-
PDF
- Split View
-
Views
-
Cite
Cite
Koji Nakagawa, Teiji Akagi, Satoshi Nagase, Yoichi Takaya, Yasufumi Kijima, Norihisa Toh, Atsuyuki Watanabe, Nobuhiro Nishii, Kazufumi Nakamura, Hiroshi Morita, Kengo Kusano, Hiroshi Ito, Efficacy of catheter ablation for paroxysmal atrial fibrillation in patients with atrial septal defect: a comparison with transcatheter closure alone, EP Europace, Volume 21, Issue 11, November 2019, Pages 1663–1669, https://doi.org/10.1093/europace/euz207
Close - Share Icon Share
Abstract
There is no valid treatment strategy for addressing paroxysmal atrial fibrillation (AF) in patients with unclosed atrial septal defect (ASD). We aimed to assess the efficacy of catheter ablation (CA) compared with transcatheter ASD closure alone for treating pre-existing paroxysmal AF in patients with ASD.
Among 908 patients who underwent transcatheter ASD closure, we evaluated 50 consecutive patients (63 ± 12 years) with paroxysmal AF. We compared the AF outcomes of these patients after transcatheter ASD closure between those with and without CA prior to ASD closure. Thirty (60%) patients underwent CA. During the follow-up period after ASD closure (mean: 49 ± 23 months), recurrence of AF was observed in 6/30 (20%) patients with upfront CA and 12/20 (60%) patients with ASD closure alone. Kaplan–Meier analysis showed that the AF-free survival rate was significantly higher for patients with CA than for those with ASD closure alone (79% vs. 37% at 5 years, P = 0.002). Upfront CA and previous heart failure hospitalization were associated with recurrence of AF after ASD closure [hazard ratio (HR) 0.18, 95% confidence interval (CI) 0.06–0.53; P = 0.002 and HR 4.64, 95% CI 1.60–13.49; P = 0.005, respectively].
In ASD patient with paroxysmal AF, transcatheter ASD closure alone demonstrated high AF recurrence rate after ASD closure. On the other hand, upfront CA prior to ASD closure substantially suppressed AF recurrence over the long term. A combination of CA and transcatheter ASD closure may be a feasible treatment strategy for paroxysmal AF in patients with ASD.
Long-term data on atrial fibrillation (AF) outcome after transcatheter atrial septal defect (ASD) closure and efficacy of upfront catheter ablation (CA) are lacking.
The 5-year incidence of recurrent AF after transcatheter ASD closure in the patient group with transcatheter ASD closure alone was at 63% (12%/year). On the other hand, in the patient group with upfront CA was ∼20% (4%/year).
Upfront CA prior to ASD closure was associated with a reduced risk of AF recurrence after ASD closure. Our study also showed that previous heart failure hospitalization was an independent risk factor for recurrence of AF after transcatheter ASD closure.
Introduction
Atrial fibrillation (AF) is one of the most common comorbidities in middle-aged and older patients with an atrial septal defect (ASD). Atrial fibrillation develops at a younger age in those with ASD than in the normal population, and its prevalence is as high as 50% after 60 years old.1,2 Patients with ASD and AF not only remain at risk of stroke and bleeding complications due to anticoagulants, but they also can develop heart failure, especially older patients.1 Furthermore, a recent report showed that atrial tachyarrhythmia was an independent determinant of mortality, and heart failure was a major cause of death in adult patients with ASD.3 Despite this situation, there is no valid therapeutic strategy for addressing concomitant AF in patients with ASD except for surgical ASD closure with Maze procedure.
Nowadays, transcatheter ASD closure and catheter ablation (CA) are well-established. Transcatheter ASD closure achieves geometrical, functional, and electrical reverse remodelling in both atria4 and has a provide favourable effects on arrhythmia suppression.5 Pulmonary vein isolation (PVI) by means of CA is widely performed for curative treatment of paroxysmal AF in patients without structural heart disease. However, efficacy of each of or a combination of these two therapies for suppressing AF is not well validated in patients with ASD. In the present study, we aimed to assess the efficacy of upfront CA prior to transcatheter ASD closure compared with transcatheter ASD closure alone for treating pre-existing paroxysmal AF in patients with ASD.
Methods
Subjects included in the study cohort
This retrospective study included 908 patients who underwent transcatheter ASD closure at our hospital from January 2005 to May 2016. Among them, 50 consecutive patients had a history of paroxysmal AF (Figure 1). The patients were retrospectively identified based on data in their medical records and medical history. Patients who had persistent AF, long-standing persistent AF or permanent AF were not included in this study. Patients were classified according to the modified European Heart Rhythm Association (mEHRA) score6 for AF-related symptoms. The patients were then divided into two groups based on whether they had undergone PVI by means of CA for AF prior to transcatheter ASD closure. Patients who underwent transcatheter ASD closure alone did not consent to CA prior to ASD closure because their AF attack was infrequent (once or twice a year) or symptoms during AF was none or mild. We refrained from CA for one particular patient with a pacemaker due to complete atrioventricular block. For patients with upfront CA, transcatheter ASD closure was scheduled when AF had not recurred for at least 3 months after the last successful CA.
