-
PDF
- Split View
-
Views
-
Cite
Cite
Graham R McClure, Emilie P Belley-Cote, Iqbal H Jaffer, Nazari Dvirnik, Kevin R An, Gabriel Fortin, Jessica Spence, Jeff Healey, Rohit K Singal, Richard P Whitlock, Surgical ablation of atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials, EP Europace, Volume 20, Issue 9, September 2018, Pages 1442–1450, https://doi.org/10.1093/europace/eux336
Close - Share Icon Share
Abstract
The aim of this review was to assess the effect of concomitant surgical atrial fibrillation (AF) ablation on postoperative freedom from AF and patient-important outcomes.
We searched Cochrane CENTRAL, MEDLINE, and EMBASE databases from inception to May 2016 for randomized controlled trials (RCTs) evaluating surgical AF ablation using any lesion set vs. no surgical AF ablation in adults with AF undergoing cardiac surgery. We performed screening, risk-of-bias evaluation, and data collection independently and in duplicate. We evaluated risk of bias with the modified Cochrane tool, quality of evidence using GRADE framework, and pooled data with a random-effects model. Of the 23 included studies, only one was considered at low risk of bias. Surgical AF ablation was associated with more freedom from AF at 12 months [relative risk (RR) = 2.32, 95% confidence interval (CI) 1.92–2.80; P < 0.001, low quality]. However, no significant difference was seen in mortality (RR = 1.07, 95% CI 0.72–1.52; P = 0.41, moderate quality), stroke (RR = 1.19, 95% CI 0.59–2.39; P = 0.63, moderate quality), or pacemaker implantation (RR = 1.28, 95% CI 0.85–1.95; P = 0.24, high quality). Comparing biatrial and left-sided lesion sets showed no difference in mortality (P-interaction = 0.60) or stroke (P-interaction = 0.12). At 12 months, biatrial procedures led to more freedom from AF (RR = 2.80, 95% CI 2.13–3.68; P < 0.0001) when compared with left-sided ablation (RR = 2.00, 95% CI 1.68–2.39; P < 0.0001) (P-interaction = 0.04) Biatrial procedures appear to increase the risk for pacemaker (RR = 2.68, 95% CI 1.41–5.11; P = 0.002) compared with no ablation while left-sided ablation does not (RR = 1.08, 95% CI 0.67–1.74; P = 0.76) (P-interaction = 0.03).
Surgical AF ablation during cardiac surgery improves freedom from AF. However, impact on patient-important outcomes including mortality and stroke has not shown statistical significance in current RCT evidence. Biatrial compared with left-sided lesion sets showed no difference in mortality or stroke but were associated with significantly increased freedom from AF and risk for pacemaker requirement.
What’s new?
Surgical AF ablation is associated with improved freedom-from-AF at 12 months (RR = 2.32, 95% CI 1.92–2.80; P<0.001, low-quality).
Surgical AF ablation does not appear to affect mortality (RR = 1.07, 95% CI 0.72–1.52; P = 0.41, moderate-quality), stroke (RR = 1.19, 95% CI 0.59–2.39; P = 0.63, moderate-quality).
Biatrial procedures appear to increase risk for pacemaker (RR = 2.68, 95% CI 1.41–5.11; P = 0.002) compared to no ablation while left-sided ablation does not (RR = 1.08, 95% CI 0.67–1.74; P = 0.76) (P-interaction = 0.03).
Introduction
Atrial fibrillation (AF) affects an estimated 2.8% of the general population1 and 10% of patients undergoing cardiac surgery.2 Atrial fibrillation is an independent risk factor for all-cause mortality, ischaemic stroke, and heart failure.3,4 Over time, AF leads to an increasing number of hospitalizations.3,5
Surgical AF ablation aims to eliminate AF and maintain atrial contraction using surgical lesions to block electrical conduction to inhibit the generation and propagation of macro-re-entry circuits in the atria.6,7 The technique has been shown to reduce the burden of AF on follow-up.7–9
The Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society consensus statement and the American College of Cardiology guidelines currently consider surgical AF ablation concomitant to cardiac surgery to be a reasonable treatment, indicated for patients with persistent or permanent AF.8,10 Recent European Society of Cardiology guidelines in collaboration with the European Association for Cardio-Thoracic Surgery go further recommending concomitant AF ablation for all patients with a history of AF if the heart team believes added rate control may be beneficial.11 Studies to date have focused on the maintenance of sinus rhythm at 6 and 12 months. However, the benefit of forcing sinus rhythm onto these diseased atria is not given, and further research is required for long-term, patient-important outcomes such as mortality, stroke, heart failure, and health-related quality of life. The individual trials conducted to date are too small to accurately assess these outcomes.8,10,12–14 Of further concern, a recent randomized trial suggested harm by surgical ablation with an almost three-fold increase in the requirement for permanent pacemaker (PPM) post-ablation.9
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to assess the benefits and risks associated with surgical AF ablation in patients undergoing cardiac surgery.
Methods
A detailed description of the methods has been published elsewhere.15 We searched CENTRAL, MEDLINE, and EMBASE databases from inception to May 2016 for randomized trials, including adult patients undergoing any type of cardiac surgery with a history of AF and comparing surgical ablation utilizing any lesion set to no ablation. We also reviewed trial registries, conference proceedings, the references of included studies, and previous systematic reviews on the topic for other potentially relevant articles. No language constraints were placed.
Outcomes of interest included freedom from AF at 6 and 12 months, health-related quality of life as reported by any standardized and validated instrument, mortality, ischaemic stroke, transient ischaemic attack (TIA), pulmonary embolism, rehospitalization, cardiovascular rehospitalization, emergency room visits, length of stay during index hospitalization, need for antiarrhythmic therapy, myocardial infarction, worsening heart failure, pacemaker implementation, atrio-oesophageal perforation, postoperative bleeding, and deep sternal wound infection.
As possible, a priori defined subgroup analyses were performed, including comparison of bilateral vs left-sided only lesion sets. Further sensitivity analysis of this comparison was performed by comparing maze vs pulmonary vein isolation (PVI), with studies categorized into ‘Maze’ or ‘PVI’ based on each study’s included definition.
Study selection process
Titles and abstracts of each reference were reviewed in duplicate to assess for relevance to the review. Eligibility for inclusion was assessed using specific criteria and agreement between reviewers was measured using the kappa statistic. Any reference deemed relevant by either reviewer was included for full article review, which was again performed independently and in duplicate.
Data analyses and assessment of heterogeneity
A random-effects model was used to pool the relevant studies and summarize the evidence. The results were presented as relative risk (RR) with 95% confidence intervals (CIs) for dichotomous outcomes and as mean difference (MD) with 95% CI for continuous outcomes. We assessed for heterogeneity using the χ2 test for homogeneity and the I2 statistic for inconsistency as recommended by the Cochrane collaboration.16 We conducted subgroup analyses to assess clinical and methodological sources of heterogeneity in intervention effect and looked for potential publication bias using funnel plots and Egger’s test.
Assessment of risk of bias
Using the Cochrane Collaboration’s tool for assessing risk of bias, two reviewers assessed the risk of bias for each included study in duplicate. The reviewers evaluated the risk of bias as ‘low’, ‘high’, or ‘unclear’ for six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting, and other sources of bias.
