-
PDF
- Split View
-
Views
-
Cite
Cite
Masateru Takigawa, Taishi Kuwahara, Atsushi Takahashi, Atsushi Kobori, Yoshihide Takahashi, Kenji Okubo, Yuji Watari, Tomoyo Sugiyama, Shigeki Kimura, Katsumasa Takagi, Hiroyuki Hikita, Kenzo Hirao, Mitsuaki Isobe, The impact of haemodialysis on the outcomes of catheter ablation in patients with paroxysmal atrial fibrillation, EP Europace, Volume 16, Issue 3, March 2014, Pages 327–334, https://doi.org/10.1093/europace/eut230
Close - Share Icon Share
Abstract
The outcomes of catheter ablation (CA) in patients with paroxysmal atrial fibrillation (PAF) who are undergoing haemodialysis (HD) have not been fully elucidated. This study aimed to determine the impact of HD on CA outcome in these patients.
We examined 1364 consecutive PAF patients (mean age, 61 ± 10 years) who underwent CA, including 32 (2.3%) patients undergoing HD. The patients undergoing HD had a significantly lower body mass index (P < 0.0001), higher CHADS2 score (P = 0.006), and higher prevalence of structural heart disease (P < 0.0001), hypertension (P = 0.002), and congestive heart failure (P = 0.02). Echocardiography indicated a larger left atrial diameter (P < 0.0001) and left ventricular diameter (P = 0.0002) in the HD patients. Haemodialysis was a significant predictor of AF recurrence (hazard ratio 2.56; 95% confidence interval 1.56–4.03; P = 0.0004) in the overall population. Sinus rhythm maintenance rates in the HD patients at 1, 3, and 5 years were 42.3, 37.6, and 19.7%, respectively, after the first procedure, and 64.7, 54.9, and 47.1%, respectively, after the final procedure (median, 2; range, 1–2 procedures); these rates were significantly lower than those in the non-HD patients (P < 0.0001). The 5-year survival rate was 78.1% in the HD patients.
Haemodialysis was significantly associated with AF recurrence after CA for PAF. However, an ∼50% success rate for sinus rhythm maintenance without antiarrhythmic drug therapy in HD patients suggested that CA could be an option for the treatment of AF.
We studied 1364 consecutive paroxysmal atrial fibrillation (AF) patients including 32 patients on haemodialysis (HD), who underwent catheter abalation (CA), and found that:
Although the success rate of CA for AF patients with HD was worse than that without HD, sinus rhythm was maintained in as many as 50% of the patients without the use of any antiarrhythmic drugs (AADs) during a 5-year follow-up period.
Haemodialysis remained as one of the significant predictors associated with AF recurrence after the first CA for paroxysmal AF by multivariate analysis, and HD increased the risk of AF recurrence 2.6-fold.
The duration of AF and number of failed AADs were significant predictors of AF recurrence in the HD patients after an adjustment for the age, gender, body mass index, and duration of the HD.
The 5-year survival rate after the CA of paroxysmal AF was ∼80% in the HD patients.
Introduction
Atrial fibrillation (AF) is observed in 11–23% of patients undergoing haemodialysis (HD), of which more than half is paroxysmal AF (PAF).1–4 Haemodialysis patients with AF exhibit an extremely higher mortality as compared with those without AF.1,2 In addition, the development of an episode of AF with a rapid ventricular response during HD sessions can result in haemodynamic compromise or occurrence of palpitations. However, the treatment of AF in HD patients by using antiarrhythmic drugs (AADs) is often difficult.5 The use of catheter ablation (CA) as a standard therapy for treating patients with AF is increasing.6–8 However, the outcomes of CA in patients with PAF who are undergoing HD remain unclear. In the present study, we aimed to elucidate the impact of HD on the outcomes of CA in PAF patients.
Methods
Study population
We enroled 1364 consecutive patients (mean age, 61 ± 10 years; 1047 males), who were referred to our institution between February 2003 and March 2010, for undergoing their first CA session for the treatment of symptomatic PAF that was refractory to AADs. Atrial fibrillation was considered as PAF when it terminated spontaneously and persisted for <7 days.9 Our institutional review board approved the study protocol, and all patients provided written, informed consent before the procedures.
Electrophysiological study
All AADs were discontinued for >7 days (amiodarone was discontinued for >1 month) before the ablation. All patients received anticoagulation medication for >1 month. A 7-F 20- or 14-pole, 2-site mapping catheter (Irvine Biomedical Inc.) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. These procedures were performed under sedation with intravenous propofol or dexmedetomidine in the fasting state.
Catheter ablation
Our ablation strategy has been described in detail in previous reports.10,11 Briefly, after performing a transseptal puncture using a one-puncture technique, two long sheaths were inserted into the left atrium (LA). Pulmonary venography and contrast esophagography were performed to determine the anatomical relationships of the pulmonary vein (PV) ostia, LA, and oesophagus. An activated clotting time of 250–350 s was maintained with a continuous infusion of heparin during the procedure. Two circular mapping catheters were placed in the superior and inferior PVs, and ipsilateral PVs were circumferentially and extensively ablated under fluoroscopic and electrophysiological guidance. All radiofrequency (RF) applications were delivered using an 8 mm-tip ablation catheter (Japan Lifeline Inc.) in the temperature control mode, at a target temperature of 55°C and maximum power of 35 W on the LA posterior wall and 40 W at the anterior aspect of the PVs. The RF energy was delivered for 30–40 s at each site. Since 2005, the oesophageal temperature has been routinely measured during RF applications to avoid oesophagus-related complications.11–13 However, the applications were discontinued in cases where the oesophageal temperature reached 42°C, and was initiated again when the temperature reduced to <38°C.11 The endpoint was the elimination of all PV potentials following a 20–40 mg bolus injection of adenosine triphosphate, which unmasked any dormant PV conduction.14 Subsequently, a cavotricuspid isthmus line was created, with the endpoint being a bidirectional conduction block.15 Isoproterenol (5–20 μg/min) was injected intravenously before the procedure was completed. If sustained or non-sustained AF was reproducibly induced from non-PV foci, they were focally ablated.16 When non-PV foci were located in the superior vena cava (SVC), the SVC was electrically isolated.17 If no spontaneous AF occurred, rapid atrial pacing was performed to induce AF. After an episode of pacing-induced AF became sustained, internal cardioversion was attempted to convert the AF to sinus rhythm (SR) and to confirm whether a spontaneous reinitiation of AF occurred. Linear ablation was also performed, if required, only when an AF episode from an undetermined origin or macroreentrant atrial tachycardia spontaneously occurred.