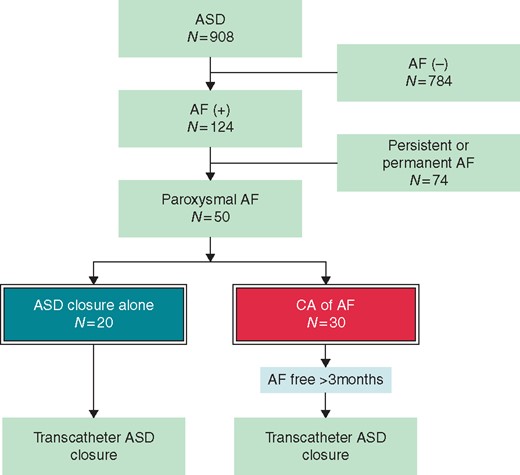
Flowchart for selection of study patients. AF, atrial fibrillation; ASD, atrial septal defect; CA, catheter ablation.
We compared the AF outcomes of these patients after transcatheter ASD closure between the patient groups with upfront CA and with ASD closure alone and evaluated associated factors with recurrence of AF.
Catheter ablation
In all patients, PVI was performed as a standard method of CA with the assistance of a three-dimensional mapping system (Carto, Biosense Webstar, Irvine, CA, USA). A 20-polar circular mapping catheter was placed within ostia of the pulmonary veins and a 20-polar electrode catheter was inserted via the right subclavian vein into the coronary sinus. Mapping and ablation were performed using a 4-mm non-irrigated-tip catheter (Navistar; Biosense Webstar) for cases before 2011 and a 3.5-mm irrigated-tip catheter (ThermoCool Navistar; Biosense Webstar) for cases in or after 2011. The endpoint of PVI was the achievement of bidirectional block between the left atrium and each pulmonary vein. Acute procedural complications of CA were defined as stroke, transient ischaemic attack, cardiac tamponade, pericardial effusion, bleeding requiring transfusion, oesophageal injury, gastric hypomotility, phrenic nerve paralysis, pericarditis, pulmonary vein stenosis, and vascular access complications needing intervention.
Transcatheter atrial septal defect closure
Indications for ASD closure were right ventricular enlargement or a history of systemic embolism including embolic stroke. Exclusion criteria included a maximum defect diameter of >38 mm (evaluated by transoesophageal echocardiography), other concomitant congenital heart diseases, and pulmonary hypertension with pulmonary vascular resistance of >8 Wood units. Transcatheter ASD closure was conducted under general anaesthesia with fluoroscopic guidance and transoesophageal echocardiography. The Amplatzer® Septal Occluder (St. Jude Medical; St. Paul, MN, USA) or Figulla® Flex II (Occlutech International AB, Helsingborg, Sweden) was used for all closures. Acute procedural complications of CA were defined as device migration/malposition, cardiac erosion, cardiac tamponade, pericardial effusion, arrhythmias, stroke, transient ischaemic attack, acute heart failure, bleeding requiring transfusion, and vascular access complications needing intervention. All antiarrhythmic medications, except for Class II antiarrhythmic drugs (β-blockers), were discontinued following ASD closure in all patients with CA. Aspirin was continued for 6 months after ASD closure. Anticoagulation was stopped if the sinus rhythm had been maintained for more than 6 months after ASD closure unless otherwise indicated. Follow-up examination after ASD closure in our hospital was scheduled at 1, 3, 6, and 12 months and annually thereafter. At each outpatient visit, the patient’s symptoms and a 12-lead electrocardiogram (ECG) were recorded. These follow-up schedules after transcatheter ASD closure are mandatory in Japan. In addition, all patients received a consultation by a family doctor every month or every other month. Holter ECG was recorded more than once annually with or without symptoms. Additionally, Holter or ambulatory ECGs were obtained based on the patient’s symptoms. Identification of recurrent AF was based on documentation of AF on any ECG.
Statistical analysis
Data are expressed as mean ± standard deviation or median and range. Continuous variables were compared using the Student’s t-test or Mann–Whitney U test. Categorical variables were analysed by the χ2 or Fisher’s exact test, as appropriate. The Kaplan–Meier method was used to calculate AF-free survival, which was compared using the log-rank test. The Cox proportional hazards regression model was used to determine the independent predictors of recurrence of AF after transcatheter ASD closure in patients with and without recurrence of AF. A value of P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS version 24 software (SPSS Inc., Chicago, IL, USA). The institutional ethics review committee approved the study protocol. Written informed consent was obtained from all of the patients.
Results
Patients’ characteristics
The patients’ characteristics before ASD closure are shown in Table 1. This study included 22 men and 28 women (age: 25–83 years, mean age: 63 ± 12 years). Among them, 30 (60%) underwent CA. The mean ASD diameter was 19 ± 7 mm and the mean pulmonary/systemic flow ratio was 2.5 ± 0.8. The mean dimension of the left atrium was 41 ± 6 mm. Eighteen (90%) of patients who underwent transcatheter ASD closure were in mEHRA Score 2b and less. On the other hand, 18 (60%) of who underwent upfront CA were in mEHRA Score 2b and more. All clinical variables, except for ASD diameter and mEHRA score, were not significantly different between the two groups (with and without upfront CA). Patients with ASD closure alone had a larger ASD (21 ± 10 mm vs. 17 ± 5 mm, P = 0.046) and a lower mEHRA score (P = 0.007) than those with CA.
