S | h45, 53, 60 |
| Setrox S, Safio S | h53 |
| Device type . | Connectorb . | Device family . | Model options . |
|---|---|---|---|
| ICD device (ProMRI 3T-conditional) | DF-1 or DF4 | Generation 1: Ilestoc, Iforiac, Idovad | eVR-T, DR-T, VR-T DX (for all three generations) |
| Generation 2: Iperiac, Itreviac, Inventrad | |||
| Generation 3: Inticac, Iliviad | |||
| ICD leadf (in the right ventricle) | DF-1 | Linoxsmart (ProMRI)g S | h65, 75 |
| Linoxsmart (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart (ProMRI)g S DX | j65/15, 65/17 | ||
| Protego DF-1 (ProMRI)g S | h65, 75 | ||
| Protego DF-1 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Protego DF-1 (ProMRI)g S DX | j65/15, 65/17 | ||
| ICD leadf (in the right ventricle) | DF4 | Protego (ProMRI)g S | h65, 75 |
| Protego (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Linoxsmart DF4 (ProMRI)g SD | i65/16, 65/18, 75/18 | ||
| Plexa ProMRI S | h65, 75 | ||
| Plexa ProMRI SD | i65/16, 65/18, 75/18 | ||
| Pacing/sensing leadf (in the right atrium) | IS-1 | Solia S, Siello S | h45, 53, 60 |
| Setrox S, Safio S | h53 |
DX, single-chamber ICD with atrial diagnostics (atrial sensing via floating dipole)14; ICD, implantable cardioverter-defibrillator; MRI, magnetic resonance imaging; S models of ICD leads have one shock coil; SD models have two shock coils; S DX models have one shock coil and atrial sensing dipole.
All devices were CE (European Conformity) approved for MRI scans at 3 T under conditions defined by the Biotronik ProMRI manual. Investigators were free to choose among listed ICD and lead options.
DF4 connector (four-pole in-line) gradually replaces the bulkier DF-1 connector (bi- or trifurcated).15 IS-1 is the low-profile international lead connector standard 1.
Models 5 or 7.
Model 7.
Suffix ‘-T’ indicates Biotronik® Home Monitoring function. ‘VR’ denotes single-chamber device; ‘DR’, dual-chamber device; ‘VR-T DX’, DX device.
Endocardial, bipolar, with a steroid-eluting screw-in tip electrode made of fractally-coated iridium.
Optional possibility.
Length of the lead in cm.
Length of the lead in cm/distance of the proximal shock coil to the lead tip in cm.
Length of the lead/distance of the centre of the atrial floating sensing dipole to the lead tip in cm.
Study flowchart
Study timelines are summarized in Figure 1. Implanted devices were interrogated and device-based measurements of atrial and ventricular bipolar sensing amplitudes, pacing thresholds at 0.4 ms, and lead impedances were performed at enrolment, at the MRI procedure follow-up (before and after MRI), and at the 1- and 3-month post-MRI visits. All adverse events were recorded. The Home Monitoring option was used throughout the study to collect pacing threshold and sensing measurements automatically on a daily basis and to monitor any arrhythmia episodes.
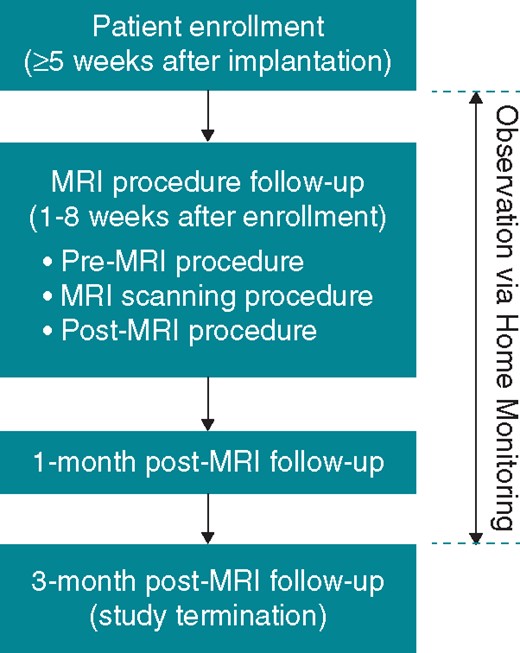
Magnetic resonance imaging procedure
Before the MRI scans, the ICD devices were programmed to MRI mode to disable detection and therapy of ventricular tachyarrhythmia. During the scans, the patients were continuously monitored by electrocardiogram (ECG), pulse oximetry, blood pressure unit, or a combination of these methods. At least one physician had to be present during the procedure (radiologist, cardiologist, or electrophysiologist), and a second person (that could also be an authorized technical MRI assistant or cardiology technician) in order that one staff member could focus on the MRI aspects (e.g. radiologist, cardiologist experienced with MRI, or authorized technical MRI assistant) and that the other staff member could focus on the monitoring and the patient.