Follow-up
Anticoagulation was discontinued after 3–6 months in patients who were free of AF recurrence and had no risk factors for thromboembolism. No AAD medication was prescribed. All patients were followed up at 2, 6, 10, 14, 24, 36, and 48 weeks after the ablation procedure; 12-lead electrocardiograms were recorded at every visit and 24 h monitoring was performed every 3 months. Thereafter, the patients were followed up every 1–3 months at our institution, or by their general physician. Patients with any symptoms were encouraged to undergo a 1-month event recorder. Successful ablation was defined as the absence of any atrial tachyarrhythmias lasting at least 30 s, without any AADs, after a blanking period of 1 month. A repeat ablation was recommended for patients who experienced atrial tachyarrhythmias after this time point.
Statistical analysis
The data were expressed as means ± standard deviations for continuous variables, and frequencies and percentages for categorical variables. To compare the two groups, χ2 analysis or Fisher's exact test were used for categorical variables and an unpaired t-test or Wilcoxon analysis was used for continuous variables. A multivariate Cox proportional analysis was performed to clarify the significant risk factors and calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). The follow-up period was calculated from the date of the procedure to the date of AF recurrence or censoring. The AF recurrence-free rates were calculated using Kaplan–Meier analysis and log-rank statistics were used for comparisons between the groups. A P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the study population. Among the 1364 patients (mean age, 61 ± 10 years; 1047 males), 32 (2.3%) were undergoing HD. The patients undergoing HD had a significantly lower body mass index (BMI; P < 0.0001), higher CHADS2 score (P = 0.006), and higher prevalence of structural heart disease (P < 0.0001), hypertension (P = 0.002), and congestive heart failure (P = 0.02) than those who were not undergoing HD. Echocardiography revealed that the patients undergoing HD had a larger LA diameter (P < 0.0001) and left ventricular diameter (P = 0.0002). The mean HD duration was 13.6 years in the patients undergoing HD.
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| Patient age (year) | 61 ± 10 | 61 ± 11 | 62 ± 7 | 0.97 |
| Male gender (%) | 1047 (76.8%) | 1025 (77.0%) | 22 (68.8%) | 0.29 |
| BMI | 23.5 ± 3.0 | 23.6 ± 3.0 | 20.4 ± 2.2 | <0.0001 |
| Duration of AF (year) | 5.0 ± 5.4 | 5.0 ± 5.4 | 3.2 ± 2.8 | 0.08 |
| Structural heart disease (%) | 232 (17.0%) | 217 (16.3%) | 15 (46.9%) | <0.0001 |
| Congestive heart failure (%) | 95 (7.0%) | 89 (6.7%) | 6 (18.8%) | 0.02 |
| Hypertension (%) | 604 (44.3%) | 581 (43.6%) | 23 (71.9%) | 0.002 |
| Diabetes (%) | 144 (10.6%) | 139 (10.4%) | 5 (15.6%) | 0.37 |
| Stroke (%) | 105 (7.7%) | 103 (7.7%) | 2 (6.3%) | 0.99 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 1.2 ± 0.9 | 0.006 |
| Failed AADs (n) | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.7 ± 1.6 | 0.44 |
| Echocardiography | ||||
| LAD (mm) | 37.7 ± 5.1 | 37.6 ± 5.0 | 43.1 ± 5.3 | <0.0001 |
| LVD (mm) | 47.4 ± 4.6 | 47.3 ± 4.6 | 50.9 ± 6.0 | 0.0002 |
| LVEF (%) | 66.1 ± 7.3 | 66.2 ± 7.3 | 63.0 ± 10.1 | 0.13 |
| Duration of HD (year) | – | – | 13.6 ± 10.9 | – |
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| Patient age (year) | 61 ± 10 | 61 ± 11 | 62 ± 7 | 0.97 |
| Male gender (%) | 1047 (76.8%) | 1025 (77.0%) | 22 (68.8%) | 0.29 |
| BMI | 23.5 ± 3.0 | 23.6 ± 3.0 | 20.4 ± 2.2 | <0.0001 |
| Duration of AF (year) | 5.0 ± 5.4 | 5.0 ± 5.4 | 3.2 ± 2.8 | 0.08 |
| Structural heart disease (%) | 232 (17.0%) | 217 (16.3%) | 15 (46.9%) | <0.0001 |
| Congestive heart failure (%) | 95 (7.0%) | 89 (6.7%) | 6 (18.8%) | 0.02 |
| Hypertension (%) | 604 (44.3%) | 581 (43.6%) | 23 (71.9%) | 0.002 |
| Diabetes (%) | 144 (10.6%) | 139 (10.4%) | 5 (15.6%) | 0.37 |
| Stroke (%) | 105 (7.7%) | 103 (7.7%) | 2 (6.3%) | 0.99 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 1.2 ± 0.9 | 0.006 |
| Failed AADs (n) | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.7 ± 1.6 | 0.44 |
| Echocardiography | ||||
| LAD (mm) | 37.7 ± 5.1 | 37.6 ± 5.0 | 43.1 ± 5.3 | <0.0001 |
| LVD (mm) | 47.4 ± 4.6 | 47.3 ± 4.6 | 50.9 ± 6.0 | 0.0002 |
| LVEF (%) | 66.1 ± 7.3 | 66.2 ± 7.3 | 63.0 ± 10.1 | 0.13 |
| Duration of HD (year) | – | – | 13.6 ± 10.9 | – |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; HD, haemodialysis, LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| Patient age (year) | 61 ± 10 | 61 ± 11 | 62 ± 7 | 0.97 |
| Male gender (%) | 1047 (76.8%) | 1025 (77.0%) | 22 (68.8%) | 0.29 |
| BMI | 23.5 ± 3.0 | 23.6 ± 3.0 | 20.4 ± 2.2 | <0.0001 |
| Duration of AF (year) | 5.0 ± 5.4 | 5.0 ± 5.4 | 3.2 ± 2.8 | 0.08 |