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Variables | ||||
| Age (years) | 63 ± 12 | 67 ± 11 | 61 ± 12 | 0.071 |
| Gender (male) | 22 (44) | 10 (50) | 12 (40) | 0.485 |
| BMI | 22 ± 4 | 22 ± 4 | 22 ± 4 | 0.908 |
| LVEF (%) | 68 ± 7 | 69 ± 10 | 67 ± 5 | 0.503 |
| ASD diameter (mm) | 19 ± 7 | 21 ± 10 | 17 ± 5 | 0.046 |
| Qp/Qs | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.4 ± 0.8 | 0.146 |
| Pulmonary hypertension | 13 (26) | 7 (35) | 6 (20) | 0.236 |
| Hypertension | 23 (46) | 8 (40) | 15 (50) | 0.487 |
| Coronary artery disease | 0 (0) | 0 (0) | 0 (0) | – |
| Previous HF hospitalization | 15 (30) | 6 (30) | 9 (30) | 1.000 |
| Antiarrhythmic drug usage | 29 (58) | 13 (65) | 16 (53) | 0.413 |
| LA dimension (mm) | 41 ± 6 | 42 ± 6 | 40 ± 6 | 0.252 |
| mEHRA score (1/2a/2b/3/4) | 9/15/10/7/9 | 5/7/6/2/0 | 4/8/4/5/9 | 0.007 |
| Catheter ablation | ||||
| PVI alone | – | – | 9 (30) | – |
| +CTI ablation | – | – | 19 (63) | – |
| +CTI ablation + SVC isolation | – | – | 1 (3) | – |
| +CTI ablation + GP ablation | – | – | 1 (3) | – |
| Use of irrigated-tip ablation catheter | 23 (77) | |||
| Repeated catheter ablation | – | – | 6 (20) | – |
| Acute procedural complication | – | – | 0 | – |
| Transcatheter ASD closure | ||||
| Time from last PVI (months) | – | – | 5.7 ± 6.3 | – |
| ASD closure device (ASO/OFFII) | 47/3 | 20/0 | 27/3 | 0.145 |
| Size of device (mm) | 22 ± 7 | 24 ± 9 | 20 ± 5 | 0.060 |
| Successful deployment | 50 (100) | 20 (100) | 30 (100) | 1.000 |
| Acute procedural complication | 0 | 0 | 0 | – |
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Variables | ||||
| Age (years) | 63 ± 12 | 67 ± 11 | 61 ± 12 | 0.071 |
| Gender (male) | 22 (44) | 10 (50) | 12 (40) | 0.485 |
| BMI | 22 ± 4 | 22 ± 4 | 22 ± 4 | 0.908 |
| LVEF (%) | 68 ± 7 | 69 ± 10 | 67 ± 5 | 0.503 |
| ASD diameter (mm) | 19 ± 7 | 21 ± 10 | 17 ± 5 | 0.046 |
| Qp/Qs | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.4 ± 0.8 | 0.146 |
| Pulmonary hypertension | 13 (26) | 7 (35) | 6 (20) | 0.236 |
| Hypertension | 23 (46) | 8 (40) | 15 (50) | 0.487 |
| Coronary artery disease | 0 (0) | 0 (0) | 0 (0) | – |
| Previous HF hospitalization | 15 (30) | 6 (30) | 9 (30) | 1.000 |
| Antiarrhythmic drug usage | 29 (58) | 13 (65) | 16 (53) | 0.413 |
| LA dimension (mm) | 41 ± 6 | 42 ± 6 | 40 ± 6 | 0.252 |
| mEHRA score (1/2a/2b/3/4) | 9/15/10/7/9 | 5/7/6/2/0 | 4/8/4/5/9 | 0.007 |
| Catheter ablation | ||||
| PVI alone | – | – | 9 (30) | – |
| +CTI ablation | – | – | 19 (63) | – |
| +CTI ablation + SVC isolation | – | – | 1 (3) | – |
| +CTI ablation + GP ablation | – | – | 1 (3) | – |
| Use of irrigated-tip ablation catheter | 23 (77) | |||
| Repeated catheter ablation | – | – | 6 (20) | – |
| Acute procedural complication | – | – | 0 | – |
| Transcatheter ASD closure | ||||
| Time from last PVI (months) | – | – | 5.7 ± 6.3 | – |
| ASD closure device (ASO/OFFII) | 47/3 | 20/0 | 27/3 | 0.145 |
| Size of device (mm) | 22 ± 7 | 24 ± 9 | 20 ± 5 | 0.060 |
| Successful deployment | 50 (100) | 20 (100) | 30 (100) | 1.000 |
| Acute procedural complication | 0 | 0 | 0 | – |
Values are expressed as n (%) or mean ± SD.
AF, atrial fibrillation; ASD, atrial septal defect; ASO, Amplatzer® Septal Occluder; BMI, body mass index; CA, catheter ablation; CTI, cavotricuspid isthmus; GP, ganglionated plexi; HF, heart failure; LA, left atrium; mEHRA, modified European Heart Rhythm Association; OFFII, Occlutech® Figulla Flex II; PVI, pulmonary vein isolation; Qp/Qs, the ratio of pulmonary to systemic flow; SD, standard deviation; SVC, superior vena cava.