The lower lumbar and head MRI scans were performed with an exclusion zone described in Figure 2. Siemens, General Electric, and Philips MRI systems were used, with cylindrical magnets and a static magnetic field strength of 3 T. The predefined scanning sequences (Table 2) ensured comparability across different sites and MRI systems, and allowed evaluation of the implanted systems at the maximum allowed burden according to labelling conditions. Total MRI scan time per patient was ≤30 min, comprising six to seven sequences for the lower lumbar (≈14 min) and eight to nine sequences for head (≈16 min) scan. The MRI protocol was designed to maximize the specific absorption rate within the constraints of the ‘normal scanning mode’, i.e. maximum 2 W/kg for whole-body MRI and 3.2 W/kg for head scans. The allowed slew rate of the MRI scanners’ gradient fields was ≤200 T/m/s per axis.
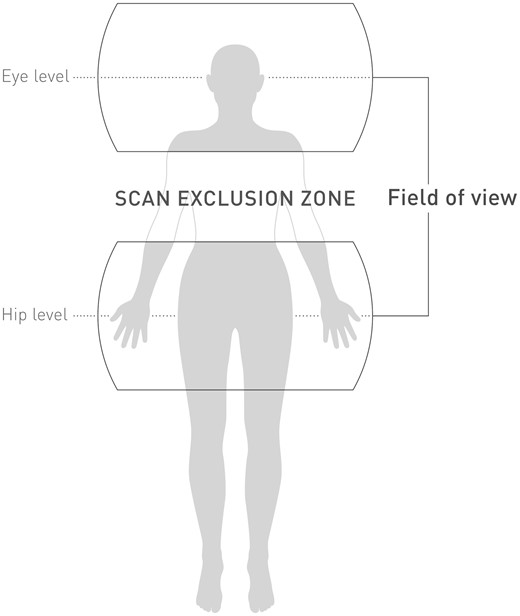
Isocenters at eye and hip level of magnetic resonance imaging scans. The shaded area in between depicts the scan exclusion zone.
| Body region . | Types of scan sequences . | ||
|---|---|---|---|
| . | Siemens . | General Electric . | Philips . |
| Lumbar (landmark on trochanter) | Localizer | Localizer | Localizer |
| SAG T1 | SAG T1 | Reference scan | |
| SAG T2 | SAG T2 | SAG T2 | |
| AX T1 | AX T1 | COR T2 | |
| AX T2 | AX T2 | SAG T1 | |
| SAG diffusion | SAG T1 | AX T2 | |
| STIR | |||
| Head (landmark on eyes) | 3-plane localizer | 3-plane localizer | 3-plane localizer |
| SAG SE T1 | ASSET calibration | Reference scan | |
| AX TSE T2 | SAG SE T1 | SAG SE T1 | |
| T2 TIRM | AX FSE T2 | AX FSE T2 | |
| Diffusion | AX T2 FLAIR | T2 FLAIR | |
| 3D TOF MT | AX diffusion | Diffusion | |
| CE-MRA | AX 3D TOF MT | 3D TOF MT | |
| Perfusion | CE-MRA | CE-MRA | |
| AX perfusion | COR FSE T2 | ||
| Body region . | Types of scan sequences . | ||
|---|---|---|---|
| . | Siemens . | General Electric . | Philips . |
| Lumbar (landmark on trochanter) | Localizer | Localizer | Localizer |
| SAG T1 | SAG T1 | Reference scan | |
| SAG T2 | SAG T2 | SAG T2 | |
| AX T1 | AX T1 | COR T2 | |
| AX T2 | AX T2 | SAG T1 | |
| SAG diffusion | SAG T1 | AX T2 | |
| STIR | |||
| Head (landmark on eyes) | 3-plane localizer | 3-plane localizer | 3-plane localizer |
| SAG SE T1 | ASSET calibration | Reference scan | |
| AX TSE T2 | SAG SE T1 | SAG SE T1 | |
| T2 TIRM | AX FSE T2 | AX FSE T2 | |
| Diffusion | AX T2 FLAIR | T2 FLAIR | |
| 3D TOF MT | AX diffusion | Diffusion | |
| CE-MRA | AX 3D TOF MT | 3D TOF MT | |
| Perfusion | CE-MRA | CE-MRA | |
| AX perfusion | COR FSE T2 | ||
ASSET, array spatial sensitivity encoding technique; AX, axial; CE, contrast enhanced; COR, coronal; 3D, three-dimensional; FLAIR, fluid attenuated inversion recovery; FSE, fast spin echo; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MT, magnetization transfer; SAG, sagittal; SE, spin echo; STIR, short tau (inversion time) inversion recovery; T1, T1-weighted (short repetition time and short echo time sequence); T2, T2-weighted (long repetition time and long echo time sequence); TIRM, turbo inversion recovery magnitude; TOF, time of flight; TSE, turbo spin echo.