Quality of evidence
Confidence in the pooled effects estimates was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.17 According to GRADE, data from RCTs are considered high-quality evidence but can be rated down according to the risk of bias, imprecision, inconsistency, indirectness, or publication bias.
Results
Our search strategy identified 4352 studies for review, from which 23 published studies were included (see Supplementary material online, Appendix A) with a kappa agreement value of 0.98. Review of trial registries identified five ongoing and two terminated studies relevant to our review (see Supplementary material online, Appendix B).
The 23 studies, outlined in Table 1, included 1965 patients with a mean follow-up ranging from 10 months to 44 months. Fifteen studies included PVI in their protocol, 13 included Maze, 14 included left-sided lesions only, and 14 included biatrial lesions. One study included both biatrial and left-sided Maze in a single group without allowing extraction of data for each lesion set, causing it to be excluded from subgroup analyses.
| Study ID . | n . | Design . | Population . | Lesion set . | Energy . | Rhythm assessment tool . | Length of F/U (months) . |
|---|---|---|---|---|---|---|---|
| Abreu et al.18 | 70 | Single centre | Permanent AF and rheumatic mitral valve disease | Group 1: modified Maze III | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Akpinar et al.19 | 67 | Single centre | Permanent AF and mitral valve disease for minimally invasive access | Group 1: modified Maze for LA | Radiofrequency ablation | 72 h of Holter monitor | 10 |
| Group 1 subset—biatrial ablation | |||||||
| Gillinov et al.9 | 260 | Multicentre | Persistent or long-standing persistent AF and mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 72 h of Holter monitor | 12 |
| Group 2: bi-atrial Maze | |||||||
| Budera et al.20,21 | 224 | Multicentre | All types, on-pump, non-emergency | Group 1: PVI | 96.6% surgical cryoablation | ECG and 24 h of Holter monitor | 12 |
| 3.4% radiofrequency ablation | |||||||
| Albrecht et al.22 | 60 | Single centre | Mitral valve surgery | Group 1: PVI | Cut and sew | ECG | 5 |
| Group 2: Maze | |||||||
| Blomstrom et al.23 | 71 | Multicentre | Permanent AF+mitral valve surgery | Group 1: PVI | Cryoablation | ECG | 12 |
| Deneke et al.24 | 30 | Single centre | Chronic AF+mitral valve replacement | Group 1: modified Maze III | Cooled tip radiofrequency ablation | ECG and Holter (time not specified) | 22 |
| Jonsson et al.25 | 72 | Multicentre | Mitral valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Knaut et al.26 | 45 | Single centre | Permanent AF+CABG or aortic valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Khargi et al.27 | 30 | Single centre | Mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Cherniavsky et al.28 | 95 | Single centre | Persistent AF+CABG | Group 1: PVI | Irrigated radiofrequency ablation | Implantable loop recorder | 24 |
| Group 2: mini Maze | |||||||
| Chevalier et al.29 | 43 | Multicentre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Jessurun et al.30 | 35 | Single centre | Symptomatic AF+mitral valve surgery | Group 1: Maze III | Cut and sew | ECG | 12 |
| De Lima et al.31 | 30 | Single centre | Permanent AF+mitral valve surgery | Group 1: PVI | Incision and electrocautery | ECG and 24 h Holter | 24 |
| Group 2: modified Maze III | |||||||
| Pokushalov et al.32 | 35 | Single centre | Paroxysmal+CABG | Group 1: PVI | Radiofrequency ablation | Implantable loop recorder | 18 |
| Schuetz et al.33 | 43 | Single centre | Permanent AF+ABG and/or valvular surgery | Group 1: PVI | Microwave ablation | ECG+Holter | 12 |
| Srivastava et al.34 | 160 | Single centre | Chronic rheumatic AF undergoing valvular heart surgery | Group 1: biatrial Maze | Radiofrequency ablation and cryoablation | ECG and Echo | 44 |
| Group 2: left atrial Maze | |||||||
| Group 3: PVI | |||||||
| von Oppell et al.35 | 49 | Single centre | Persistent and permanent AF+mitral valve surgery | Group 1: modified biatrial MAZE | Radiofrequency ablation | ECG | 12 |
| Vasconcelos et al.36 | 29 | Single centre | Persistent AF+rheumatic valvular surgery | Group 1: modified MAZE | Cut and sew | ECG | 12 |
| Van Breugel et al.37 | 132 | Multicenter | All AF+CABG and/or valvular surgery | Group 1: PVI | Not reported | ECG and 24 h Holter | 12 |
| Wang et al.38 | 210 | Single centre | Long-standing persistent AF+rheumatic mitral valve surgery | Group 1: left sided MAZE | Radiofrequency ablation and cut and sew lesions | ECG and Holter (time not specified) | 12 |
| Group 2: biatrial MAZE | |||||||
| Doukas et al.39 | 97 | Single centre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG and 48 h Holter | 12 |
| Bahar et al.40 | 17 | Single centre | Chronic AF+rheumatic mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG | 12 |
| Study ID . | n . | Design . | Population . | Lesion set . | Energy . | Rhythm assessment tool . | Length of F/U (months) . |
|---|---|---|---|---|---|---|---|
| Abreu et al.18 | 70 | Single centre | Permanent AF and rheumatic mitral valve disease | Group 1: modified Maze III | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Akpinar et al.19 | 67 | Single centre | Permanent AF and mitral valve disease for minimally invasive access | Group 1: modified Maze for LA | Radiofrequency ablation | 72 h of Holter monitor | 10 |
| Group 1 subset—biatrial ablation | |||||||
| Gillinov et al.9 | 260 | Multicentre | Persistent or long-standing persistent AF and mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 72 h of Holter monitor | 12 |
| Group 2: bi-atrial Maze | |||||||
| Budera et al.20,21 | 224 | Multicentre | All types, on-pump, non-emergency | Group 1: PVI | 96.6% surgical cryoablation | ECG and 24 h of Holter monitor | 12 |
| 3.4% radiofrequency ablation | |||||||
| Albrecht et al.22 | 60 | Single centre | Mitral valve surgery | Group 1: PVI | Cut and sew | ECG | 5 |
| Group 2: Maze | |||||||
| Blomstrom et al.23 | 71 | Multicentre | Permanent AF+mitral valve surgery | Group 1: PVI | Cryoablation | ECG | 12 |
| Deneke et al.24 | 30 | Single centre | Chronic AF+mitral valve replacement | Group 1: modified Maze III | Cooled tip radiofrequency ablation | ECG and Holter (time not specified) | 22 |
| Jonsson et al.25 | 72 | Multicentre | Mitral valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Knaut et al.26 | 45 | Single centre | Permanent AF+CABG or aortic valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Khargi et al.27 | 30 | Single centre | Mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Cherniavsky et al.28 | 95 | Single centre | Persistent AF+CABG | Group 1: PVI | Irrigated radiofrequency ablation | Implantable loop recorder | 24 |
| Group 2: mini Maze | |||||||
| Chevalier et al.29 | 43 | Multicentre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Jessurun et al.30 | 35 | Single centre | Symptomatic AF+mitral valve surgery | Group 1: Maze III | Cut and sew | ECG | 12 |
| De Lima et al.31 | 30 | Single centre | Permanent AF+mitral valve surgery | Group 1: PVI | Incision and electrocautery | ECG and 24 h Holter | 24 |
| Group 2: modified Maze III | |||||||
| Pokushalov et al.32 | 35 | Single centre | Paroxysmal+CABG | Group 1: PVI | Radiofrequency ablation | Implantable loop recorder | 18 |
| Schuetz et al.33 | 43 | Single centre | Permanent AF+ABG and/or valvular surgery | Group 1: PVI | Microwave ablation | ECG+Holter | 12 |
| Srivastava et al.34 | 160 | Single centre | Chronic rheumatic AF undergoing valvular heart surgery | Group 1: biatrial Maze | Radiofrequency ablation and cryoablation | ECG and Echo | 44 |
| Group 2: left atrial Maze | |||||||
| Group 3: PVI | |||||||
| von Oppell et al.35 | 49 | Single centre | Persistent and permanent AF+mitral valve surgery | Group 1: modified biatrial MAZE | Radiofrequency ablation | ECG | 12 |
| Vasconcelos et al.36 | 29 | Single centre | Persistent AF+rheumatic valvular surgery | Group 1: modified MAZE | Cut and sew | ECG | 12 |
| Van Breugel et al.37 | 132 | Multicenter | All AF+CABG and/or valvular surgery | Group 1: PVI | Not reported | ECG and 24 h Holter | 12 |
| Wang et al.38 | 210 | Single centre | Long-standing persistent AF+rheumatic mitral valve surgery | Group 1: left sided MAZE | Radiofrequency ablation and cut and sew lesions | ECG and Holter (time not specified) | 12 |
| Group 2: biatrial MAZE | |||||||
| Doukas et al.39 | 97 | Single centre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG and 48 h Holter | 12 |
| Bahar et al.40 | 17 | Single centre | Chronic AF+rheumatic mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG | 12 |
Primary outcome of all trials was freedom from AF. Lesions used in each category non-homogenous between studies. All groups compared against a control of ‘no surgical ablation’.