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Variables | ||||
| Age (years) | 63 ± 12 | 67 ± 11 | 61 ± 12 | 0.071 |
| Gender (male) | 22 (44) | 10 (50) | 12 (40) | 0.485 |
| BMI | 22 ± 4 | 22 ± 4 | 22 ± 4 | 0.908 |
| LVEF (%) | 68 ± 7 | 69 ± 10 | 67 ± 5 | 0.503 |
| ASD diameter (mm) | 19 ± 7 | 21 ± 10 | 17 ± 5 | 0.046 |
| Qp/Qs | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.4 ± 0.8 | 0.146 |
| Pulmonary hypertension | 13 (26) | 7 (35) | 6 (20) | 0.236 |
| Hypertension | 23 (46) | 8 (40) | 15 (50) | 0.487 |
| Coronary artery disease | 0 (0) | 0 (0) | 0 (0) | – |
| Previous HF hospitalization | 15 (30) | 6 (30) | 9 (30) | 1.000 |
| Antiarrhythmic drug usage | 29 (58) | 13 (65) | 16 (53) | 0.413 |
| LA dimension (mm) | 41 ± 6 | 42 ± 6 | 40 ± 6 | 0.252 |
| mEHRA score (1/2a/2b/3/4) | 9/15/10/7/9 | 5/7/6/2/0 | 4/8/4/5/9 | 0.007 |
| Catheter ablation | ||||
| PVI alone | – | – | 9 (30) | – |
| +CTI ablation | – | – | 19 (63) | – |
| +CTI ablation + SVC isolation | – | – | 1 (3) | – |
| +CTI ablation + GP ablation | – | – | 1 (3) | – |
| Use of irrigated-tip ablation catheter | 23 (77) | |||
| Repeated catheter ablation | – | – | 6 (20) | – |
| Acute procedural complication | – | – | 0 | – |
| Transcatheter ASD closure | ||||
| Time from last PVI (months) | – | – | 5.7 ± 6.3 | – |
| ASD closure device (ASO/OFFII) | 47/3 | 20/0 | 27/3 | 0.145 |
| Size of device (mm) | 22 ± 7 | 24 ± 9 | 20 ± 5 | 0.060 |
| Successful deployment | 50 (100) | 20 (100) | 30 (100) | 1.000 |
| Acute procedural complication | 0 | 0 | 0 | – |
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Variables | ||||
| Age (years) | 63 ± 12 | 67 ± 11 | 61 ± 12 | 0.071 |
| Gender (male) | 22 (44) | 10 (50) | 12 (40) | 0.485 |
| BMI | 22 ± 4 | 22 ± 4 | 22 ± 4 | 0.908 |
| LVEF (%) | 68 ± 7 | 69 ± 10 | 67 ± 5 | 0.503 |
| ASD diameter (mm) | 19 ± 7 | 21 ± 10 | 17 ± 5 | 0.046 |
| Qp/Qs | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.4 ± 0.8 | 0.146 |
| Pulmonary hypertension | 13 (26) | 7 (35) | 6 (20) | 0.236 |
| Hypertension | 23 (46) | 8 (40) | 15 (50) | 0.487 |
| Coronary artery disease | 0 (0) | 0 (0) | 0 (0) | – |
| Previous HF hospitalization | 15 (30) | 6 (30) | 9 (30) | 1.000 |
| Antiarrhythmic drug usage | 29 (58) | 13 (65) | 16 (53) | 0.413 |
| LA dimension (mm) | 41 ± 6 | 42 ± 6 | 40 ± 6 | 0.252 |
| mEHRA score (1/2a/2b/3/4) | 9/15/10/7/9 | 5/7/6/2/0 | 4/8/4/5/9 | 0.007 |
| Catheter ablation | ||||
| PVI alone | – | – | 9 (30) | – |
| +CTI ablation | – | – | 19 (63) | – |
| +CTI ablation + SVC isolation | – | – | 1 (3) | – |
| +CTI ablation + GP ablation | – | – | 1 (3) | – |
| Use of irrigated-tip ablation catheter | 23 (77) | |||
| Repeated catheter ablation | – | – | 6 (20) | – |
| Acute procedural complication | – | – | 0 | – |
| Transcatheter ASD closure | ||||
| Time from last PVI (months) | – | – | 5.7 ± 6.3 | – |
| ASD closure device (ASO/OFFII) | 47/3 | 20/0 | 27/3 | 0.145 |
| Size of device (mm) | 22 ± 7 | 24 ± 9 | 20 ± 5 | 0.060 |
| Successful deployment | 50 (100) | 20 (100) | 30 (100) | 1.000 |
| Acute procedural complication | 0 | 0 | 0 | – |
Values are expressed as n (%) or mean ± SD.
AF, atrial fibrillation; ASD, atrial septal defect; ASO, Amplatzer® Septal Occluder; BMI, body mass index; CA, catheter ablation; CTI, cavotricuspid isthmus; GP, ganglionated plexi; HF, heart failure; LA, left atrium; mEHRA, modified European Heart Rhythm Association; OFFII, Occlutech® Figulla Flex II; PVI, pulmonary vein isolation; Qp/Qs, the ratio of pulmonary to systemic flow; SD, standard deviation; SVC, superior vena cava.