| Body region . | Types of scan sequences . | ||
|---|---|---|---|
| . | Siemens . | General Electric . | Philips . |
| Lumbar (landmark on trochanter) | Localizer | Localizer | Localizer |
| SAG T1 | SAG T1 | Reference scan | |
| SAG T2 | SAG T2 | SAG T2 | |
| AX T1 | AX T1 | COR T2 | |
| AX T2 | AX T2 | SAG T1 | |
| SAG diffusion | SAG T1 | AX T2 | |
| STIR | |||
| Head (landmark on eyes) | 3-plane localizer | 3-plane localizer | 3-plane localizer |
| SAG SE T1 | ASSET calibration | Reference scan | |
| AX TSE T2 | SAG SE T1 | SAG SE T1 | |
| T2 TIRM | AX FSE T2 | AX FSE T2 | |
| Diffusion | AX T2 FLAIR | T2 FLAIR | |
| 3D TOF MT | AX diffusion | Diffusion | |
| CE-MRA | AX 3D TOF MT | 3D TOF MT | |
| Perfusion | CE-MRA | CE-MRA | |
| AX perfusion | COR FSE T2 | ||
| Body region . | Types of scan sequences . | ||
|---|---|---|---|
| . | Siemens . | General Electric . | Philips . |
| Lumbar (landmark on trochanter) | Localizer | Localizer | Localizer |
| SAG T1 | SAG T1 | Reference scan | |
| SAG T2 | SAG T2 | SAG T2 | |
| AX T1 | AX T1 | COR T2 | |
| AX T2 | AX T2 | SAG T1 | |
| SAG diffusion | SAG T1 | AX T2 | |
| STIR | |||
| Head (landmark on eyes) | 3-plane localizer | 3-plane localizer | 3-plane localizer |
| SAG SE T1 | ASSET calibration | Reference scan | |
| AX TSE T2 | SAG SE T1 | SAG SE T1 | |
| T2 TIRM | AX FSE T2 | AX FSE T2 | |
| Diffusion | AX T2 FLAIR | T2 FLAIR | |
| 3D TOF MT | AX diffusion | Diffusion | |
| CE-MRA | AX 3D TOF MT | 3D TOF MT | |
| Perfusion | CE-MRA | CE-MRA | |
| AX perfusion | COR FSE T2 | ||
ASSET, array spatial sensitivity encoding technique; AX, axial; CE, contrast enhanced; COR, coronal; 3D, three-dimensional; FLAIR, fluid attenuated inversion recovery; FSE, fast spin echo; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MT, magnetization transfer; SAG, sagittal; SE, spin echo; STIR, short tau (inversion time) inversion recovery; T1, T1-weighted (short repetition time and short echo time sequence); T2, T2-weighted (long repetition time and long echo time sequence); TIRM, turbo inversion recovery magnitude; TOF, time of flight; TSE, turbo spin echo.
After the MRI scans, ICD programming was restored to the initial parameters or modified at the discretion of the investigator. All MR images were reviewed for any relevant abnormalities.
Study endpoints
Primary endpoint 1 was freedom from serious adverse device effects (SADEs) between the pre-scan MRI-mode programming and the 1-month post-MRI visit, related or possibly related to both the MRI procedure and the implanted system, leading to death, life-threatening condition, prolonged or new hospitalization, medical or surgical intervention (to prevent life-threatening condition), a permanent impairment of a body structure or a body function, or serious deterioration of health status.
Primary endpoints 2 and 3 were pacing threshold invariance and sensing amplitude invariance. The value of interest was the geometric mean (TR) of the patient-wise pacing threshold and sensing amplitude ratios between the 1-month post-MRI visit and the pre-MRI measurements. This was analysed for each lead. The geometric mean was chosen because patient-wise ratios have a log-normal distribution for which TR is the appropriate centre estimation method. TR will be increased if any systematic change after MRI is observed, either a slight change in a large amount of leads or a large change in a small amount of leads. Pacing and sensing values were primarily retrieved from Home Monitoring data and replaced if available by measurements from in-office follow-ups in both the pre-MRI and the 1-month post-MRI data.
Secondary endpoints were the proportion of ICD leads with a pacing threshold increase of >0.5 V, or a R-wave amplitude decrease of >50%, comparing the pre-MRI with the 1-month post-MRI measurements. Additional data of interest were ventricular pacing impedance; atrial pacing threshold, sensing amplitudes, pacing impedance and painless shock impedance; battery capacity; success of ICD therapies; non-serious adverse device effects potentially related to MRI scan; and functionality of the MRI mode. Analysis of secondary endpoints and additional data of interest was exclusively based on measurements from in-office follow-ups.