| Structural heart disease (%) | 232 (17.0%) | 217 (16.3%) | 15 (46.9%) | <0.0001 |
| Congestive heart failure (%) | 95 (7.0%) | 89 (6.7%) | 6 (18.8%) | 0.02 |
| Hypertension (%) | 604 (44.3%) | 581 (43.6%) | 23 (71.9%) | 0.002 |
| Diabetes (%) | 144 (10.6%) | 139 (10.4%) | 5 (15.6%) | 0.37 |
| Stroke (%) | 105 (7.7%) | 103 (7.7%) | 2 (6.3%) | 0.99 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 1.2 ± 0.9 | 0.006 |
| Failed AADs (n) | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.7 ± 1.6 | 0.44 |
| Echocardiography | ||||
| LAD (mm) | 37.7 ± 5.1 | 37.6 ± 5.0 | 43.1 ± 5.3 | <0.0001 |
| LVD (mm) | 47.4 ± 4.6 | 47.3 ± 4.6 | 50.9 ± 6.0 | 0.0002 |
| LVEF (%) | 66.1 ± 7.3 | 66.2 ± 7.3 | 63.0 ± 10.1 | 0.13 |
| Duration of HD (year) | – | – | 13.6 ± 10.9 | – |
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| Patient age (year) | 61 ± 10 | 61 ± 11 | 62 ± 7 | 0.97 |
| Male gender (%) | 1047 (76.8%) | 1025 (77.0%) | 22 (68.8%) | 0.29 |
| BMI | 23.5 ± 3.0 | 23.6 ± 3.0 | 20.4 ± 2.2 | <0.0001 |
| Duration of AF (year) | 5.0 ± 5.4 | 5.0 ± 5.4 | 3.2 ± 2.8 | 0.08 |
| Structural heart disease (%) | 232 (17.0%) | 217 (16.3%) | 15 (46.9%) | <0.0001 |
| Congestive heart failure (%) | 95 (7.0%) | 89 (6.7%) | 6 (18.8%) | 0.02 |
| Hypertension (%) | 604 (44.3%) | 581 (43.6%) | 23 (71.9%) | 0.002 |
| Diabetes (%) | 144 (10.6%) | 139 (10.4%) | 5 (15.6%) | 0.37 |
| Stroke (%) | 105 (7.7%) | 103 (7.7%) | 2 (6.3%) | 0.99 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 1.2 ± 0.9 | 0.006 |
| Failed AADs (n) | 1.8 ± 1.4 | 1.8 ± 1.4 | 1.7 ± 1.6 | 0.44 |
| Echocardiography | ||||
| LAD (mm) | 37.7 ± 5.1 | 37.6 ± 5.0 | 43.1 ± 5.3 | <0.0001 |
| LVD (mm) | 47.4 ± 4.6 | 47.3 ± 4.6 | 50.9 ± 6.0 | 0.0002 |
| LVEF (%) | 66.1 ± 7.3 | 66.2 ± 7.3 | 63.0 ± 10.1 | 0.13 |
| Duration of HD (year) | – | – | 13.6 ± 10.9 | – |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; HD, haemodialysis, LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
Outcomes after the initial catheter ablation session
The endpoint was achieved in all patients. The mean duration of the procedure was 178 ± 42 min (177 ± 42 min in non-HD patients vs. 193 ± 38 min in HD patients; P = 0.03), whereas the mean duration of fluoroscopy was 76 ± 23 min (75 ± 23 min in non-HD patients vs. 84 ± 19 min in HD patients, P = 0.02). The details of the first and second CA sessions are summarized in Table 2. The HD patients underwent additional focal ablations (P = 0.01) more frequently than the non-HD patients during the first CA session.
| . | Non-HD . | HD . | . |
|---|---|---|---|
| First CA session | (N = 1332) | (N = 32) | P values |
| EPVI | 1332 (100%) | 32 (100%) | – |
| SVCI | 156 (11.7%) | 4 (12.5%) | 0.89 |
| Focal ablation | 141 (10.6%) | 8 (25.0%) | 0.01 |
| Linear ablation | 12 (0.9%) | 1 (3.1%) | 0.20 |
| Second CA session | (N = 332) | (N = 17) | P values |
| EPVI | 254 (76.5%) | 15 (88.2%) | 0.26 |
| SVCI | 89 (26.8%) | 5 (29.4%) | 0.81 |
| Focal ablation | 98 (29.5%) | 9 (52.9%) | 0.04 |
| Linear ablation | 35 (10.5%) | 5 (29.4%) | 0.02 |
| . | Non-HD . | HD . | . |
|---|---|---|---|
| First CA session | (N = 1332) | (N = 32) | P values |
| EPVI | 1332 (100%) | 32 (100%) | – |
| SVCI | 156 (11.7%) | 4 (12.5%) | 0.89 |
| Focal ablation | 141 (10.6%) | 8 (25.0%) | 0.01 |
| Linear ablation | 12 (0.9%) | 1 (3.1%) | 0.20 |
| Second CA session | (N = 332) | (N = 17) | P values |
| EPVI | 254 (76.5%) | 15 (88.2%) | 0.26 |
| SVCI | 89 (26.8%) | 5 (29.4%) | 0.81 |
| Focal ablation | 98 (29.5%) | 9 (52.9%) | 0.04 |
| Linear ablation | 35 (10.5%) | 5 (29.4%) | 0.02 |
CA, catheter ablation; EPVI, extensive pulmonary vein isolation; HD, hemodialysis; SVCI, superior vena cava isolation.
| . | Non-HD . | HD . | . |
|---|---|---|---|
| First CA session | (N = 1332) | (N = 32) | P values |
| EPVI | 1332 (100%) | 32 (100%) | – |
| SVCI | 156 (11.7%) | 4 (12.5%) | 0.89 |
| Focal ablation | 141 (10.6%) | 8 (25.0%) | 0.01 |
| Linear ablation | 12 (0.9%) | 1 (3.1%) | 0.20 |
| Second CA session | (N = 332) | (N = 17) | P values |
| EPVI | 254 (76.5%) | 15 (88.2%) | 0.26 |
| SVCI | 89 (26.8%) | 5 (29.4%) | 0.81 |
| Focal ablation | 98 (29.5%) | 9 (52.9%) | 0.04 |
| Linear ablation | 35 (10.5%) | 5 (29.4%) | 0.02 |
| . | Non-HD . | HD . | . |
|---|---|---|---|
| First CA session | (N = 1332) | (N = 32) | P values |
| EPVI | 1332 (100%) | 32 (100%) | – |
| SVCI | 156 (11.7%) | 4 (12.5%) | 0.89 |
| Focal ablation | 141 (10.6%) | 8 (25.0%) | 0.01 |
| Linear ablation | 12 (0.9%) | 1 (3.1%) | 0.20 |
| Second CA session | (N = 332) | (N = 17) | P values |
| EPVI | 254 (76.5%) | 15 (88.2%) | 0.26 |
| SVCI | 89 (26.8%) | 5 (29.4%) | 0.81 |
| Focal ablation | 98 (29.5%) | 9 (52.9%) | 0.04 |
| Linear ablation | 35 (10.5%) | 5 (29.4%) | 0.02 |
CA, catheter ablation; EPVI, extensive pulmonary vein isolation; HD, hemodialysis; SVCI, superior vena cava isolation.