Catheter ablation of atrial fibrillation and transcatheter atrial septal defect closure
Procedural details of CA and transcatheter ASD closure are shown in Table 1. Pulmonary vein isolation was completed via ASD without acute complications in any of the patients. Pulmonary vein isolation alone was performed in nine (30%) of the 30 patients. The other 21 (70%) patients underwent cavotricuspid isthmus (CTI) linear ablation in addition to PVI, 13 of whom were treated empirically. Left atrial ganglionated plexi ablation was added to PVI and CTI ablation in one (3%) patient at the first CA. Six (20%) patients underwent a second CA because AF had recurred within 3 months after the first CA before ASD closure. At the second CA, a repeated PVI was performed in all six patients because of reconnection of the pulmonary vein and isolation of the superior vena cava (SVC) was added in one of those patients. The mean time from the last CA to transcatheter ASD closure was 5.7 ± 6.3 months. Transcatheter ASD closure was successful without acute complications in all patients using the Amplatzer® Septal Occluder in 27 (90%) patients and the Figulla® Flex II in three (10%) patients. The mean size of the closure device was 22 ± 7 mm.
Outcome after transcatheter atrial septal defect closure
Outcomes after transcatheter ASD closure are shown in Table 2. During a mean follow-up period of 49 ± 23 months after ASD closure, 18 (36%) of the 50 patients developed recurrent AF. Patients with upfront CA had a significantly lower recurrence rate of AF than did those with ASD closure alone (20% vs. 60%, P = 0.006). Furthermore, time to AF recurrence after ASD closure tended to be longer in patients with upfront CA. On the other hand, antiarrhythmic drug usage was significantly fewer in patients with CA than those without (17% vs. 70%, P < 0.001).
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Follow-up after ASD closure (months) | 49 ± 23 | 50 ± 24 | 49 ± 22 | 0.782 |
| Recurrence of AF | 18 (36) | 12 (60) | 6 (20) | 0.006 |
| Time to AF recurrence after ASD closure (months) | 23 (1–47) | 16 (1–47) | 38 (12–102) | 0.068 |
| Antiarrhythmic drug usage | 19 (38) | 14 (70) | 5 (17) | <0.001 |
| Class I/II/III/IV/digoxin | 6/7/0/3/3 | 6/2/0/3/3 | 0/5/0/0/0 |
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Follow-up after ASD closure (months) | 49 ± 23 | 50 ± 24 | 49 ± 22 | 0.782 |
| Recurrence of AF | 18 (36) | 12 (60) | 6 (20) | 0.006 |
| Time to AF recurrence after ASD closure (months) | 23 (1–47) | 16 (1–47) | 38 (12–102) | 0.068 |
| Antiarrhythmic drug usage | 19 (38) | 14 (70) | 5 (17) | <0.001 |
| Class I/II/III/IV/digoxin | 6/7/0/3/3 | 6/2/0/3/3 | 0/5/0/0/0 |
Values are expressed as n (%), mean ± SD, or median (range).
AF, atrial fibrillation; ASD, atrial septal defect; CA, catheter ablation; SD, standard deviation.
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Follow-up after ASD closure (months) | 49 ± 23 | 50 ± 24 | 49 ± 22 | 0.782 |
| Recurrence of AF | 18 (36) | 12 (60) | 6 (20) | 0.006 |
| Time to AF recurrence after ASD closure (months) | 23 (1–47) | 16 (1–47) | 38 (12–102) | 0.068 |
| Antiarrhythmic drug usage | 19 (38) | 14 (70) | 5 (17) | <0.001 |
| Class I/II/III/IV/digoxin | 6/7/0/3/3 | 6/2/0/3/3 | 0/5/0/0/0 |
| . | Entire cohort (n = 50) . | ASD closure alone (n = 20) . | Upfront CA (n = 30) . | ASD closure alone vs. upfront CA (P value) . |
|---|---|---|---|---|
| Follow-up after ASD closure (months) | 49 ± 23 | 50 ± 24 | 49 ± 22 | 0.782 |
| Recurrence of AF | 18 (36) | 12 (60) | 6 (20) | 0.006 |
| Time to AF recurrence after ASD closure (months) | 23 (1–47) | 16 (1–47) | 38 (12–102) | 0.068 |
| Antiarrhythmic drug usage | 19 (38) | 14 (70) | 5 (17) | <0.001 |
| Class I/II/III/IV/digoxin | 6/7/0/3/3 | 6/2/0/3/3 | 0/5/0/0/0 |
Values are expressed as n (%), mean ± SD, or median (range).
AF, atrial fibrillation; ASD, atrial septal defect; CA, catheter ablation; SD, standard deviation.
Figure 2 shows the results of Kaplan–Meier analysis of long-term AF-free survival after transcatheter ASD closure in patients with CA and those with ASD closure alone. The 5-year AF-free survival rate was significantly higher in patients with CA than in those with ASD closure alone (79% vs. 37% P = 0.002, log-rank test).