Study hypotheses and sample size
Corresponding to the three primary endpoints, the following three primary hypotheses were predefined for MRI safety: (i) an SADE-free rate >90%, calculated as 100% × (1 − number of SADEs divided by the number of ICD systems subjected to the study-specific MRI procedure); (ii) a TR < 1.07 (<7.0% increase) for pacing threshold; and (iii) a TR > 0.933 (<6.7% decrease) for sensing amplitude. The calculation of the sample size was based on all primary endpoints. For a significance level of α = 0.05, a statistical power of 1−β = 0.8, the total of 116 MRI procedures were required. Assuming a drop-out rate of 10%, 129 patients had to be enrolled.
Statistical analysis
Data were evaluated according to per-protocol analysis. The SADE-related hypothesis was tested by an exact binomial test, with the 95% confidence interval (CI) calculated according to the Agresti–Coull method. The other two primary hypotheses were analysed by one-sample t-tests of the logarithm (base 2) of the data, because patient-wise pacing threshold and sensing amplitude ratios are not normally distributed without log-transformation. The geometric mean of ratios mentioned above now converts to the arithmetic mean of log-transformed ratios, and the pre-defined cut-off values of <1.07 (pacing threshold) and >0.933 (sensing amplitude) convert to log-transformed values of <0.0976 (i.e. <0.10 when rounded to 2 decimal points) and >−0.10, respectively.
Continuous variables are shown as means, standard deviation, median, interquartile range (IQR), and total range. Nominal or ordinal variables are shown as frequencies and proportions. Calculations were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) or S+ 8.2 (Solution Metrics, Sydney, Australia) statistical software.
Results
Between 31 July 2015 and 25 September 2017, a total of 129 patients were enrolled at 12 investigational sites in Germany (5 sites), Australia (3), Austria (2), Switzerland (1), and Singapore (1). Sixteen patients did not undergo MRI due to consent withdrawal (n = 8), death (n = 1), not fulfilling MR-scan requirements (n = 5), health deterioration (n = 1), or lack of time (n = 1), and the remaining 113 patients underwent MRI. After exclusion of one patient because of protocol violation [too early MR scan (<6 weeks) after implantation], 112 patients constituted the analysis population described in Table 3.
| Characteristic . | Analysis population (n = 112) . |
|---|---|
| Age (years) | 62 ± 11 (range 35–86) |
| Males | 96 (86%) |
| Body mass index (kg/m2) | 28.2 ± 4.5 (range 19.0–40.4) |
| Height (cm) | 175 ± 9 (range 150–200) |
| Weight (kg) | 87 ± 18 (range 51–150) |
| Cardiovascular history | |
| Coronary artery disease | 83 (74%) |
| Hypertension | 71 (63%) |
| Dilated cardiomyopathy | 26 (23%) |
| Hypertrophic cardiomyopathy | 3 (3%) |
| Long QT syndrome | 4 (4%) |
| Pacing dependency | 4 (4%) |
| Diabetes mellitus | 35 (31%) |
| Renal insufficiency | 18 (16%) |
| Drugs affecting pacing threshold | |
| Class I antiarrhythmics | 0 (0%) |
| Class II antiarrhythmics | 98 (88%) |
| Class III antiarrhythmics | 12 (11%) |
| Class IV antiarrhythmics | 2 (2%) |
| Glucocorticoids | 4 (4%) |
| Implanted system | |
| ICD type | |
| Single chambera | 56 (50%) |
| Dual chamberb | 31 (28%) |
| DXc | 25 (22%) |
| Connector type | |
| DF-1 | 43 (38%) |
| DF4 | 69 (62%) |
| Leads | |
| ICDd | 112 (100%) |
| Atriale | 31 (28%) |
| Characteristic . | Analysis population (n = 112) . |
|---|---|
| Age (years) | 62 ± 11 (range 35–86) |
| Males | 96 (86%) |
| Body mass index (kg/m2) | 28.2 ± 4.5 (range 19.0–40.4) |
| Height (cm) | 175 ± 9 (range 150–200) |
| Weight (kg) | 87 ± 18 (range 51–150) |
| Cardiovascular history | |
| Coronary artery disease | 83 (74%) |
| Hypertension | 71 (63%) |
| Dilated cardiomyopathy | 26 (23%) |
| Hypertrophic cardiomyopathy | 3 (3%) |
| Long QT syndrome | 4 (4%) |
| Pacing dependency | 4 (4%) |
| Diabetes mellitus | 35 (31%) |
| Renal insufficiency | 18 (16%) |
| Drugs affecting pacing threshold | |
| Class I antiarrhythmics | 0 (0%) |
| Class II antiarrhythmics | 98 (88%) |
| Class III antiarrhythmics | 12 (11%) |
| Class IV antiarrhythmics | 2 (2%) |
| Glucocorticoids | 4 (4%) |
| Implanted system | |
| ICD type | |
| Single chambera | 56 (50%) |
| Dual chamberb | 31 (28%) |
| DXc | 25 (22%) |
| Connector type | |
| DF-1 | 43 (38%) |
| DF4 | 69 (62%) |
| Leads | |
| ICDd | 112 (100%) |
| Atriale | 31 (28%) |
Values are presented as mean ± standard deviation.
