-
PDF
- Split View
-
Views
-
Cite
Cite
Loreto Bravo, Felipe Atienza, Gabriel Eidelman, Pablo Ávila, Mauricio Pelliza, Evaristo Castellanos, Gerard Loughlin, Tomás Datino, Esteban G Torrecilla, Jesús Almendral, Pedro Luis Sánchez, Ángel Arenal, Nieves Martínez-Alzamora, Francisco Fernández-Avilés, Safety and efficacy of cryoablation vs. radiofrequency ablation of septal accessory pathways: systematic review of the literature and meta-analyses, EP Europace, Volume 20, Issue 8, August 2018, Pages 1334–1342, https://doi.org/10.1093/europace/eux269
Close - Share Icon Share
Abstract
Radiofrequency ablation (RFA) of septal accessory pathways (APs) is associated with a significant rate of first procedure failures and complications. Cryoablation is an alternative energy source but there are no studies comparing both ablation techniques. We aimed to systematically review the literature and compare the efficacy and safety of cryoablation vs. RFA of septal APs.
We conducted two separate meta-analysis of cryoablation and RFA of septal APs and calculated the global estimates of the efficacy and safety. Sixty-four articles were included: 38 articles reporting RFA and 27 articles reporting cryoablation procedures. Additionally, we included the previously non-published cryoablation registry of septal APs performed at our institution. Overall, 4244 septal APs constitute our study population, 3495 in the RFA cohort and 749 in the cryoablation cohort. Acute procedural success rate of cryoablation was 86.0% (95% CI 81.6–89.4%) and RFA 89.0% (95% CI 86.8–91.0%). Recurrence rate of cryoablation was 18.1% (95% CI 14.8–21.8%) and RFA 9.9% (95% CI 8.2–12.0%). Long-term success rate after multiple ablation procedures of cryoablation was 75.9% (95% CI 68.2–82.3%) and RFA 88.4% (95% CI 84.7–91.3%). There were no reported cases of persistent atrioventricular block (AVB) with cryoablation and 2.7% (95% CI 2.2–3.4%) with RFA.
Studies of RFA for treatment of septal APs report higher efficacy rates than do studies using cryoablation, but a significantly higher rate of AVB.
This is the first meta-analyses that calculated the global estimates of the efficacy and safety of cryoablation and radiofrequency ablation (RFA) for septal accessory pathways (APs) elimination.
Radiofrequency ablation for treatment of septal AP report higher overall acute and long-term efficacy rates than do studies using cryoenergy, due to the higher recurrence rate after acutely successful cryoablation.
Cryoablation long-term efficacy outcomes for para-hisian APs are comparable with those achieved by RFA, while efficacy diminishes at other septal locations compared with RFA.
In contrast, RFA studies report a higher incidence of permanent atrioventricular block as compared with cryoablation studies.
Introduction
Radiofrequency catheter ablation (RFA) is the treatment of choice for eliminating accessory pathways (APs).1 However, RFA of septal APs is associated with higher rates of first procedure failures, complications, and recurrences as compared with other locations.2–4 Mid-septal and para-hisian APs ablation pose an increased risk of atrioventricular block (AVB) because of their close proximity to the normal AV conduction system, although occasionally AV block can also occur in other septal locations.5 On the other hand, the complex anatomy of the posteroseptal space reduces the efficacy of RFA and portends a risk of coronary injury. These issues are especially relevant when treating paediatric patients, in whom the dimensions of the triangle of Koch and overall tissue thickness are reduced.2,5 Consequently, a significant proportion of patients are left untreated because of safety concerns.
Cryoablation has evolved as a safe alternative to RFA in several arrhythmogenic substrates, and particularly in the septal region. That is so for several reasons related to the characteristics of the cryolesion: (i) the reversible nature related to both temperature and application duration; (ii) catheter stability due to catheter adherence during cryoablation delivery; and (iii) the formation of well-circumscribed discrete lesions with less collateral tissue damage.6–8 However, since the first reports in 2000 and across multicentre registries and single-centre studies, there is concern about recurrence rates of cryoablation, which seem to be higher than those of RFA.9–13
Although a significant number of studies have reported the efficacy and safety of catheter ablation of septal APs using one of these energy sources, most are either single-centre studies, retrospective case series, or registries. To date, there have been no large, prospective, randomized multicentre clinical trials comparing the relative efficacy and safety of these two ablation energy sources in this specific setting. Moreover, the feasibility of such a trial seems questionable. Here, we aim to provide accurate and broadly representative estimates of the relative efficacy and safety of these two treatment options; and to analyse their strengths, and limitations.
To address these objectives we systematically reviewed the available literature in order to compare the efficacy and safety of cryoablation vs. RFA of septal APs.