During a mean follow-up of 29.2 ± 21.4 months after the first CA session, 475 of 1364 patients (453 of 1332 patients with non-HD and 22of 32 patients with HD) had AF recurrence. The SR maintenance rates at 1, 3, and 5 years after the first CA session were 42.3, 37.6, and 19.7% in the HD patients, and 73.2, 66.8, and 61.7% in the non-HD patients. Atrial fibrillation recurred more frequently in the HD patients than in the non-HD patients (P < 0.0001), as shown in Figure 1. In addition, the recurrence of AF within 1 year was markedly noted in HD patients.
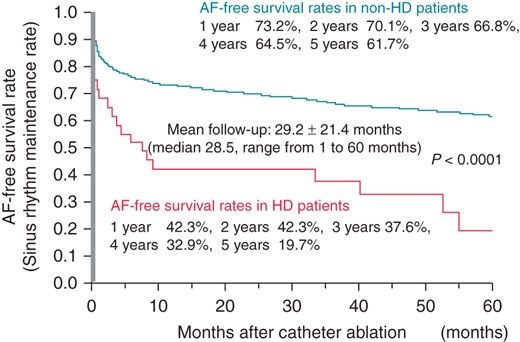
Clinical predictors of atrial fibrillation recurrence after the first catheter ablation session in the overall population
In the present study, HD itself was one of the significant predictors of AF recurrence after the first CA session in the overall population (HR 2.56; 95% CI 1.56–4.03; P = 0.0004). Additionally, the duration of AF (HR 1.03/year; 95% CI 1.02–1.05; P < 0.0001), the number of failed AADs (HR 1.10; 95% CI 1.03–1.17; P = 0.003), and a larger LA diameter (HR 1.43/10 mm, 95% CI 1.16–1.76, P = 0.0007) remained as significant predictors after the multivariate analysis, as shown in Table 3.
Clinical predictors of AF recurrences after the initial CA in the overall population
| . | Univariate P values . | Multivariate P values . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age (/year) | 0.75 | 0.10 | 0.99 | 0.98–1.00 |
| Female gender | 0.34 | 0.47 | 1.09 | 0.87–1.35 |
| BMI | 0.46 | 0.27 | 0.98 | 0.95–1.02 |
| Duration of AF (/year) | <0.0001 | 0.0001 | 1.03 | 1.02–1.05 |
| Structural heart disease | 0.005 | 0.05 | 1.31 | 1.00–1.69 |
| HD | <0.0001 | 0.0004 | 2.56 | 1.56–4.03 |
| Failed AADs | 0.0004 | 0.003 | 1.10 | 1.03–1.17 |
| Congestive heart failure | 0.15 | 0.29 | 0.74 | 0.41–1.29 |
| Hypertension | 0.66 | 0.20 | 0.74 | 0.47–1.18 |
| Diabetes | 0.015 | 0.47 | 1.20 | 0.74–1.98 |
| Stroke | 0.41 | 0.62 | 0.80 | 0.34–1.98 |
| CHADS2 score | 0.14 | 0.39 | 1.20 | 0.78–1.77 |
| Echocardiography | ||||
| LAD (/10 mm increase) | 0.0006 | 0.0007 | 1.43 | 1.16–1.76 |
| LVD (/10 mm increase) | 0.91 | 0.24 | 0.87 | 0.69–1.10 |
| LVEF (per 10% increase) | 0.84 | 0.41 | 1.06 | 0.92–1.22 |
| . | Univariate P values . | Multivariate P values . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age (/year) | 0.75 | 0.10 | 0.99 | 0.98–1.00 |
| Female gender | 0.34 | 0.47 | 1.09 | 0.87–1.35 |
| BMI | 0.46 | 0.27 | 0.98 | 0.95–1.02 |
| Duration of AF (/year) | <0.0001 | 0.0001 | 1.03 | 1.02–1.05 |
| Structural heart disease | 0.005 | 0.05 | 1.31 | 1.00–1.69 |
| HD | <0.0001 | 0.0004 | 2.56 | 1.56–4.03 |
| Failed AADs | 0.0004 | 0.003 | 1.10 | 1.03–1.17 |
| Congestive heart failure | 0.15 | 0.29 | 0.74 | 0.41–1.29 |
| Hypertension | 0.66 | 0.20 | 0.74 | 0.47–1.18 |
| Diabetes | 0.015 | 0.47 | 1.20 | 0.74–1.98 |
| Stroke | 0.41 | 0.62 | 0.80 | 0.34–1.98 |
| CHADS2 score | 0.14 | 0.39 | 1.20 | 0.78–1.77 |
| Echocardiography | ||||
| LAD (/10 mm increase) | 0.0006 | 0.0007 | 1.43 | 1.16–1.76 |
| LVD (/10 mm increase) | 0.91 | 0.24 | 0.87 | 0.69–1.10 |
| LVEF (per 10% increase) | 0.84 | 0.41 | 1.06 | 0.92–1.22 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CA, catheter ablation; CI, confidential interval; HD, haemodialysis; HR, hazard ratio; LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
Clinical predictors of AF recurrences after the initial CA in the overall population
| . | Univariate P values . | Multivariate P values . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age (/year) | 0.75 | 0.10 | 0.99 | 0.98–1.00 |
| Female gender | 0.34 | 0.47 | 1.09 | 0.87–1.35 |
| BMI | 0.46 | 0.27 | 0.98 | 0.95–1.02 |
| Duration of AF (/year) | <0.0001 | 0.0001 | 1.03 | 1.02–1.05 |
| Structural heart disease | 0.005 | 0.05 | 1.31 | 1.00–1.69 |
| HD | <0.0001 | 0.0004 | 2.56 | 1.56–4.03 |
| Failed AADs | 0.0004 | 0.003 | 1.10 | 1.03–1.17 |
| Congestive heart failure | 0.15 | 0.29 | 0.74 | 0.41–1.29 |
| Hypertension | 0.66 | 0.20 | 0.74 | 0.47–1.18 |
| Diabetes | 0.015 | 0.47 | 1.20 | 0.74–1.98 |
| Stroke | 0.41 | 0.62 | 0.80 | 0.34–1.98 |
| CHADS2 score | 0.14 | 0.39 | 1.20 | 0.78–1.77 |
| Echocardiography | ||||
| LAD (/10 mm increase) | 0.0006 | 0.0007 | 1.43 | 1.16–1.76 |
| LVD (/10 mm increase) | 0.91 | 0.24 | 0.87 | 0.69–1.10 |
| LVEF (per 10% increase) | 0.84 | 0.41 | 1.06 | 0.92–1.22 |
| . | Univariate P values . | Multivariate P values . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age (/year) | 0.75 | 0.10 | 0.99 | 0.98–1.00 |
| Female gender | 0.34 | 0.47 | 1.09 | 0.87–1.35 |
| BMI | 0.46 | 0.27 | 0.98 | 0.95–1.02 |
| Duration of AF (/year) | <0.0001 | 0.0001 | 1.03 | 1.02–1.05 |
| Structural heart disease | 0.005 | 0.05 | 1.31 | 1.00–1.69 |
| HD | <0.0001 | 0.0004 | 2.56 | 1.56–4.03 |
| Failed AADs | 0.0004 | 0.003 | 1.10 | 1.03–1.17 |
| Congestive heart failure | 0.15 | 0.29 | 0.74 | 0.41–1.29 |