DF-1, old-standard connector type; DF4, new-standard connector type; DX, single-chamber ICD with atrial diagnostics (atrial sensing via floating dipole)14; ICD, implantable cardioverter-defibrillator.
Iforia 5 (n = 33), Itrevia 5 (n = 9), Iperia 7 (n = 6), Itrevia 7 (n = 5), Ilesto 7 (n = 3).
Iforia 5 (n = 12), Itrevia 5 (n = 10), Iperia 7 (n = 4), Ilesto 7 (n = 4), Intica 7 (n = 1).
Itrevia 7 (n = 9), Itrevia 5 (n = 6), Iperia 7 (n = 3), Ilesto 7 (n = 3), Iforia 5 (n = 2), Intica 7 (n = 1), Ilivia 7 (n = 1).
Protego ProMRI (n = 58), Linoxsmart ProMRI (n = 21), Protego DF-1 ProMRI (n = 19), Linoxsmart DF4 ProMRI (n = 5), Plexa ProMRI (n = 5), Protego DF-1 (n = 2), Protego (n = 1), Linoxsmart (n = 1).
Solia S (n = 27), Safio S (n = 4).
| Characteristic . | Analysis population (n = 112) . |
|---|---|
| Age (years) | 62 ± 11 (range 35–86) |
| Males | 96 (86%) |
| Body mass index (kg/m2) | 28.2 ± 4.5 (range 19.0–40.4) |
| Height (cm) | 175 ± 9 (range 150–200) |
| Weight (kg) | 87 ± 18 (range 51–150) |
| Cardiovascular history | |
| Coronary artery disease | 83 (74%) |
| Hypertension | 71 (63%) |
| Dilated cardiomyopathy | 26 (23%) |
| Hypertrophic cardiomyopathy | 3 (3%) |
| Long QT syndrome | 4 (4%) |
| Pacing dependency | 4 (4%) |
| Diabetes mellitus | 35 (31%) |
| Renal insufficiency | 18 (16%) |
| Drugs affecting pacing threshold | |
| Class I antiarrhythmics | 0 (0%) |
| Class II antiarrhythmics | 98 (88%) |
| Class III antiarrhythmics | 12 (11%) |
| Class IV antiarrhythmics | 2 (2%) |
| Glucocorticoids | 4 (4%) |
| Implanted system | |
| ICD type | |
| Single chambera | 56 (50%) |
| Dual chamberb | 31 (28%) |
| DXc | 25 (22%) |
| Connector type | |
| DF-1 | 43 (38%) |
| DF4 | 69 (62%) |
| Leads | |
| ICDd | 112 (100%) |
| Atriale | 31 (28%) |
| Characteristic . | Analysis population (n = 112) . |
|---|---|
| Age (years) | 62 ± 11 (range 35–86) |
| Males | 96 (86%) |
| Body mass index (kg/m2) | 28.2 ± 4.5 (range 19.0–40.4) |
| Height (cm) | 175 ± 9 (range 150–200) |
| Weight (kg) | 87 ± 18 (range 51–150) |
| Cardiovascular history | |
| Coronary artery disease | 83 (74%) |
| Hypertension | 71 (63%) |
| Dilated cardiomyopathy | 26 (23%) |
| Hypertrophic cardiomyopathy | 3 (3%) |
| Long QT syndrome | 4 (4%) |
| Pacing dependency | 4 (4%) |
| Diabetes mellitus | 35 (31%) |
| Renal insufficiency | 18 (16%) |
| Drugs affecting pacing threshold | |
| Class I antiarrhythmics | 0 (0%) |
| Class II antiarrhythmics | 98 (88%) |
| Class III antiarrhythmics | 12 (11%) |
| Class IV antiarrhythmics | 2 (2%) |
| Glucocorticoids | 4 (4%) |
| Implanted system | |
| ICD type | |
| Single chambera | 56 (50%) |
| Dual chamberb | 31 (28%) |
| DXc | 25 (22%) |
| Connector type | |
| DF-1 | 43 (38%) |
| DF4 | 69 (62%) |
| Leads | |
| ICDd | 112 (100%) |
| Atriale | 31 (28%) |
Values are presented as mean ± standard deviation.
DF-1, old-standard connector type; DF4, new-standard connector type; DX, single-chamber ICD with atrial diagnostics (atrial sensing via floating dipole)14; ICD, implantable cardioverter-defibrillator.
Iforia 5 (n = 33), Itrevia 5 (n = 9), Iperia 7 (n = 6), Itrevia 7 (n = 5), Ilesto 7 (n = 3).
Iforia 5 (n = 12), Itrevia 5 (n = 10), Iperia 7 (n = 4), Ilesto 7 (n = 4), Intica 7 (n = 1).