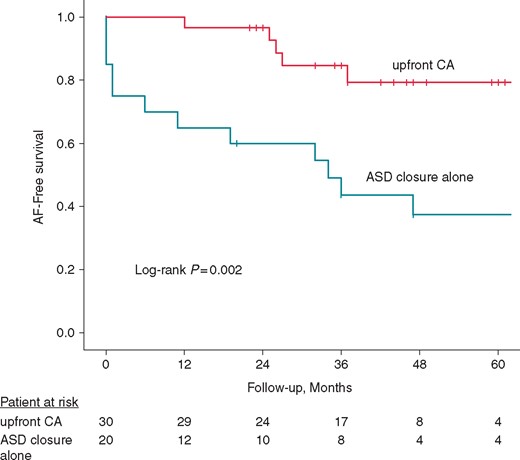
Kaplan–Meier analysis of long-term AF-free survival after transcatheter ASD closure. The AF-free survival rate was significantly higher in patients with upfront CA than in those with ASD closure alone (P = 0.002, log-rank test). AF, atrial fibrillation; ASD, atrial septal defect; CA, catheter ablation.
Effect of catheter ablation on reducing recurrence of atrial fibrillation after atrial septal defect closure
The univariate and multivariate Cox proportional hazard models for recurrence of AF after transcatheter ASD closure are shown in Table 3. Multivariate analyses showed that upfront CA significantly decreased the risk of recurrent AF following transcatheter ASD closure [hazard ratio (HR) 0.18, 95% confidence interval (CI) 0.06–0.53; P = 0.002]. They also showed that previous heart failure hospitalization was a risk factor for recurrence of AF (HR 4.64, 95% CI 1.60–13.49; P = 0.005).
Univariate and multivariate cox proportional hazard analyses of AF recurrence
| . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.01 | 0.97–1.05 | 0.643 |
| Gender (male) | 1.14 | 0.44–2.96 | 0.788 |
| BMI | 1.04 | 0.92–1.18 | 0.536 |
| LVEF (mm) | 0.98 | 0.92–1.05 | 0.573 |
| ASD diameter (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Qp/Qs | 1.26 | 0.70–2.25 | 0.444 |
| Pulmonary hypertension | 1.88 | 0.72–4.91 | 0.198 |
| Hypertension | 0.57 | 0.22–1.53 | 0.265 |
| Previous HF hospitalization | 3.51 | 1.29–9.58 | 0.014 |
| Antiarrhythmic drug usage | 1.99 | 0.77–5.16 | 0.159 |
| LA dimension (mm) | 1.04 | 0.96–1.12 | 0.371 |
| Upfront CA | 0.22 | 0.08–0.63 | 0.005 |
| Closure device size (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Multivariate analysis | |||
| Previous HF hospitalization | 4.64 | 1.60–13.49 | 0.005 |
| Upfront CA | 0.18 | 0.06–0.53 | 0.002 |
| . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.01 | 0.97–1.05 | 0.643 |
| Gender (male) | 1.14 | 0.44–2.96 | 0.788 |
| BMI | 1.04 | 0.92–1.18 | 0.536 |
| LVEF (mm) | 0.98 | 0.92–1.05 | 0.573 |
| ASD diameter (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Qp/Qs | 1.26 | 0.70–2.25 | 0.444 |
| Pulmonary hypertension | 1.88 | 0.72–4.91 | 0.198 |
| Hypertension | 0.57 | 0.22–1.53 | 0.265 |
| Previous HF hospitalization | 3.51 | 1.29–9.58 | 0.014 |
| Antiarrhythmic drug usage | 1.99 | 0.77–5.16 | 0.159 |
| LA dimension (mm) | 1.04 | 0.96–1.12 | 0.371 |
| Upfront CA | 0.22 | 0.08–0.63 | 0.005 |
| Closure device size (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Multivariate analysis | |||
| Previous HF hospitalization | 4.64 | 1.60–13.49 | 0.005 |
| Upfront CA | 0.18 | 0.06–0.53 | 0.002 |
AF, atrial fibrillation; ASD, atrial septal defect; BMI, body mass index; CA, catheter ablation; HF, heart failure; LA, left atrium; Qp/Qs, the ratio of pulmonary to systemic flow.