| Hypertension | 0.66 | 0.20 | 0.74 | 0.47–1.18 |
| Diabetes | 0.015 | 0.47 | 1.20 | 0.74–1.98 |
| Stroke | 0.41 | 0.62 | 0.80 | 0.34–1.98 |
| CHADS2 score | 0.14 | 0.39 | 1.20 | 0.78–1.77 |
| Echocardiography | ||||
| LAD (/10 mm increase) | 0.0006 | 0.0007 | 1.43 | 1.16–1.76 |
| LVD (/10 mm increase) | 0.91 | 0.24 | 0.87 | 0.69–1.10 |
| LVEF (per 10% increase) | 0.84 | 0.41 | 1.06 | 0.92–1.22 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CA, catheter ablation; CI, confidential interval; HD, haemodialysis; HR, hazard ratio; LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
Outcomes after multiple catheter ablation sessions
As shown in Table 2, the HD patients more frequently underwent additional focal ablations (P = 0.04), as well as linear ablations (P = 0.02) than the non-HD patients during the second CA session.
Among the 453 of the 1332 non-HD patients who had AF recurrence after the first CA session, 332 (73.3%) underwent a second CA session. An electrical re-conduction between the LA and PVs was observed in 254 of 332 (76.5%) patients during the second CA session. Among the 82 of these 332 patients who had AF recurrence after the second session, 42 (51.2%) underwent a third CA session, of whom 14 had a later AF recurrence.
On the other hand, among the 32 patients undergoing HD, AF recurred in 22 after the first CA session, and 17 (77.3%) of these patients underwent a second session. Electrical re-conduction between the LA and PVs were observed in 15 of 17 patients (88.2%) during the second CA session. Although 10 patients had AF recurrence after the second CA session, all of them rejected a third session. Finally, SR was maintained in 22 of 32 patients (68.7%), including 17 (53.1%) without the use of AADs and 5 (15.6%) who were receiving AADs. Four patients (12.5%) who had suffered from severe dialysis hypotension because of tachyarrhythmic attacks during HD could undergo stable HD after the CA procedures. Two patients (6.3%) had no relief of their symptoms after the CA procedures. One of them required atrioventricular nodal ablation and pacemaker implantation to control the symptoms.
During a mean follow-up of 35.9 ± 18.6 months after the final CA session, 209 patients (14 of the HD patients and 195 of the non-HD patients) had AF recurrence. The SR maintenance rate at 1, 3, and 5 years after the final CA session was 64.7, 54.9, and 47.1% in the HD patients, and 90.4, 86.0, and 81.8% in the non-HD patients, respectively. Atrial fibrillation recurred more frequently in the HD patients than in the non-HD patients (P < 0.0001) after the final CA session, as shown in Figure 2. None of the patients underwent renal transplantation during the follow-up period.
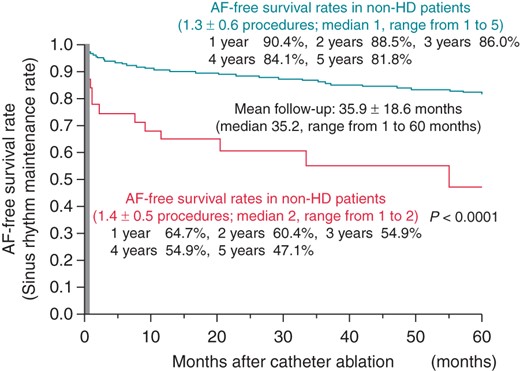
Procedure-related complications
Table 4 describes the 1758 procedures that were performed in 1364 patients (49 in 32 HD vs. 1709 in 1332 non-HD patients). The incidence of total complications did not significantly differ between the two groups (HD vs. non-HD: 6.1 vs. 3.8%; P = 0.43).
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| (1758 procedures) . | (1709 procedures) . | (49 procedures) . | ||
| Cardiac tamponade/effusion | 23 (1.3%) | 23 (1.3%) | 0 (0%) | 0.99 |
| Air embolism | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Cerebral infarction/TIA | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Myocardial infarction | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0.99 |
| DVT/PE | 5 (0.3%) | 5 (0.3%) | 0 (0%) | 0.99 |
| Phrenic nerve injury | 10 (0.6%) | 10 (0.6%) | 0 (0%) | 0.99 |
| Vagal nerve injury | 5 (0.3%) | 4 (0.2%) | 1 (2.0%) | 0.13 |
| Pulmonary vein injury/stenosis | 3 (0.2%) | 3 (0.2%) | 0 (0.3%) | 0.99 |
| Pneumothorax | 2 (0.1%) | 1 (0.1%) | 1 (2.0%) | 0.06 |
| Vascular injury | 4 (0.2%) | 3 (0.2%) | 1 (2.0%) | 0.11 |
| Total | 67 (3.8%) | 64 (3.7%) | 3 (6.1%) | 0.43 |
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| (1758 procedures) . | (1709 procedures) . | (49 procedures) . | ||
| Cardiac tamponade/effusion | 23 (1.3%) | 23 (1.3%) | 0 (0%) | 0.99 |
| Air embolism | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Cerebral infarction/TIA | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Myocardial infarction | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0.99 |
| DVT/PE | 5 (0.3%) | 5 (0.3%) | 0 (0%) | 0.99 |
| Phrenic nerve injury | 10 (0.6%) | 10 (0.6%) | 0 (0%) | 0.99 |
| Vagal nerve injury | 5 (0.3%) | 4 (0.2%) | 1 (2.0%) | 0.13 |
| Pulmonary vein injury/stenosis | 3 (0.2%) | 3 (0.2%) | 0 (0.3%) | 0.99 |
| Pneumothorax | 2 (0.1%) | 1 (0.1%) | 1 (2.0%) | 0.06 |
| Vascular injury | 4 (0.2%) | 3 (0.2%) | 1 (2.0%) | 0.11 |
| Total | 67 (3.8%) | 64 (3.7%) | 3 (6.1%) | 0.43 |
CA, catheter ablation; DVT, deep vein thrombosis; HD, haemodialysis; PE, pulmonary embolism; TIA, transient ischaemic attack.