Itrevia 7 (n = 9), Itrevia 5 (n = 6), Iperia 7 (n = 3), Ilesto 7 (n = 3), Iforia 5 (n = 2), Intica 7 (n = 1), Ilivia 7 (n = 1).
Protego ProMRI (n = 58), Linoxsmart ProMRI (n = 21), Protego DF-1 ProMRI (n = 19), Linoxsmart DF4 ProMRI (n = 5), Plexa ProMRI (n = 5), Protego DF-1 (n = 2), Protego (n = 1), Linoxsmart (n = 1).
Solia S (n = 27), Safio S (n = 4).
Mean age of the patients was 62 ± 11 years, mean height 175 ± 9 cm, and mean weight 87 ± 18 kg. Male patients prevailed largely (86%). Pacing dependency was infrequent (4%). The most common ICD type implanted was single chamber (50%), followed by dual chamber (28%), and DX (22%). The newer, DF4 connector prevailed over DF-1 (62% vs. 38%).
Magnetic resonance imaging scans
The programmed MRI pacing mode was VOO in 25 patients (22%), DOO in 14 (13%), and OFF in 73 (65%). The time between MRI mode programming and reactivation of the initial programming was 69 ± 31 min (median 62, IQR 51–75). Siemens MRI systems were used in 84 patients (75%), General Electric in 14 (13%), and Philips in 14 (13%). Patient monitoring involved ECG (alone or combined with other methods) in 78 patients (70%), pulse oximetry (alone or combined with blood pressure) in 33 patients (29%), and blood pressure monitoring alone in one patient (1%).
Follow-up
During the MR scan, one patient had a panic attack and prematurely terminated study participation after the MRI procedure follow-up. The remaining 111 patients contributed to the primary endpoint 1 (SADE-free rate). After the 1-month follow-up, one patient withdrew consent and one patient was lost to follow-up due to relocation. The remaining 109 patients underwent the 3-month follow-up. The mean time from the MRI scan to study termination was 97 ± 23 days (median 95, IQR 87–107).
Primary endpoints
No MRI-related SADE occurred, translating into a 100% MRI-related SADE-free rate (95% CI 95.98–100%). The lower limit of the 95% CI is above 90% (P < 0.0001, exact binomial test), fulfilling the primary hypothesis 1.
Primary hypotheses 2 and 3 are also met with high statistical significance (Table 4). The raw (non-transformed) difference in pacing thresholds between 1 month and pre-MRI measurement was −0.1 ± 0.1 V in the right atrium (median 0.0, IQR −0.2 to 0.0, range −0.3 to 0.2), and 0.0 ± 0.1 V in the right ventricle (median 0.0, IQR −0.1 to 0.0, range −0.3 to 0.2). The raw difference in atrial and ventricular sensing amplitudes at 1 month vs. pre-MRI was 0.0 ± 0.5 mV (median −0.1, IQR −0.3 to 0.1, range −1.2 to 2.0) and 0.0 ± 1.1 mV (median 0.0, IQR −0.5 to 0.4, range −4.3 to 5.8).
| Hypothesis . | Value . |
|---|---|
| MRI-related SADE | |
| Number of patients included | 111a |
| Number of SADEs | 0 |
| SADE-free rate (95% CI) | 100% (95.98–100) |
| P-value for ‘>90% hypothesis’ | <0.0001 |
| Pacing threshold invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 28b |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.132 ± 0.226 |
| 95% CI | Infinity to −0.059 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Right ventricle | |
| Number of patients included | 108e |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.021 ± 0.189 |
| 95% CI | Infinity to 0.009 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Sensing amplitude invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 31f |
| Mean ± SD of log-transformed patient-wise ratiosc | 0.009 ± 0.184 |
| 95% CI | −0.047 to infinity |
| P-value for ‘>−0.10 hypothesis’g | 0.0012 |
| Right ventricle | |
| Number of patients included | 107h |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.005 ± 0.133 |
| 95% CI | −0.026 to infinity |
| P-value for ‘>−0.10 hypothesis’g | <0.0001 |
| Hypothesis . | Value . |
|---|---|
| MRI-related SADE | |
| Number of patients included | 111a |
| Number of SADEs | 0 |
| SADE-free rate (95% CI) | 100% (95.98–100) |
| P-value for ‘>90% hypothesis’ | <0.0001 |
| Pacing threshold invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 28b |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.132 ± 0.226 |
| 95% CI | Infinity to −0.059 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Right ventricle | |
| Number of patients included | 108e |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.021 ± 0.189 |
| 95% CI | Infinity to 0.009 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Sensing amplitude invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 31f |
| Mean ± SD of log-transformed patient-wise ratiosc | 0.009 ± 0.184 |
| 95% CI | −0.047 to infinity |
| P-value for ‘>−0.10 hypothesis’g | 0.0012 |
| Right ventricle | |
| Number of patients included | 107h |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.005 ± 0.133 |
| 95% CI | −0.026 to infinity |
| P-value for ‘>−0.10 hypothesis’g | <0.0001 |
CI, confidence interval; ICD, implantable cardioverter-defibrillator; MRI, magnetic resonance imaging; SADE, serious adverse device effect; SD, standard deviation.