AF, atrial fibrillation; ECG, electrocardiogram; echo, echocardiogram; LA, left atria; PVI, pulmonary vein isolation.
| Study ID . | n . | Design . | Population . | Lesion set . | Energy . | Rhythm assessment tool . | Length of F/U (months) . |
|---|---|---|---|---|---|---|---|
| Abreu et al.18 | 70 | Single centre | Permanent AF and rheumatic mitral valve disease | Group 1: modified Maze III | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Akpinar et al.19 | 67 | Single centre | Permanent AF and mitral valve disease for minimally invasive access | Group 1: modified Maze for LA | Radiofrequency ablation | 72 h of Holter monitor | 10 |
| Group 1 subset—biatrial ablation | |||||||
| Gillinov et al.9 | 260 | Multicentre | Persistent or long-standing persistent AF and mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 72 h of Holter monitor | 12 |
| Group 2: bi-atrial Maze | |||||||
| Budera et al.20,21 | 224 | Multicentre | All types, on-pump, non-emergency | Group 1: PVI | 96.6% surgical cryoablation | ECG and 24 h of Holter monitor | 12 |
| 3.4% radiofrequency ablation | |||||||
| Albrecht et al.22 | 60 | Single centre | Mitral valve surgery | Group 1: PVI | Cut and sew | ECG | 5 |
| Group 2: Maze | |||||||
| Blomstrom et al.23 | 71 | Multicentre | Permanent AF+mitral valve surgery | Group 1: PVI | Cryoablation | ECG | 12 |
| Deneke et al.24 | 30 | Single centre | Chronic AF+mitral valve replacement | Group 1: modified Maze III | Cooled tip radiofrequency ablation | ECG and Holter (time not specified) | 22 |
| Jonsson et al.25 | 72 | Multicentre | Mitral valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Knaut et al.26 | 45 | Single centre | Permanent AF+CABG or aortic valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Khargi et al.27 | 30 | Single centre | Mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Cherniavsky et al.28 | 95 | Single centre | Persistent AF+CABG | Group 1: PVI | Irrigated radiofrequency ablation | Implantable loop recorder | 24 |
| Group 2: mini Maze | |||||||
| Chevalier et al.29 | 43 | Multicentre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Jessurun et al.30 | 35 | Single centre | Symptomatic AF+mitral valve surgery | Group 1: Maze III | Cut and sew | ECG | 12 |
| De Lima et al.31 | 30 | Single centre | Permanent AF+mitral valve surgery | Group 1: PVI | Incision and electrocautery | ECG and 24 h Holter | 24 |
| Group 2: modified Maze III | |||||||
| Pokushalov et al.32 | 35 | Single centre | Paroxysmal+CABG | Group 1: PVI | Radiofrequency ablation | Implantable loop recorder | 18 |
| Schuetz et al.33 | 43 | Single centre | Permanent AF+ABG and/or valvular surgery | Group 1: PVI | Microwave ablation | ECG+Holter | 12 |
| Srivastava et al.34 | 160 | Single centre | Chronic rheumatic AF undergoing valvular heart surgery | Group 1: biatrial Maze | Radiofrequency ablation and cryoablation | ECG and Echo | 44 |
| Group 2: left atrial Maze | |||||||
| Group 3: PVI | |||||||
| von Oppell et al.35 | 49 | Single centre | Persistent and permanent AF+mitral valve surgery | Group 1: modified biatrial MAZE | Radiofrequency ablation | ECG | 12 |
| Vasconcelos et al.36 | 29 | Single centre | Persistent AF+rheumatic valvular surgery | Group 1: modified MAZE | Cut and sew | ECG | 12 |
| Van Breugel et al.37 | 132 | Multicenter | All AF+CABG and/or valvular surgery | Group 1: PVI | Not reported | ECG and 24 h Holter | 12 |
| Wang et al.38 | 210 | Single centre | Long-standing persistent AF+rheumatic mitral valve surgery | Group 1: left sided MAZE | Radiofrequency ablation and cut and sew lesions | ECG and Holter (time not specified) | 12 |
| Group 2: biatrial MAZE | |||||||
| Doukas et al.39 | 97 | Single centre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG and 48 h Holter | 12 |
| Bahar et al.40 | 17 | Single centre | Chronic AF+rheumatic mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG | 12 |
| Study ID . | n . | Design . | Population . | Lesion set . | Energy . | Rhythm assessment tool . | Length of F/U (months) . |
|---|---|---|---|---|---|---|---|
| Abreu et al.18 | 70 | Single centre | Permanent AF and rheumatic mitral valve disease | Group 1: modified Maze III | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Akpinar et al.19 | 67 | Single centre | Permanent AF and mitral valve disease for minimally invasive access | Group 1: modified Maze for LA | Radiofrequency ablation | 72 h of Holter monitor | 10 |
| Group 1 subset—biatrial ablation | |||||||
| Gillinov et al.9 | 260 | Multicentre | Persistent or long-standing persistent AF and mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 72 h of Holter monitor | 12 |
| Group 2: bi-atrial Maze | |||||||
| Budera et al.20,21 | 224 | Multicentre | All types, on-pump, non-emergency | Group 1: PVI | 96.6% surgical cryoablation | ECG and 24 h of Holter monitor | 12 |
| 3.4% radiofrequency ablation | |||||||
| Albrecht et al.22 | 60 | Single centre | Mitral valve surgery | Group 1: PVI | Cut and sew | ECG | 5 |
| Group 2: Maze | |||||||
| Blomstrom et al.23 | 71 | Multicentre | Permanent AF+mitral valve surgery | Group 1: PVI | Cryoablation | ECG | 12 |
| Deneke et al.24 | 30 | Single centre | Chronic AF+mitral valve replacement | Group 1: modified Maze III | Cooled tip radiofrequency ablation | ECG and Holter (time not specified) | 22 |
| Jonsson et al.25 | 72 | Multicentre | Mitral valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Knaut et al.26 | 45 | Single centre | Permanent AF+CABG or aortic valve surgery | Group 1: PVI | Microwave ablation | ECG and 24 h of Holter monitor | 12 |
| Khargi et al.27 | 30 | Single centre | Mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Cherniavsky et al.28 | 95 | Single centre | Persistent AF+CABG | Group 1: PVI | Irrigated radiofrequency ablation | Implantable loop recorder | 24 |
| Group 2: mini Maze | |||||||
| Chevalier et al.29 | 43 | Multicentre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | 24 h of Holter monitor | 12 |