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| (1758 procedures) . | (1709 procedures) . | (49 procedures) . | ||
| Cardiac tamponade/effusion | 23 (1.3%) | 23 (1.3%) | 0 (0%) | 0.99 |
| Air embolism | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Cerebral infarction/TIA | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Myocardial infarction | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0.99 |
| DVT/PE | 5 (0.3%) | 5 (0.3%) | 0 (0%) | 0.99 |
| Phrenic nerve injury | 10 (0.6%) | 10 (0.6%) | 0 (0%) | 0.99 |
| Vagal nerve injury | 5 (0.3%) | 4 (0.2%) | 1 (2.0%) | 0.13 |
| Pulmonary vein injury/stenosis | 3 (0.2%) | 3 (0.2%) | 0 (0.3%) | 0.99 |
| Pneumothorax | 2 (0.1%) | 1 (0.1%) | 1 (2.0%) | 0.06 |
| Vascular injury | 4 (0.2%) | 3 (0.2%) | 1 (2.0%) | 0.11 |
| Total | 67 (3.8%) | 64 (3.7%) | 3 (6.1%) | 0.43 |
| . | Total (N = 1364) . | Non-HD (N = 1332) . | HD (N = 32) . | P values . |
|---|---|---|---|---|
| (1758 procedures) . | (1709 procedures) . | (49 procedures) . | ||
| Cardiac tamponade/effusion | 23 (1.3%) | 23 (1.3%) | 0 (0%) | 0.99 |
| Air embolism | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Cerebral infarction/TIA | 7 (0.4%) | 7 (0.4%) | 0 (0%) | 0.99 |
| Myocardial infarction | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0.99 |
| DVT/PE | 5 (0.3%) | 5 (0.3%) | 0 (0%) | 0.99 |
| Phrenic nerve injury | 10 (0.6%) | 10 (0.6%) | 0 (0%) | 0.99 |
| Vagal nerve injury | 5 (0.3%) | 4 (0.2%) | 1 (2.0%) | 0.13 |
| Pulmonary vein injury/stenosis | 3 (0.2%) | 3 (0.2%) | 0 (0.3%) | 0.99 |
| Pneumothorax | 2 (0.1%) | 1 (0.1%) | 1 (2.0%) | 0.06 |
| Vascular injury | 4 (0.2%) | 3 (0.2%) | 1 (2.0%) | 0.11 |
| Total | 67 (3.8%) | 64 (3.7%) | 3 (6.1%) | 0.43 |
CA, catheter ablation; DVT, deep vein thrombosis; HD, haemodialysis; PE, pulmonary embolism; TIA, transient ischaemic attack.
Clinical predictors of atrial fibrillation recurrence in patients with paroxysmal atrial fibrillation undergoing haemodialysis
Although the duration of AF, the number of AADs, and history of stroke were associated with AF recurrence in the univariate analysis in the HD patients, only the duration of AF (HR 1.30/year; 95% CI 1.07–1.63, P = 0.01) and the number of failed AADs (HR 1.71; 95% CI 1.26–2.32, P = 0.0007) remained significant after adjustment for age, gender, BMI, and HD duration, as shown in Tables 5 and 6. The HD duration was not associated with the incidence of AF recurrence in this study population.
| . | Univariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Patient age/year | 0.71 | 1.01 | 0.95–1.07 |
| Gender, female | 0.66 | 0.81 | 0.29–1.99 |
| BMI | 0.71 | 0.96 | 0.79–1.17 |
| Duration of HD | 0.28 | 0.98 | 0.94–1.02. |
| Duration of AF (/year) | 0.046 | 1.18 | 1.00–1.38 |
| Structural heart disease | 0.96 | 1.02 | 0.44–2.39 |
| Congestive heart failure | 0.94 | 1.04 | 0.30–2.92 |
| Hypertension | 0.49 | 0.72 | 0.30–1.90 |
| Diabetes | 0.77 | 1.19 | 0.34–3.25 |
| Stroke | 0.047 | 6.96 | 1.03–29.0 |
| CHADS2 score | 0.5 | 1.20 | 0.70–1.97 |
| Failed AADs | 0.0005 | 1.70 | 1.27–2.29 |
| Echocardiography | |||
| LAD (/10 mm increase) | 0.12 | 1.94 | 0.84–4.42 |
| LVD (/10 mm increase) | 0.72 | 0.86 | 0.37–1.91 |
| LVEF (per 10% increase) | 0.45 | 0.84 | 0.55–1.35 |
| . | Univariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Patient age/year | 0.71 | 1.01 | 0.95–1.07 |
| Gender, female | 0.66 | 0.81 | 0.29–1.99 |
| BMI | 0.71 | 0.96 | 0.79–1.17 |
| Duration of HD | 0.28 | 0.98 | 0.94–1.02. |
| Duration of AF (/year) | 0.046 | 1.18 | 1.00–1.38 |
| Structural heart disease | 0.96 | 1.02 | 0.44–2.39 |
| Congestive heart failure | 0.94 | 1.04 | 0.30–2.92 |
| Hypertension | 0.49 | 0.72 | 0.30–1.90 |
| Diabetes | 0.77 | 1.19 | 0.34–3.25 |
| Stroke | 0.047 | 6.96 | 1.03–29.0 |
| CHADS2 score | 0.5 | 1.20 | 0.70–1.97 |
| Failed AADs | 0.0005 | 1.70 | 1.27–2.29 |
| Echocardiography | |||
| LAD (/10 mm increase) | 0.12 | 1.94 | 0.84–4.42 |
| LVD (/10 mm increase) | 0.72 | 0.86 | 0.37–1.91 |
| LVEF (per 10% increase) | 0.45 | 0.84 | 0.55–1.35 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CI, confidential interval; HD, haemodialysis; HR, hazard ratio; LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
| . | Univariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Patient age/year | 0.71 | 1.01 | 0.95–1.07 |
| Gender, female | 0.66 | 0.81 | 0.29–1.99 |
| BMI | 0.71 | 0.96 | 0.79–1.17 |
| Duration of HD | 0.28 | 0.98 | 0.94–1.02. |
| Duration of AF (/year) | 0.046 | 1.18 | 1.00–1.38 |
| Structural heart disease | 0.96 | 1.02 | 0.44–2.39 |
| Congestive heart failure | 0.94 | 1.04 | 0.30–2.92 |
| Hypertension | 0.49 | 0.72 | 0.30–1.90 |
| Diabetes | 0.77 | 1.19 | 0.34–3.25 |
| Stroke | 0.047 | 6.96 | 1.03–29.0 |
| CHADS2 score | 0.5 | 1.20 | 0.70–1.97 |
| Failed AADs | 0.0005 | 1.70 | 1.27–2.29 |
| Echocardiography | |||
| LAD (/10 mm increase) | 0.12 | 1.94 | 0.84–4.42 |
| LVD (/10 mm increase) | 0.72 | 0.86 | 0.37–1.91 |