All patients reaching the 1-month follow-up. One patient of 112 who underwent study-specific MRI had a panic attack and prematurely terminated the study after the MRI-procedure follow-up.
Of 31 patients with atrial lead and 1-month follow-up, 3 did not contribute because of missing data.
Corresponding to the geometric mean of non-log-transformed ratios.
Corresponding to ‘<1.07 hypothesis’ for non-log-transformed ratios.
Of 111 patients with ICD lead and 1-month follow-up, 3 did not contribute because of missing data.
All 31 patients with atrial lead and 1-month follow-up contributed.
Corresponding to ‘>0.933 hypothesis’ for non-log-transformed ratios.
Of 111 patients with ICD lead and 1-month follow-up, 4 did not contribute because of missing data.
| Hypothesis . | Value . |
|---|---|
| MRI-related SADE | |
| Number of patients included | 111a |
| Number of SADEs | 0 |
| SADE-free rate (95% CI) | 100% (95.98–100) |
| P-value for ‘>90% hypothesis’ | <0.0001 |
| Pacing threshold invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 28b |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.132 ± 0.226 |
| 95% CI | Infinity to −0.059 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Right ventricle | |
| Number of patients included | 108e |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.021 ± 0.189 |
| 95% CI | Infinity to 0.009 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Sensing amplitude invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 31f |
| Mean ± SD of log-transformed patient-wise ratiosc | 0.009 ± 0.184 |
| 95% CI | −0.047 to infinity |
| P-value for ‘>−0.10 hypothesis’g | 0.0012 |
| Right ventricle | |
| Number of patients included | 107h |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.005 ± 0.133 |
| 95% CI | −0.026 to infinity |
| P-value for ‘>−0.10 hypothesis’g | <0.0001 |
| Hypothesis . | Value . |
|---|---|
| MRI-related SADE | |
| Number of patients included | 111a |
| Number of SADEs | 0 |
| SADE-free rate (95% CI) | 100% (95.98–100) |
| P-value for ‘>90% hypothesis’ | <0.0001 |
| Pacing threshold invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 28b |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.132 ± 0.226 |
| 95% CI | Infinity to −0.059 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Right ventricle | |
| Number of patients included | 108e |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.021 ± 0.189 |
| 95% CI | Infinity to 0.009 |
| P-value for ‘<0.10 hypothesis’d | <0.0001 |
| Sensing amplitude invariance (1-month vs. pre-MRI) | |
| Right atrium | |
| Number of patients included | 31f |
| Mean ± SD of log-transformed patient-wise ratiosc | 0.009 ± 0.184 |
| 95% CI | −0.047 to infinity |
| P-value for ‘>−0.10 hypothesis’g | 0.0012 |
| Right ventricle | |
| Number of patients included | 107h |
| Mean ± SD of log-transformed patient-wise ratiosc | −0.005 ± 0.133 |
| 95% CI | −0.026 to infinity |
| P-value for ‘>−0.10 hypothesis’g | <0.0001 |
CI, confidence interval; ICD, implantable cardioverter-defibrillator; MRI, magnetic resonance imaging; SADE, serious adverse device effect; SD, standard deviation.
All patients reaching the 1-month follow-up. One patient of 112 who underwent study-specific MRI had a panic attack and prematurely terminated the study after the MRI-procedure follow-up.
Of 31 patients with atrial lead and 1-month follow-up, 3 did not contribute because of missing data.
Corresponding to the geometric mean of non-log-transformed ratios.
Corresponding to ‘<1.07 hypothesis’ for non-log-transformed ratios.
Of 111 patients with ICD lead and 1-month follow-up, 3 did not contribute because of missing data.
All 31 patients with atrial lead and 1-month follow-up contributed.
Corresponding to ‘>0.933 hypothesis’ for non-log-transformed ratios.
Of 111 patients with ICD lead and 1-month follow-up, 4 did not contribute because of missing data.
Secondary endpoints
One of the secondary endpoints was defined as a rise of the right ventricular threshold between pre-MRI procedure and 1-month follow-up to be ≤0.5 V. For all 89 ICD leads with data available for this analysis, the threshold rise was ≤0.5 V, resulting in a success rate of 100% (95% CI 95.94–100%). Likewise, no R-wave amplitude attenuation >50% was observed in 88 ICD leads with available data, yielding sensing amplitude success rate of 100% (95% CI 95.89–100%).