| Jessurun et al.30 | 35 | Single centre | Symptomatic AF+mitral valve surgery | Group 1: Maze III | Cut and sew | ECG | 12 |
| De Lima et al.31 | 30 | Single centre | Permanent AF+mitral valve surgery | Group 1: PVI | Incision and electrocautery | ECG and 24 h Holter | 24 |
| Group 2: modified Maze III | |||||||
| Pokushalov et al.32 | 35 | Single centre | Paroxysmal+CABG | Group 1: PVI | Radiofrequency ablation | Implantable loop recorder | 18 |
| Schuetz et al.33 | 43 | Single centre | Permanent AF+ABG and/or valvular surgery | Group 1: PVI | Microwave ablation | ECG+Holter | 12 |
| Srivastava et al.34 | 160 | Single centre | Chronic rheumatic AF undergoing valvular heart surgery | Group 1: biatrial Maze | Radiofrequency ablation and cryoablation | ECG and Echo | 44 |
| Group 2: left atrial Maze | |||||||
| Group 3: PVI | |||||||
| von Oppell et al.35 | 49 | Single centre | Persistent and permanent AF+mitral valve surgery | Group 1: modified biatrial MAZE | Radiofrequency ablation | ECG | 12 |
| Vasconcelos et al.36 | 29 | Single centre | Persistent AF+rheumatic valvular surgery | Group 1: modified MAZE | Cut and sew | ECG | 12 |
| Van Breugel et al.37 | 132 | Multicenter | All AF+CABG and/or valvular surgery | Group 1: PVI | Not reported | ECG and 24 h Holter | 12 |
| Wang et al.38 | 210 | Single centre | Long-standing persistent AF+rheumatic mitral valve surgery | Group 1: left sided MAZE | Radiofrequency ablation and cut and sew lesions | ECG and Holter (time not specified) | 12 |
| Group 2: biatrial MAZE | |||||||
| Doukas et al.39 | 97 | Single centre | Persistent AF+mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG and 48 h Holter | 12 |
| Bahar et al.40 | 17 | Single centre | Chronic AF+rheumatic mitral valve surgery | Group 1: PVI | Radiofrequency ablation | ECG | 12 |
Primary outcome of all trials was freedom from AF. Lesions used in each category non-homogenous between studies. All groups compared against a control of ‘no surgical ablation’.
AF, atrial fibrillation; ECG, electrocardiogram; echo, echocardiogram; LA, left atria; PVI, pulmonary vein isolation.
Risk of bias was considered low in one identified study (see Supplementary material online, Appendix A). All other studies had either high risk of bias in one or more criteria or a significant uncertainty in the risk of bias ascertainment that remained unresolved after contacting the authors. In general, allocation concealment and randomized sequence generation were poorly reported.
Surgical ablation of atrial fibrillation vs. no surgical ablation
Freedom from atrial fibrillation
Twenty studies (n = 1407) reported on freedom from AF at 12 months, showing significant benefit from surgical ablation (RR 2.32, 95% CI 1.92–2.80; P < 0.0001, I2 = 44%, low quality) (Figure 1). At this time, 70% of ablated patients and 30% of control patients were free from AF. This benefit was also seen at 6 months postoperatively and at 3 months no significant difference was observed. (Table 1) On visual inspection, the funnel plots of all time points for freedom from AF assessment appeared asymmetrical (see Supplementary material online, Appendix A). Egger analysis for publication bias confirmed significant bias in reporting freedom from AF at three (P = 0.014), six months (P = 0.026) and 12 months (P < 0.001), favouring the publication of significantly positive results. Tools used to assess freedom from AF were also quite heterogeneous across studies with 30% of studies only utilizing electrocardiography (ECG), 39% using a combination of ECG and Holter assessment, 13% using 24 h Holter assesment, 9% using 72 h Holter assessment, and 9% using implantable loop recorder.
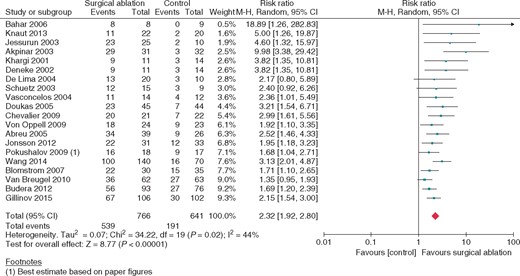
Freedom from atrial fibrillation at 12 months; surgical atrial fibrillation ablation vs. no ablation. Square markers represent the point estimate of RR for each primary study, with size of each square proportional to the weight of the given study in meta-analysis. Horizontal lines indicate 95% CIs. The solid diamond represents the estimated 95% confidence interval for effect size of all meta-analyzed data.
Mortality
All studies (n = 1869) reported mortality, and no significant difference was demonstrated (RR 1.07, 95% CI 0.75–1.52; P = 0.72, I2= 0%, moderate quality) (see Supplementary material online, Appendix A). The funnel plot was not suggestive of publication bias.
Stroke
Fifteen studies (n = 1326) reported on stroke during follow-up with no significant difference observed (RR 1.19, 95% CI 0.59–2.39; P = 0.63, I2 = 0%, moderate quality) (see Supplementary material online, Appendix A). No publication bias was suspected on inspection of the funnel plot.
Pacemaker implantation
Pacemaker implantation at latest follow-up was reported in 15 studies (n = 1485). When pooled, no significant difference in risk of pacemaker requirement was found (RR 1.28, 95% CI 0.85–1.95; P = 0.24, I2 = 0%, high quality) (see Supplementary material online, Appendix A). No significant publication bias was observed on funnel plot analysis.
Hospital length of stay
Eleven studies (n = 930) reported postoperative hospital length of stay, which was found to be significantly longer for patients who underwent ablation (MD 1.67 days, 95%CI 0.22–3.12; P = 0.02, I2 = 68%, low quality).
Myocardial infarction
Five studies (n = 675) reported on myocardial infarction during follow-up; no significant difference was observed (RR = 1.01, 95%, CI 0.32–3.15; P = 0.99, I2 = 0%, moderate quality).
Health-related quality of life
Five studies captured health-related quality of life using various measurement tools. SF-36 was used in four of the studies; however, two studies did not report variance to questionnaire scores or number of patients at each assessment point for inclusion in analysis. Meta-analysis of SF-36 scores thus included two studies (n = 181) and showed significant difference in a single SF-36 domain preoperatively and postoperatively (one of eight domains), physical role functioning (MD19.12, 95% CI 1.62–36.61; P = 0.03, I2 = 10%, high quality).