| LVEF (per 10% increase) | 0.45 | 0.84 | 0.55–1.35 |
| . | Univariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Patient age/year | 0.71 | 1.01 | 0.95–1.07 |
| Gender, female | 0.66 | 0.81 | 0.29–1.99 |
| BMI | 0.71 | 0.96 | 0.79–1.17 |
| Duration of HD | 0.28 | 0.98 | 0.94–1.02. |
| Duration of AF (/year) | 0.046 | 1.18 | 1.00–1.38 |
| Structural heart disease | 0.96 | 1.02 | 0.44–2.39 |
| Congestive heart failure | 0.94 | 1.04 | 0.30–2.92 |
| Hypertension | 0.49 | 0.72 | 0.30–1.90 |
| Diabetes | 0.77 | 1.19 | 0.34–3.25 |
| Stroke | 0.047 | 6.96 | 1.03–29.0 |
| CHADS2 score | 0.5 | 1.20 | 0.70–1.97 |
| Failed AADs | 0.0005 | 1.70 | 1.27–2.29 |
| Echocardiography | |||
| LAD (/10 mm increase) | 0.12 | 1.94 | 0.84–4.42 |
| LVD (/10 mm increase) | 0.72 | 0.86 | 0.37–1.91 |
| LVEF (per 10% increase) | 0.45 | 0.84 | 0.55–1.35 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CI, confidential interval; HD, haemodialysis; HR, hazard ratio; LAD, left atrial dimension at end-systole; LVD, left ventricular dimension at end-diastole; LVEF, left ventricular ejection fraction.
Clinical predictors of AF recurrences after an adjustment for the age, sex, BMI, and HD duration in patients on HD
| . | Multivariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Duration of AF/year | 0.01 | 1.3 | 1.07–1.63 |
| Failed AADs | 0.0007 | 1.71 | 1.26–2.32 |
| . | Multivariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Duration of AF/year | 0.01 | 1.3 | 1.07–1.63 |
| Failed AADs | 0.0007 | 1.71 | 1.26–2.32 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CI, confidential interval; HD, hemodialysis; HR, hazard ratio.
Clinical predictors of AF recurrences after an adjustment for the age, sex, BMI, and HD duration in patients on HD
| . | Multivariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Duration of AF/year | 0.01 | 1.3 | 1.07–1.63 |
| Failed AADs | 0.0007 | 1.71 | 1.26–2.32 |
| . | Multivariate analysis . | HR . | 95% CI . |
|---|---|---|---|
| Duration of AF/year | 0.01 | 1.3 | 1.07–1.63 |
| Failed AADs | 0.0007 | 1.71 | 1.26–2.32 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CI, confidential interval; HD, hemodialysis; HR, hazard ratio.
Survival rates after catheter ablation in patients with paroxysmal atrial fibrillation undergoing haemodialysis
Five (15.6%) of 32 patients undergoing HD died during the 5-year follow-up period. The survival rates at 1, 3, and 5 years after the first CA session were 96.9, 90.1, and 78.1%, respectively, as shown in Figure 3. Only one of those five patients had AF recurrence and that patient died of sepsis due to gangrene of an extremity. The causes of death of the other four patients without AF recurrences were: cerebral haemorrhage (in a patient receiving anticoagulation treatment), sepsis due to gangrene of an extremity, ventricular fibrillation secondary to a myocardial infarction, and malignancy. None of these patients died due to heart failure or a cerebral infarction associated with AF.
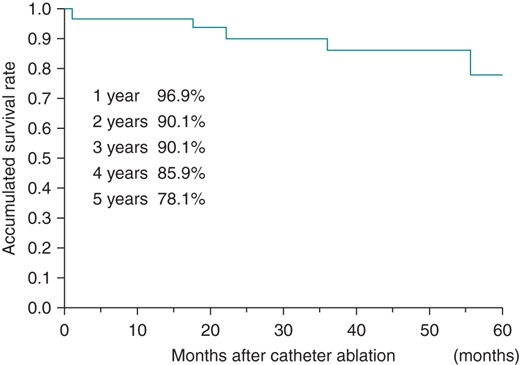
Discussion
Major findings
In the present study, we determined that: (i) although the success rate of CA for AF patients undergoing HD was worse than for those without HD, SR was maintained in almost 50% of the patients, without the use of any AADs, during a 5-year follow-up period; (ii) HD itself was one of the significant predictors associated with AF recurrence after the first CA for PAF; (iii) the duration of AF and the number of failed AADs were significant predictors of AF recurrence in the HD patients, after adjustment for age, gender, BMI, and duration of the HD; and (iv) the 5-year survival rate after the CA was ∼80% in the HD patients.
Sinus rhythm maintenance after catheter ablation in patients with paroxysmal atrial fibrillation undergoing haemodialysis
The SR maintenance rates in the HD patients after the first and final CA sessions were both significantly lower than those in the non-HD patients. Haemodialysis was significantly associated with AF recurrence after CA for PAF. However, ∼50% success rate of SR maintenance was achieved without AAD therapy in HD patients during the 5-year follow-up period after the final (second) CA session. In addition, the incidence of procedure-related complications did not differ significantly between the two groups. These findings indicate that CA could be a suitable option for the treatment of drug-refractory AF among HD patients. In a recent report by Sairaku et al., 54% of HD patients remained free from AF recurrence after the initial ablation session, without the use of any AADs, during a follow-up duration of 821 ± 218 days. Furthermore, 67% were in SR after the last ablation session, during a follow-up duration of 747 ± 221 days.18 Although the SR maintenance rate at 2 years after the final procedure in that study was similar to that in the present study, we noted that this value kept decreasing over time, as shown in Figure 2.