Univariate and multivariate cox proportional hazard analyses of AF recurrence
| . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.01 | 0.97–1.05 | 0.643 |
| Gender (male) | 1.14 | 0.44–2.96 | 0.788 |
| BMI | 1.04 | 0.92–1.18 | 0.536 |
| LVEF (mm) | 0.98 | 0.92–1.05 | 0.573 |
| ASD diameter (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Qp/Qs | 1.26 | 0.70–2.25 | 0.444 |
| Pulmonary hypertension | 1.88 | 0.72–4.91 | 0.198 |
| Hypertension | 0.57 | 0.22–1.53 | 0.265 |
| Previous HF hospitalization | 3.51 | 1.29–9.58 | 0.014 |
| Antiarrhythmic drug usage | 1.99 | 0.77–5.16 | 0.159 |
| LA dimension (mm) | 1.04 | 0.96–1.12 | 0.371 |
| Upfront CA | 0.22 | 0.08–0.63 | 0.005 |
| Closure device size (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Multivariate analysis | |||
| Previous HF hospitalization | 4.64 | 1.60–13.49 | 0.005 |
| Upfront CA | 0.18 | 0.06–0.53 | 0.002 |
| . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.01 | 0.97–1.05 | 0.643 |
| Gender (male) | 1.14 | 0.44–2.96 | 0.788 |
| BMI | 1.04 | 0.92–1.18 | 0.536 |
| LVEF (mm) | 0.98 | 0.92–1.05 | 0.573 |
| ASD diameter (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Qp/Qs | 1.26 | 0.70–2.25 | 0.444 |
| Pulmonary hypertension | 1.88 | 0.72–4.91 | 0.198 |
| Hypertension | 0.57 | 0.22–1.53 | 0.265 |
| Previous HF hospitalization | 3.51 | 1.29–9.58 | 0.014 |
| Antiarrhythmic drug usage | 1.99 | 0.77–5.16 | 0.159 |
| LA dimension (mm) | 1.04 | 0.96–1.12 | 0.371 |
| Upfront CA | 0.22 | 0.08–0.63 | 0.005 |
| Closure device size (mm) | 1.02 | 0.96–1.08 | 0.557 |
| Multivariate analysis | |||
| Previous HF hospitalization | 4.64 | 1.60–13.49 | 0.005 |
| Upfront CA | 0.18 | 0.06–0.53 | 0.002 |
AF, atrial fibrillation; ASD, atrial septal defect; BMI, body mass index; CA, catheter ablation; HF, heart failure; LA, left atrium; Qp/Qs, the ratio of pulmonary to systemic flow.
Discussion
The main finding of this study was long-term superiority of upfront CA compared with transcatheter ASD closure alone for preventing recurrence of AF after transcatheter ASD closure. Our findings provide new information regarding the management of patients with ASD and concomitant paroxysmal AF.
Transcatheter atrial septal defect closure alone and recurrence of atrial fibrillation
The high incidence of AF in patients with ASD is related to chronic volume overload due to a left-to-right atrial shunt, resulting in geometrical and electrical remodelling of both atria.7,8 ASD closure halts this volume overload and causes geometrical, functional, and electrical reverse remodelling in both atria, which can contribute to AF prevention. The incidence of AF after transcatheter ASD closure in patients with a history of atrial arrhythmias has reported to be at 17%/year and is about 3–4 times higher than that in the general ASD population.9–11 Consistent with this, our study also demonstrated that the 5-year incidence of recurrent AF in patients with transcatheter ASD closure alone was high at 63% (12%/year). A meta-analysis of 26 studies included 1841 surgical closures and 945 transcatheter closures showed that ASD closure, whether performed surgically or by transcatheter, was associated with a reduction in the post-closure prevalence of pre-existing atrial tachyarrhythmias in the short to medium term.12 However, in this study, when only AF was considered in the transcatheter closure group, the salutary efficacy no longer reached statistical significance. These findings also suggest that transcatheter ASD closure alone is insufficient to prevent recurrent AF for the long-term in patients with ASD and pre-existing AF.
Catheter ablation for paroxysmal atrial fibrillation in patients with atrial septal defect
Achieving durable PVI is now the cornerstone of CA of AF. A meta-analysis demonstrated that the rate of freedom from AF with multiple PVI procedures was ∼70% at a long-term follow-up.13 However, little is known regarding the efficacy of PVI in patients with congenital heart disease including ASD. To the best of our knowledge, there have been only two reports on the efficacy of PVI with CA to suppress AF in patients with unclosed ASD. Crandall et al.14 reported that, in their case series, recurrent medically refractory AF was controlled after CA in three of four patients with ASD. Nie et al.15 evaluated and compared the efficacy of CA for AF in 18 patients with unclosed ASD vs. 72 control subjects. In their study, non-paroxysmal AF was observed in 28% of patients with ASD, with adjunctive linear ablations of the left atrial roof and mitral isthmus in 33% and 44% of patients, respectively. They found that patients with ASD had an AF-free survival rate of 56% at a median follow-up period of 20 months after single procedure, which was similar to that for the control subjects. Our study compared long-term outcome of paroxysmal AF after transcatheter ASD closure between two ASD patient groups: those who underwent CA prior to transcatheter ASD closure and those who underwent transcatheter ASD closure alone. We showed that patients who underwent CA had a significantly higher long-term AF-free survival rate than those who did not, despite fewer antiarrhythmic drug usages for AF after ASD closure. Furthermore, the time to recurrence of AF from ASD closure tended to be longer in patients who underwent CA than in those who underwent ASD closure alone. Our long-term (5 years) AF-free survival rate of ∼80% in patients with CA appears to be comparable with that of previous results with PVI for paroxysmal AF in the general population.12 Although older age at ASD closure and a larger ASD diameter in patients with ASD closure alone might have affected the higher recurrence rate of AF, these findings did not reach statistical significance regarding recurrence of AF after ASD closure. Furthermore, multivariable Cox hazard regression analysis showed that only upfront CA was strongly associated with a reduced risk of AF recurrence after transcatheter ASD closure. We did not directly assess whether the pulmonary vein plays a major electrical role for the genesis of AF in patients with ASD. However, our results suggest that PVI can affect the occurrence of AF, even in patients with ASD, and could be a feasible strategy of CA for patients with ASD and paroxysmal AF.