Other outcomes
Findings for all other outcomes are summarized in Table 2.
| Outcomes . | Studies . | Participants . | Effect estimate . | P-value . | I2 . | GRADE . |
|---|---|---|---|---|---|---|
| Freedom from AF at 3 months | 13 | 870 | 1.97 (0.83 to 4.68)a | 0.12 | 99% | Very low |
| Freedom from AF at 6 months | 15 | 1096 | 2.31 (1.82 to 2.93)a | <0.00001 | 56% | Low |
| Freedom from AF at 12 months | 20 | 1407 | 2.32 (1.92 to 2.80)a | <0.00001 | 44% | Low |
| All-cause mortality | 23 | 1869 | 1.07 (0.75 to 1.52)a | 0.88 | 0% | Moderate |
| Stroke | 14 | 1326 | 1.19 (0.59 to 2.39)a | 0.63 | 0% | Moderate |
| Ischaemic stroke (including TIA with positive imaging) | 10 | 1102 | 1.00 (0.39 to 2.59)a | 0.99 | 0% | Moderate |
| TIA | 8 | 677 | 1.04 (0.23 to 4.75)a | 0.95 | 16% | Moderate |
| Pulmonary embolism | 7 | 504 | 0.17 (0.01 to 3.94)a | 0.27 | n/ab | Moderate |
| Worsening NYHA classification | 1 | 236 | 1.87 (0.56 to 6.23)a | 0.31 | n/ab | Low |
| Any rehospitalization | 2 | 478 | 1.13 (0.88 to 1.44)a | 0.35 | 0% | Moderate |
| Readmission for cardiovascular causes | 2 | 478 | 1.21 (0.79 to 1.84)a | 0.38 | 22% | Moderate |
| ER visits postoperatively | 0 | 0 | n/a | n/a | n/a | |
| All-cause ICU mortality during index hospitalization | 7 | 414 | 2.44 (0.41 to 14.55)a | 0.34 | 0% | Moderate |
| All-cause hospital mortality during index hospitalization | 15 | 1030 | 1.12 (0.56 to 2.22)a | 0.88 | 0% | Moderate |
| Pacemaker implantation at latest follow up | 15 | 1485 | 1.27 (0.85 to 1.95)a | 0.24 | 0% | High |
| Pacemaker implantation at index hospital discharge | 11 | 1121 | 1.88 (0.97 to 3.62)a | 0.06 | 15% | High |
| Atrio-oesophageal perforation at latest follow-up | 2 | 203 | Not estimable—no events | n/a | n/a | Low |
| Deep sternal wound infection | 4 | 577 | 1.71 (0.51 to 5.72)a | 0.35 | 0% | Moderate |
| Myocardial infarction | 5 | 675 | 1.01 (0.32 to 3.15)a | 0.99 | 0% | Moderate |
| Hospital length of stay during index hospitalization | 11 | 930 | 1.67 (0.22 to 3.12)c | 0.02 | 59% | Low |
| ICU length of stay during index hospitalization | 4 | 238 | −12.85 (−16.32 to − 9.38)c | <0.00001 | 0% | High |
| Postoperative bleeding | 0 | 0 | Not estimable | n/a | n/a | |
| Decrease in NYHA classification at latest follow-up | 3 | 224 | 0.23 (0.01 to 0.45) | 0.04 | 0% | High |
| SF-36: general health | 2 + 2 without reported variance | 181d | 1.66 (−13.95 to 17.26)c | 0.84 | 66% | Moderate |
| SF-36: physical function | 2 + 2 without reported variance | 181d | −4.32 (−29.66 to 21.03)c | 0.74 | 77% | Moderate |
| SF-36: bodily pain | 2 + 2 without reported variance | 181d | −0.60 (−10.62 to 9.41)c | 0.91 | 0% | Moderate |
| SF-36: mental health | 2 + 2 without reported variance | 181d | 4.16 (−3.43 to 11.76)c | 0.28 | 0% | Moderate |
| SF-36: role functioning–physical | 2 + 2 without reported variance | 181d | 19.12 (1.62 to 36.61)c | 0.03 | 10% | Moderate |
| SF-36: role functioning–emotional | 2 + 2 without reported variance | 181d | −4.92 (−16.87 to 7.04)c | 0.42 | 0% | Moderate |
| SF-36: social functioning | 2 + 2 without reported variance | 181d | −1.41 (−18.24 to 15.41)c | 0.87 | 47% | Moderate |
| SF-36: vitality | 2 + 2 without reported variance | 181d | −3.03 (−12.50 to 6.45)c | 0.53 | 0% | Moderate |
| Outcomes . | Studies . | Participants . | Effect estimate . | P-value . | I2 . | GRADE . |
|---|---|---|---|---|---|---|
| Freedom from AF at 3 months | 13 | 870 | 1.97 (0.83 to 4.68)a | 0.12 | 99% | Very low |
| Freedom from AF at 6 months | 15 | 1096 | 2.31 (1.82 to 2.93)a | <0.00001 | 56% | Low |
| Freedom from AF at 12 months | 20 | 1407 | 2.32 (1.92 to 2.80)a | <0.00001 | 44% | Low |
| All-cause mortality | 23 | 1869 | 1.07 (0.75 to 1.52)a | 0.88 | 0% | Moderate |
| Stroke | 14 | 1326 | 1.19 (0.59 to 2.39)a | 0.63 | 0% | Moderate |
| Ischaemic stroke (including TIA with positive imaging) | 10 | 1102 | 1.00 (0.39 to 2.59)a | 0.99 | 0% | Moderate |
| TIA | 8 | 677 | 1.04 (0.23 to 4.75)a | 0.95 | 16% | Moderate |
| Pulmonary embolism | 7 | 504 | 0.17 (0.01 to 3.94)a | 0.27 | n/ab | Moderate |
| Worsening NYHA classification | 1 | 236 | 1.87 (0.56 to 6.23)a | 0.31 | n/ab | Low |
| Any rehospitalization | 2 | 478 | 1.13 (0.88 to 1.44)a | 0.35 | 0% | Moderate |
| Readmission for cardiovascular causes | 2 | 478 | 1.21 (0.79 to 1.84)a | 0.38 | 22% | Moderate |
| ER visits postoperatively | 0 | 0 | n/a | n/a | n/a | |
| All-cause ICU mortality during index hospitalization | 7 | 414 | 2.44 (0.41 to 14.55)a | 0.34 | 0% | Moderate |
| All-cause hospital mortality during index hospitalization | 15 | 1030 | 1.12 (0.56 to 2.22)a | 0.88 | 0% | Moderate |
| Pacemaker implantation at latest follow up | 15 | 1485 | 1.27 (0.85 to 1.95)a | 0.24 | 0% | High |
| Pacemaker implantation at index hospital discharge | 11 | 1121 | 1.88 (0.97 to 3.62)a | 0.06 | 15% | High |
| Atrio-oesophageal perforation at latest follow-up | 2 | 203 | Not estimable—no events | n/a | n/a | Low |
| Deep sternal wound infection | 4 | 577 | 1.71 (0.51 to 5.72)a | 0.35 | 0% | Moderate |
| Myocardial infarction | 5 | 675 | 1.01 (0.32 to 3.15)a | 0.99 | 0% | Moderate |
| Hospital length of stay during index hospitalization | 11 | 930 | 1.67 (0.22 to 3.12)c | 0.02 | 59% | Low |
| ICU length of stay during index hospitalization | 4 | 238 | −12.85 (−16.32 to − 9.38)c | <0.00001 | 0% | High |
| Postoperative bleeding | 0 | 0 | Not estimable | n/a | n/a | |
| Decrease in NYHA classification at latest follow-up | 3 | 224 | 0.23 (0.01 to 0.45) | 0.04 | 0% | High |
| SF-36: general health | 2 + 2 without reported variance | 181d | 1.66 (−13.95 to 17.26)c | 0.84 | 66% | Moderate |
| SF-36: physical function | 2 + 2 without reported variance | 181d | −4.32 (−29.66 to 21.03)c | 0.74 | 77% | Moderate |
| SF-36: bodily pain | 2 + 2 without reported variance | 181d | −0.60 (−10.62 to 9.41)c | 0.91 | 0% | Moderate |
| SF-36: mental health | 2 + 2 without reported variance | 181d | 4.16 (−3.43 to 11.76)c | 0.28 | 0% | Moderate |
| SF-36: role functioning–physical | 2 + 2 without reported variance | 181d | 19.12 (1.62 to 36.61)c | 0.03 | 10% | Moderate |
| SF-36: role functioning–emotional | 2 + 2 without reported variance | 181d | −4.92 (−16.87 to 7.04)c | 0.42 | 0% | Moderate |
| SF-36: social functioning | 2 + 2 without reported variance | 181d | −1.41 (−18.24 to 15.41)c | 0.87 | 47% | Moderate |
| SF-36: vitality | 2 + 2 without reported variance | 181d | −3.03 (−12.50 to 6.45)c | 0.53 | 0% | Moderate |
AF, atrial fibrillation; TIA, transient ischaemic attack; ER, emergency room; ICU, intensive care unit; NYHA, New York Heart Association; M–H, Mantel–Haenszel; IV, inverse variance.