Atrial fibrillation recurred during the early phase to a greater extent in the HD group. Haemodialysis patients usually visit a dialysis centre three times a week for 4 or 5 h sessions of HD. During HD, acute alterations in body fluid volume, plasma ionic concentrations, and autonomic balance could be triggers of PAF. Thus, HD patients have much more frequent exposure to potential triggers of AF than do patients who are not undergoing HD. This could be a reason for the earlier development of AF recurrence in HD patients as compared with non-HD patients.
Haemodialysis as a predictor of atrial fibrillation recurrence in the overall population
Several investigators have reported independent predictors associated with AF recurrences after an initial CA session,19–23 but the impact of HD on outcomes has not fully been elucidated. Since the patients undergoing HD in the present study had a clinical background that predisposed them to a higher risk of AF recurrence, such as a higher prevalence of structural heart disease, hypertension, congestive heart failure, larger LA diameter, and lower left ventricular ejection fraction, it was reasonable to assume that AF would recur more frequently in the HD patients. However, HD still remained as a significant predictor of AF recurrence after the multivariate Cox proportional analysis, which suggested a potential impact of HD itself on AF recurrences in the PAF patients undergoing CA. The present study indicated that HD increased the risk of AF recurrence 2.6-fold, according to the multivariate analysis. A recent report described a worse outcome of CA for PAF in HD patients as compared with non-HD patients in a retrospective case control study.18 The present study supported the finding of the previous study, and additionally underscored the independent association between HD and AF recurrence. The duration of AF, the number of AADs, and LA diameter also remained as significant predictors, which is consistent with previous studies of PAF and CA therapy.19,21,22
Clinical predictors of atrial fibrillation recurrence after catheter ablation in haemodialysis patients
The duration of AF and the number of failed AADs remained as significant predictors in the present study as shown in the previous reports.22,24,25 The reason that the duration of HD did not remain as a predictor of AF recurrence is unclear; however, this finding was similar to a previous report.18 The duration of HD is not always coincident with LA enlargement and remodelling; for example, patients requiring HD because of acute renal failure due to acute nephritis should not initially exhibit LA enlargement or remodelling. However, patients requiring HD because of chronic renal failure due to diabetic nephropathy or hypertensive nephrosclerosis should exhibit remarkable LA damage when HD is initiated. Although the risk of AF recurrence may be associated with LA enlargement and LA remodelling, the extent of LA enlargement and LA remodelling may not always be associated with the duration after HD initiation. However, further investigation with a larger population might be required to clarify this issue, because the number of HD patients examined in the present study was insufficient.
Mechanism underlying the higher incidence of atrial fibrillation recurrences in haemodialysis patients
Based on the findings in the present study, we believe that the higher incidence of AF recurrence in HD patients is associated with a greater extent of LA enlargement and remodelling as well as the presence of triggers and substrates of AF. This is supported by the significantly higher prevalence of structural heart disease, hypertension, congestive heart failure, and larger LA size, as well as the requirement of more frequent additional ablation procedures (such as a non-PV focal ablation and substrate modification with linear ablation) in HD patients. In addition, the markedly greater incidence of PV–LA re-conduction may indicate the difficulty in achieving complete transmural lesions in HD patients with an enlarged LA by using an 8 mm-tip non-irrigation catheter. The longer duration of the procedure and fluoroscopy may also reflect the difficulty in creating transmural lesions and ablating all AF foci in HD patients. Further, all patients who had AF recurrence after the second CA session rejected the further session. However, considering the remarkably high prevalence of PV–LA re-conduction in HD patients during the second session, third or more CA sessions may improve the outcome. In HD patients, increasing LA size26 and decreasing left ventricular relaxation,27 frequent alterations in the plasma ionic concentrations during HD, sympathetic hyperactivity,28,29 and upregulation of the renin–angiotensin–aldosterone system30 are generally observed. Although these findings in HD patients were reported to be associated with an arrhythmogenicity in HD patients,28–33 further investigation is required to confirm the relation between these factors and the outcome of CA for the treatment of PAF.
Prognosis of catheter ablation in patients with paroxysmal atrial fibrillation undergoing haemodialysis
Based on the current findings, the prognostic value of CA for PAF in HD patients cannot be specifically stated. However, CA may affect the prognosis in HD patients with PAF. The prognosis in HD patients, particularly those with AF, is generally very poor.1–4 However, the mortality rate in the HD patients in the present study was considerably low. In addition, HD patients in the present study had a significantly lower annual crude death rate (3.1% per annum; P < 0.0001) compared with that for HD patients in general (9.7% per annum in 2010), according to the Japanese Society for Dialysis Therapy.34 According to the Society, the first, second, and third most prevalent causes of death in 2010 were heart failure (27.0%), infection (20.3%), and malignancy (9.8%).34 However, no patients in the present study died of heart failure caused by AF burden. As we did not include a control group in the present study, further prospective multicentre trials with a larger population are required to elucidate the prognostic value of CA in PAF patients undergoing HD.
Clinical implications
The present findings indicated that HD was a significant predictor of AF recurrence in the overall population. Although the success rate of CA for patients with PAF undergoing HD was still not satisfactory, SR was maintained in almost 50% of patients, without the use of any AADs, and increased to 70% with AAD therapy during a 5-year follow-up period. Therefore, CA may serve as an additional therapeutic choice for patients undergoing HD who have drug-refractory PAF.
Limitations
Although the negative impact of HD on the outcome in the total population was properly demonstrated, the statistical power of multivariate analysis in HD patients was limited because of the small number of HD patients. Secondly, despite our efforts to perform 1-month event recording in any patient who reported symptoms, completely asymptomatic AF episodes could have been missed in these patients and the actual recurrence rate of atrial arrhythmias could have been higher. Thirdly, during the study period, an 8 mm tip, non-irrigation catheter was used instead of a 3.5 mm tip, irrigation catheter, because no irrigation catheters were available in Japan and no electroanatomical mapping systems were used during the procedures. Advances in these technologies could improve the outcome. Forthly, inducing non-PV AF foci under burst pacing and isoproterenol infusion may have the risk of inducing non-clinical AF foci or unmasking clinical AF foci. Identification of non-PV AF foci is certainly difficult and so far unresolved in clinical practice. Finally, cavotricuspid isthmus was routinely ablated in all patients with PAF in the study period. However, this ablation was not performed due to an expected beneficial effect on preventing AF recurrence.35
Conclusions
Haemodialysis was significantly associated with AF recurrence after CA for PAF. However, a nearly 50% success rate for SR maintenance without the use of AADs in HD patients indicated that CA could be a suitable option for the treatment of AF.
Conflict of interest: none declared.