Other findings
There are no visible effects of MRI on lead parameters (Figure 3). Battery capacity in percents was 99.5 ± 3.8 before MRI vs. 99.5 ± 4.0 after MRI, 99.2 ± 4.5 at 1 month, and 98.9 ± 5.3 at 3 months; the ranges were 66–100% (pre-MRI), 64–100% (post-MRI), 63–100% (1 month), and 60–100% (3 months), with median and IQR values of 100% at all times.

Box- and whisker plot distributions of lead parameters measured in office. Whiskers extend from the upper and lower hinge no further than 1.5*IQR from each hinge, rhombi denote mean values. The unusually large RA amplitudes (≥15 mV) were measured in patients with DX ICD devices, which amplify the atrial signal up to four-fold. DX ICD, single-chamber implantable cardioverter-defibrillator with atrial diagnostics (atrial sensing via floating dipole)14; FU, follow-up; IQR, interquartile range; M, month; MRI, magnetic resonance imaging; RA, right atrial; RV, right ventricular.
The success rate of ICD therapies was calculated for ventricular tachyarrhythmia, excluding episodes that were induced, detected in the monitoring zone not requiring treatment, or ending spontaneously. Five episodes of monomorphic ventricular tachycardia met the inclusion criteria (one before MRI, four after MRI). All were terminated successfully by antitachycardia pacing. Additional 193 episodes of atrial tachyarrhythmia and four episodes of non-sustained monomorphic ventricular tachycardia were detected by the device, with appropriate retention of ICD therapy.
Magnetic resonance imaging-related observations
One patient experienced tingling and localized heat sensation above the ICD implantation area during the study-specific MRI procedure. The subsequent ICD check was unremarkable. The event was reported as a non-serious adverse device effect.
All MR images were reviewed for any incidental findings. Abnormalities were noted in eight patients (7%), including intracranial arterial aneurysm, a lacunar infarct in the left cerebellar hemisphere and left thalamus, multiple old strokes with corresponding defects, known sequelae of a cerebellar infarction, gliosis, mild Chiari malformation with soft tissue swelling in the paranasal sinuses, and an iliopsoas bursitis.
Discussion
Our study demonstrates that performing a head and a lower lumbar MRI scan at 3 T using the investigated MR-conditional ICD systems can be safe when appropriate exclusion zones and MRI conditions are used. There was no evidence of harm to the patients or any negative influence of the MRI scan on the implanted systems.
The number of 3 T scanners in clinical practice is increasing every year. They produce more detailed images for pathological conditions involving the brain, spine, and musculoskeletal system than 1.5 T scanners. MR scanning imposes a risk of tissue damage at the electrode–tissue interface (through heating by electrical current induced in leads, potentially compromising long-term pacing and sensing performance) and a certain risk of lead dislocation (by force generated by the magnetic field), inappropriate therapy during scans (by temporary under- or oversensing), changes in device programming, or permanent electronic dysfunction.3–5 Theoretically, the risk may increase with the strength of the magnetic field.
Magnetic resonance imaging scans at 1.5 T with or without exclusion zones have been proven safe in over a dozen of studies with MR-conditional pacemaker and ICD systems.3–13 A need remains for clinical studies to verify the safety of MR-conditional devices in the more challenging environment of the stronger magnets.17 So far, one study of 15 patients with MR-conditional pacemakers undergoing a thoracic or lumbar spine 3 T MR scans showed no adverse effect of the MRI.17 Earlier, two small studies with non-conditional pacemaker (49 brain,18,19 1 jaw,19 1 left thigh,19 and 1 lumbar spine19 scans) and ICD systems (6 brain scans19) reported no clinically relevant adverse effects of 3 T MR scanning under specific conditions in a total of 58 patients.
Our study demonstrates in a large number of patients using systematic follow-up and standardized conditions and MR-protocols that patients implanted with the investigated ICD systems can safely be scanned with a 3 T MR scanner.
Study limitations
The use of MRI scanners was limited to well-defined conditions. The findings apply to the devices scanned and cannot be extrapolated to other scanning conditions or ICD manufacturers. Although MRI artefacts associated with the implanted system7,20 were not systematically analysed, lead and pulse generator artefacts were not limiting the analysis of the MR images.
Conclusion
In the present study, head and lower lumbar MRI at 3 T under consideration of the exclusion zone and a specific MRI protocol was safe in the studied Biotronik ProMRI ICD systems.
Acknowledgements
The authors thank to Ulrich Gauger, PhD, for statistical analyses and revising the manuscript, and Dejan Danilovic, PhD, for critical reading of the manuscript and editorial assistance.
Funding
This work was supported by Biotronik SE & Co. KG (Berlin, Germany).
Conflict of interest: C.W. and G.A.F. have received consultancy fees. C.W., J.M., and J.S. travel grants, and C.W. speaker’s honoraria from Biotronik. T.T. is employee of Biotronik. Other authors declared no conflict of interest.