Risk ratio (M–H, random, 95% CI).
Only one study reporting non-zero event rate.
Mean difference (IV, random, 95% CI).
Studies not reporting measures of variance not included.
| Outcomes . | Studies . | Participants . | Effect estimate . | P-value . | I2 . | GRADE . |
|---|---|---|---|---|---|---|
| Freedom from AF at 3 months | 13 | 870 | 1.97 (0.83 to 4.68)a | 0.12 | 99% | Very low |
| Freedom from AF at 6 months | 15 | 1096 | 2.31 (1.82 to 2.93)a | <0.00001 | 56% | Low |
| Freedom from AF at 12 months | 20 | 1407 | 2.32 (1.92 to 2.80)a | <0.00001 | 44% | Low |
| All-cause mortality | 23 | 1869 | 1.07 (0.75 to 1.52)a | 0.88 | 0% | Moderate |
| Stroke | 14 | 1326 | 1.19 (0.59 to 2.39)a | 0.63 | 0% | Moderate |
| Ischaemic stroke (including TIA with positive imaging) | 10 | 1102 | 1.00 (0.39 to 2.59)a | 0.99 | 0% | Moderate |
| TIA | 8 | 677 | 1.04 (0.23 to 4.75)a | 0.95 | 16% | Moderate |
| Pulmonary embolism | 7 | 504 | 0.17 (0.01 to 3.94)a | 0.27 | n/ab | Moderate |
| Worsening NYHA classification | 1 | 236 | 1.87 (0.56 to 6.23)a | 0.31 | n/ab | Low |
| Any rehospitalization | 2 | 478 | 1.13 (0.88 to 1.44)a | 0.35 | 0% | Moderate |
| Readmission for cardiovascular causes | 2 | 478 | 1.21 (0.79 to 1.84)a | 0.38 | 22% | Moderate |
| ER visits postoperatively | 0 | 0 | n/a | n/a | n/a | |
| All-cause ICU mortality during index hospitalization | 7 | 414 | 2.44 (0.41 to 14.55)a | 0.34 | 0% | Moderate |
| All-cause hospital mortality during index hospitalization | 15 | 1030 | 1.12 (0.56 to 2.22)a | 0.88 | 0% | Moderate |
| Pacemaker implantation at latest follow up | 15 | 1485 | 1.27 (0.85 to 1.95)a | 0.24 | 0% | High |
| Pacemaker implantation at index hospital discharge | 11 | 1121 | 1.88 (0.97 to 3.62)a | 0.06 | 15% | High |
| Atrio-oesophageal perforation at latest follow-up | 2 | 203 | Not estimable—no events | n/a | n/a | Low |
| Deep sternal wound infection | 4 | 577 | 1.71 (0.51 to 5.72)a | 0.35 | 0% | Moderate |
| Myocardial infarction | 5 | 675 | 1.01 (0.32 to 3.15)a | 0.99 | 0% | Moderate |
| Hospital length of stay during index hospitalization | 11 | 930 | 1.67 (0.22 to 3.12)c | 0.02 | 59% | Low |
| ICU length of stay during index hospitalization | 4 | 238 | −12.85 (−16.32 to − 9.38)c | <0.00001 | 0% | High |
| Postoperative bleeding | 0 | 0 | Not estimable | n/a | n/a | |
| Decrease in NYHA classification at latest follow-up | 3 | 224 | 0.23 (0.01 to 0.45) | 0.04 | 0% | High |
| SF-36: general health | 2 + 2 without reported variance | 181d | 1.66 (−13.95 to 17.26)c | 0.84 | 66% | Moderate |
| SF-36: physical function | 2 + 2 without reported variance | 181d | −4.32 (−29.66 to 21.03)c | 0.74 | 77% | Moderate |
| SF-36: bodily pain | 2 + 2 without reported variance | 181d | −0.60 (−10.62 to 9.41)c | 0.91 | 0% | Moderate |
| SF-36: mental health | 2 + 2 without reported variance | 181d | 4.16 (−3.43 to 11.76)c | 0.28 | 0% | Moderate |
| SF-36: role functioning–physical | 2 + 2 without reported variance | 181d | 19.12 (1.62 to 36.61)c | 0.03 | 10% | Moderate |
| SF-36: role functioning–emotional | 2 + 2 without reported variance | 181d | −4.92 (−16.87 to 7.04)c | 0.42 | 0% | Moderate |
| SF-36: social functioning | 2 + 2 without reported variance | 181d | −1.41 (−18.24 to 15.41)c | 0.87 | 47% | Moderate |
| SF-36: vitality | 2 + 2 without reported variance | 181d | −3.03 (−12.50 to 6.45)c | 0.53 | 0% | Moderate |
| Outcomes . | Studies . | Participants . | Effect estimate . | P-value . | I2 . | GRADE . |
|---|---|---|---|---|---|---|
| Freedom from AF at 3 months | 13 | 870 | 1.97 (0.83 to 4.68)a | 0.12 | 99% | Very low |
| Freedom from AF at 6 months | 15 | 1096 | 2.31 (1.82 to 2.93)a | <0.00001 | 56% | Low |
| Freedom from AF at 12 months | 20 | 1407 | 2.32 (1.92 to 2.80)a | <0.00001 | 44% | Low |
| All-cause mortality | 23 | 1869 | 1.07 (0.75 to 1.52)a | 0.88 | 0% | Moderate |
| Stroke | 14 | 1326 | 1.19 (0.59 to 2.39)a | 0.63 | 0% | Moderate |
| Ischaemic stroke (including TIA with positive imaging) | 10 | 1102 | 1.00 (0.39 to 2.59)a | 0.99 | 0% | Moderate |
| TIA | 8 | 677 | 1.04 (0.23 to 4.75)a | 0.95 | 16% | Moderate |
| Pulmonary embolism | 7 | 504 | 0.17 (0.01 to 3.94)a | 0.27 | n/ab | Moderate |
| Worsening NYHA classification | 1 | 236 | 1.87 (0.56 to 6.23)a | 0.31 | n/ab | Low |
| Any rehospitalization | 2 | 478 | 1.13 (0.88 to 1.44)a | 0.35 | 0% | Moderate |
| Readmission for cardiovascular causes | 2 | 478 | 1.21 (0.79 to 1.84)a | 0.38 | 22% | Moderate |
| ER visits postoperatively | 0 | 0 | n/a | n/a | n/a | |
| All-cause ICU mortality during index hospitalization | 7 | 414 | 2.44 (0.41 to 14.55)a | 0.34 | 0% | Moderate |
| All-cause hospital mortality during index hospitalization | 15 | 1030 | 1.12 (0.56 to 2.22)a | 0.88 | 0% | Moderate |