Our study also showed that previous heart failure hospitalization was an independent risk factor for recurrence of AF after transcatheter ASD closure. However, other variables, such as age at ASD closure, device size, hypertension, and left atrial dimension were not associated with recurrence of AF. These results are consistent with previous findings that heart failure and AF can cause and exacerbate one another via mechanisms, such as structural cardiac remodelling and activation of neurohormonal mechanisms.16 Additionally, heart failure is regarded as a more powerful risk factor for development of AF than advanced age, valvular heart disease, hypertension, or prior myocardial infarction.17 Duong et al.11 have reported worse mid-term results in which 6 (50%) of 12 paroxysmal AF patients who had CA prior to ASD closure continued to have arrhythmia in the medium term. In their report, it is interesting to note that all five patients who had repeated ablation could not maintain sinus rhythm but six of seven patients who had only once ablation could maintain sinus rhythm. On the basis of this result, they mentioned that there may be subgroups of ASD patients for which CA is less useful. Our result may suggest that heart failure is one of the subgroups mentioned by Duong et al., but further studies are needed.
Observational period from catheter ablation to atrial septal defect closure
In a case with unclosed ASD, access to the left atrium can be easily achieved via ASD. However, in the event of recurrence of AF after transcatheter ASD closure, access to the left atrium is sometimes complicated because of obstruction by the closure device.18,19 Therefore, the observational period from CA to ASD closure is difficult to determine and needs to be carefully decided. We set the observational period from the last ablation to transcatheter ASD closure at a minimum of 3 months without recurrent AF (This time ultimately resulted in being 5.7 months). There were 6 (20%) patients who had recurrence of AF within 3 months after the first procedure and thus underwent repeated CA before ASD closure in accordance with our protocol. The first 3 months after CA is generally regarded as a blanking period for assessing the efficacy of CA for AF, and AF recurrence during this period is defined as early recurrence. Early re-ablation for early recurrence is not recommended in the latest guidelines.20 Therefore, the observational period from CA to ASD closure in our study might have been relatively short if we assessed the efficacy of CA alone. On the other hand, earlier ASD closure has some advantages. Cessation of atrial volume overload due to ASD closure can contribute to electrical reverse remodelling in the atria and reduce progression of arrhythmogenic substrate.4,5 Furthermore, in patients with concomitant heart failure, earlier closure of ASD is often required for managing their heart failure. Therefore, longer observational time from CA to ASD closure may not necessarily be appropriate in all patients. In our study, six (20%) patients with upfront CA experienced recurrence of AF after ASD closure, and the median time from their ASD closure to AF recurrence was 38 months (range 12–102 months). These results suggest that 3 months may be a feasible observational period from CA to transcatheter ASD closure. In any case with concomitant AF; however, closer cooperation among arrhythmia specialists and cardiac interventionalists is mandatory to decide the timing of ASD closure.
Limitations
Our study has several limitations. The main limitation of this study is the small number of patients. However, to the best of our knowledge, this is the largest study to show the efficacy of CA for AF in patients with ASD. Another limitation of our study is that CA was not performed in a single hospital with uniform systems and strategy. During our study period, there were great improvements in the PVI technique such as the appearance of irrigated-tip ablation catheters. Among patients who underwent CA and had recurrent AF after ASD closure, four of six patients had undergone CA a using non-irrigated-tip catheter. Thus, the improvement of techniques for CA might have affected the outcome of AF ablation. In terms of the strategy of CA, PVI having been completed in all patients, and adjunctive left atrial ablation was performed in only one case. In one patient who had undergone isolation of the SVC after completion of a repeated PVI at a second CA, termination of persistent AF was observed during isolation of the SVC. Therefore, in this particular case, the SVC might have been related to recurrence of AF. Furthermore, although most patients (82%) were symptomatic before the intervention, our follow-up protocol to detect recurrence of AF might not have been enough resulting in underestimation of recurrence of AF. During the follow-up period, 11 (37%) patients in the CA group had some kind of symptom suggestive of arrhythmias. Among them, recurrent AF and premature atrial complex or premature ventricular complex were identified as the cause of symptoms in four and six patients, respectively. The one other patient once had a similar symptom with previous AF attack. However, any arrhythmia, including AF, was not identified despite careful follow-up and repeated Holter ECG examinations. Prospective, randomized comparisons with a large number of subjects and more detailed analysis are required to confirm our findings.
Conclusions
Transcatheter ASD closure alone is insufficient for preventing recurrence of AF. However, CA substantially reduces recurrence of AF after ASD closure over the long term. Our results suggest that upfront CA with subsequent transcatheter ASD closure can be a feasible treatment strategy for managing patients with ASD and concomitant paroxysmal AF. However, further larger studies are needed to confirm our findings.
Acknowledgements
The authors wish to thank Masato Murakami, Masamichi Tanaka, Tadashi Wada, Motomi Tachibana, Motoki Kubo, Satoshi Kawada, Sho Tsushima, Yoshimasa Morimoto, and Keisuke Ookawa for their contributions to the patients’ management, catheterization, and providing follow-up data.
Conflict of interest: none declared.