| Pacemaker implantation at latest follow up | 15 | 1485 | 1.27 (0.85 to 1.95)a | 0.24 | 0% | High |
| Pacemaker implantation at index hospital discharge | 11 | 1121 | 1.88 (0.97 to 3.62)a | 0.06 | 15% | High |
| Atrio-oesophageal perforation at latest follow-up | 2 | 203 | Not estimable—no events | n/a | n/a | Low |
| Deep sternal wound infection | 4 | 577 | 1.71 (0.51 to 5.72)a | 0.35 | 0% | Moderate |
| Myocardial infarction | 5 | 675 | 1.01 (0.32 to 3.15)a | 0.99 | 0% | Moderate |
| Hospital length of stay during index hospitalization | 11 | 930 | 1.67 (0.22 to 3.12)c | 0.02 | 59% | Low |
| ICU length of stay during index hospitalization | 4 | 238 | −12.85 (−16.32 to − 9.38)c | <0.00001 | 0% | High |
| Postoperative bleeding | 0 | 0 | Not estimable | n/a | n/a | |
| Decrease in NYHA classification at latest follow-up | 3 | 224 | 0.23 (0.01 to 0.45) | 0.04 | 0% | High |
| SF-36: general health | 2 + 2 without reported variance | 181d | 1.66 (−13.95 to 17.26)c | 0.84 | 66% | Moderate |
| SF-36: physical function | 2 + 2 without reported variance | 181d | −4.32 (−29.66 to 21.03)c | 0.74 | 77% | Moderate |
| SF-36: bodily pain | 2 + 2 without reported variance | 181d | −0.60 (−10.62 to 9.41)c | 0.91 | 0% | Moderate |
| SF-36: mental health | 2 + 2 without reported variance | 181d | 4.16 (−3.43 to 11.76)c | 0.28 | 0% | Moderate |
| SF-36: role functioning–physical | 2 + 2 without reported variance | 181d | 19.12 (1.62 to 36.61)c | 0.03 | 10% | Moderate |
| SF-36: role functioning–emotional | 2 + 2 without reported variance | 181d | −4.92 (−16.87 to 7.04)c | 0.42 | 0% | Moderate |
| SF-36: social functioning | 2 + 2 without reported variance | 181d | −1.41 (−18.24 to 15.41)c | 0.87 | 47% | Moderate |
| SF-36: vitality | 2 + 2 without reported variance | 181d | −3.03 (−12.50 to 6.45)c | 0.53 | 0% | Moderate |
AF, atrial fibrillation; TIA, transient ischaemic attack; ER, emergency room; ICU, intensive care unit; NYHA, New York Heart Association; M–H, Mantel–Haenszel; IV, inverse variance.
Risk ratio (M–H, random, 95% CI).
Only one study reporting non-zero event rate.
Mean difference (IV, random, 95% CI).
Studies not reporting measures of variance not included.
Biatrial vs. left-sided lesions
Comparing biatrial and left-sided lesion sets, no significant difference was seen in mortality (P =0.44), stroke (P = 0.15) or hospital length of stay (P = 0.49). Improved freedom from AF was seen at 12 months in both biatrial (RR = 2.80, 95% CI 2.13–3.68; P < 0.0001, I2 = 39%) and left-sided (RR = 2.00, 95% CI 1.68–2.39; P < 0.0001, I2= 23%) lesions sets; this difference between groups was significant for interaction (P = 0.04) (see Supplementary material online, Appendix A). Additionally, biatrial ablation conferred a significantly increased risk of pacemaker requirement (RR 2.68, 95% CI 1.41–5.11; P = 0.003, I2 = 0%) while left-sided ablation did not (RR 1.08, 95% CI 0.67–1.74; P = 0.76, I2 = 2%); this difference was significant (P for interaction = 0.03) (Figure 2).
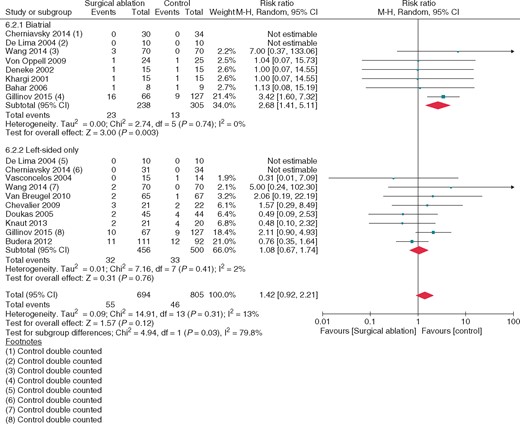
Pacemaker requirement at latest follow-up: subgroups, biatrial, and left-sided lesions sets; concomitant surgical atrial fibrillation ablation vs. no ablation. Square markers represent the point estimate of RR for each primary study, with size of each square proportional to the weight of the given study in meta-analysis. Horizontal lines indicate 95% CIs. The solid diamond represents the estimated 95% confidence interval for effect size of all meta-analyzed data.
Maze vs. pulmonary vein isolation
Comparing either Maze or PVI lesion sets, as defined above, no difference was seen in mortality (P-0.41), stroke (P = 0.21), or hospital length of stay (P = 0.83). Improved freedom from AF was seen at 12 months in both Maze (RR = 2.82, 95%CI 2.15–3.68; P < 0.0001, I2 = 29%) and PVI (RR = 1.94, 95% CI 1.62–2.33; P < 0.0001, I2 = 24%); this difference between groups was significant for interaction (P = 0.02). Additionally, Maze procedures conferred a significantly increased risk of pacemaker requirement while PVI did not (RR 2.82, 95% CI 1.47–5.40; P = 0.002, I2 = 0% and RR 1.04, 95% CI 0.65–1.66; P = 0.86, I2 = 0%, P for interaction = 0.01, respectively).
Other subgroup analyses
Stratification based on concomitant left atrial appendage occlusion was performed and showed no significant difference in freedom from AF at 12 months, all-cause mortality, stroke, or pacemaker requirement. Several additional a priori hypotheses explaining heterogeneity specified in the study protocol15 were not pursued due to insufficient number of studies per subgroup. No studies of stand-alone AF ablation were identified.
Sensitivity analyses
Plausible worst-case scenario analysis did not alter the results for freedom from AF at 12 months, mortality, or pacemaker requirement at latest follow-up.
Quality of evidence
We reviewed the quality of evidence for each outcome, and downgraded the quality based on the GRADE framework. The quality of evidence for length of hospitalization and worsening New York Health Association (NYHA) classification was downgraded for risk of bias due to consistent limitations in patient and assessor blinding. Quality of evidence for freedom from AF assessments at 3, 6, and 12 months were downgraded for inconsistency, given their significant heterogeneity evidenced by elevated I2 values on meta-analysis and for the strong suspicion of publication bias as explained above. Results for mortality and stroke were downgraded for imprecision due to significantly widened confidence intervals indicating both benefit and harm. Full detailed assessment of quality of evidence for each outcome is presented in Table 2.
Discussion
In this systematic review and meta-analysis of RCTs, surgical AF ablation concomitant to cardiac surgery in patients with a known history of AF significantly increased freedom from AF postoperatively. However, the evidence as yet fails to demonstrate an impact on mortality, thrombo-embolism, and stroke (moderate quality evidence). The overall rate of PPM requirement did not differ significantly between ablation and no ablation. However, subgroup analysis suggests a significant increase in PPM implantation at latest follow-up in patients undergoing Maze and biatrial ablation; this was not observed with PVI and left-sided ablation. Hospital length of stay was significantly longer in patients undergoing concomitant ablation. The limited data on the quality of life so far suggest there may be an improvement in the physical functional domain of the SF-36 scale with surgical AF ablation.
Surgical AF ablation is often viewed as a minor concomitant procedure and perceived as an opportunity to provide additional benefit without increasing risk appreciably.7 Our meta-analysis corroborates the efficacy of surgical ablation in promoting sinus rhythm at 6 and 12 months. However, with more than a quarter of the studies using ECG only as the method of assessment for recurrence of AF, there is a significant concern that the increased freedom from AF may be significantly overestimated. The Discerning the Incidence of Symptomatic and Asymptomatic Episodes of Atrial Fibrillation Before and After Catheter Ablation (DISCERN AF) study examined rhythm assessment post-AF ablation and demonstrated that the intensity of monitoring is directly correlated with detection of AF.41 Perhaps more importantly, from the available RCTs in this field, clinical benefits beyond maintenance of sinus rhythm, such as survival and cerebrovascular accident, have not been demonstrated. Although there may be a temptation to infer that maintenance of sinus rhythm instead of AF must inherently be correlated with clinical benefits, this has not been corroborated in large trials on other populations.12,13,42,43 The ablation literature (catheter and surgical) consists of many trials not powered to detect important differences in outcomes such as mortality and stroke. Even in meta-analysing the surgical ablation RCTs, we underpowered to exclude an important effect on these patient-important outcomes. However, establishing this evidence should be paramount. The notion that surgical ablation is benign is challenged by the suggestion of a three-fold increase in pacemaker requirement with biatrial and Maze lesions. This finding has been explained as the unmasking of pre-existing sinus node dysfunction is these patients.44–46 However, the fact that the increased risk is seen with biatrial lesion sets and not in isolated pulmonary vein isolation, with only a minimal difference in efficacy of suppressing AF between these two approaches challenges this hypothesis.
Our results differ from those in the systematic review by Huffman et al.47 who suggested a significant increase in PPM requirement in the overall ablation population even before subgroup analysis. This article elected to use a fixed-effects model for analysis when I2 < 50%, which may explain this finding. The use of a fixed-effects model assumes that there is little heterogeneity between studies both quantitatively in their results and qualitatively in their methods and in the absence of small trial effects will commonly give narrower CIs for a given analysis.48–50 The studies combined in this article were methodologically heterogeneous, both in terms of lesion sets, lesion types, concomitant surgical procedures, postoperative antiarrhythmic therapy and in terms of means of assessing outcome. Thus, it stands to reason that the a priori choice to use a fixed-effects model was inappropriate and did not account for the significant inter-study methodological variability. Further, in review of their data sets, particularly for PPM requirement, the authors combined PPM requirement rates reported at hospital discharge with PPM requirement rates reported at latest follow-up, which our results suggest inappropriately overestimates the magnitude of relative risk increase.
This meta-analysis provides the most methodologically rigorous analysis of surgical AF ablation published to date. An a priori protocol was produced, screening and data collection were carried out independently and in duplicate, GRADE assessment was completed for all outcomes, and results are reported as per PRISMA guidelines.51 However, the large number of single-centre trials included may limit generalizability of findings, and surgeon expertise is an uncontrollable variable between trials with high impact on heterogeneity. The high rates of heterogeneity observed particularly in freedom from AF outcomes, not explained by subgroup analysis, may indicate the introduction of significant heterogeneity based on surgical technique, energy choice, postoperative antiarrhythmic management, or methods of sinus rhythm assessment. This may call to question the appropriateness of pooling these statistics; however, given that this heterogeneity reflects the state of current practice with regard to surgical AF ablation, we believe that the comparison is valid at this time.
Conclusion
Based on low-quality evidence, surgical ablation of AF during concomitant cardiac surgery results in increased freedom from AF through the 1st year postoperatively. The RCT evidence is as yet insufficient to detect any impact on patient-important outcomes such as mortality, thrombo-embolic events, or neurovascular events. Further, the evidence suggests an increased risk for pacemaker requirement during follow-up when using Maze or biatrial lesion sets but not isolated left-sided lesion sets. Maze and biatrial lesion sets did, however, show improved rates of freedom from AF. The findings of this meta-analysis highlight the need for the currently reported RCTs to report on long-term follow-up of their patients, which would generate precise effect estimates from the greater numbers of events.
Acknowledgements
The authors of this work would like to thank Zorbey Turkalp who assisted in document translation and Laura Banfield, the librarian consulted in search strategy preparation, for their help with this work.
Conflict of interest: I.H.J. reports grants from Bayer-CCS Cardiovascular Resident Prize, grants from Boehringer Ingelheim, non-financial support from LivaNova Canada, personal fees from Cryolife, outside the submitted work; J.H. reports grants from Medtronic, grants from Bristol-Meyers-Squibb/Pfizer, outside the submitted work; R.P.W. reports personal fees from Atricure, during the conduct of the study, personal fees from Armetheon, personal fees from Boehringer Ingelheim, outside the submitted work. All other authors reported no conflicts of interest.
References
9.5.2 Identifying and Measuring Heterogeneity. http://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm (17 September 2017, date last accessed).
9.5.4 Incorporating Heterogeneity into Random-Effects Models. http://handbook.cochrane.org/chapter_9/9_5_4_incorporating_heterogeneity_into_random_effects_models.htm (6 October 2016, date last accessed).
9.4.4.1 Mantel-Haenszel Methods. http://handbook.cochrane.org/chapter_9/9_4_4_1_mantel_haenszel_methods.htm (6 October 2016, date last accessed).
10.4.4.1 Comparing Fixed and Random-Effects Estimates. http://handbook.cochrane.org/chapter_10/10_4_4_1_comparing_fixed_and_random_effects_estimates.htm (6 October 2016, date last accessed).