Methods
We conducted a literature search in MEDLINE guided by key terms as well as by the bibliographic references of retrieved papers. In addition, manual review of editorials, review articles, textbooks and guidelines was undertaken. Search terms: ‘catheter ablation’ OR ‘radiofrequency ablation’ OR ‘cryoablation’ ‘septal accessory pathways’ OR ‘anteroseptal accessory pathways’ OR ‘posteroseptal accessory pathways’ OR ‘mid-septal accessory pathways’ OR ‘para-hisian accessory pathways’ [MeSH and All Fields] and ‘cryoablation’ [MeSH]. The search was limited to publication date between January 1991 and April 2017. The language was not restricted to English. Data extraction was performed by two investigators (L.B., G.E.) and was independently verified by a third investigator (F.A.). Disagreements were resolved by consensus with the entire group.
Identified abstracts were selected if they made specific reference to RFA or cryoablation of accessory pathways. Articles retained from first abstract screening underwent full-text review to determine eligibility for data extraction based on the following inclusion criteria: original data in humans reported; study design consisting of a case series, case–control study, cohort study, or controlled trial; and absolute numbers for study endpoints available. Case reports, letters, or editorial comments and studies without extractable outcome data referring septal location of accessory pathways were excluded. Additionally, we conducted a pooled analysis by combining the results of the selected publications using cryoablation with the data from the non-published cryoablation registry of septal APs performed at our institution between 2002 and 2012 (excluding the cases reported in a previous publication).10
We classified the accessory pathways location according to the traditional terminology.14 The following information was obtained: institutional setting, number of patients, demographics [adult or paediatric population <18 years)], procedural data (duration, fluoroscopy time, ablation time, number of applications, catheter tip size), procedural complications, and acute and long-term efficacy outcome data. Efficacy outcomes were defined as acute procedural success, recurrence of AP conduction or tachycardia during follow-up, and long-term success after multiple procedures. Safety outcomes were chosen among the most frequently reported adverse events (i.e. AV block). Efficacy and safety outcomes were extracted as proportions, and exact binomial confidence intervals (CIs) were calculated.
Statistical analysis
We conducted two separate meta-analysis of RFA and cryoablation of septal APs and calculated the global estimates of the efficacy and safety of each ablation technique. We used the Q statistic proposed by DerSimonian and Laird to assess study heterogeneity, with a statistical significance level of 10%.15 When significant heterogeneity was present, we explore potential causes for heterogeneity through stratified analyses and meta-regression. We have considered random effects models with intra and inter-study variability to calculate pooled effects and afterwards the corresponding binomial Cis have been obtained. Additionally, we conducted a sensitivity analysis to assess the influence of each individual study on the overall result of the meta-analyses and performed cumulative analyses to evaluate the evolution of the combined pooled effect through the time with the addition of subsequent studies. Funnel plot, Duval and Tweedle’s trim and fill, and Egger tests were used to detect the presence of publication bias and assess its impact on the analysis.16
Calculations were done using the Comprehensive Meta-Analysis software, version 2 (Biostat, Englewood, NJ, USA) and SPSS software version 17.0.
Results
Of the 938 articles screened, 138 were selected for detailed review and 54 were finally retained for analysis: 38 articles reporting RFA between 1991 and 2017; and 27 articles reporting cryoablation between 2002 and 2017 (Figure 1). Overall, 4244 septal APs constitute the study population, 3495 in the RFA treatment cohort and 749 in the cryoablation cohort. The latter includes the prospective experience of our centre in cryoablation of septal APs, with 119 non-selected consecutive patients treated between 2002 and 2012. Tables 1 and 2 show the procedural and clinical characteristics of the selected studies.
Procedural and patients characteristics of radiofrequency ablation of septal accessory pathways studies
| RF studies . | Accessory pathways (number) . | Follow-up (months) . | Age (months) . | Complete AV block . | Other complications . |
|---|---|---|---|---|---|
| Jackman (1991) | 56 | 8 ± 5.4 | NA | 1 | 0 |
| Calkins (1992) | 59 | 10 ± 4 | NA | 2 | 0 |
| Kuck (1992) | 6 | 16.2 ± 2.9 | NA | 0 | 0 |
| Schlüter (1992) | 12 | 5.7 (0.5–27.8) | NA | 0 | 0 |
| Langberg (1992) | 26 | 4 ± 3 | 35 ± 15 | 1 | 0 |
| Swartz (1993) | 34 | 21.2 ± 4.6 | NA | 1 | 1 |
| Van Hare (1994) | 18 | 21.5(6–50) | 0.16–17 | 0 | 0 |
| Haissaguerre (1994) | 8 | 7 ± 6 | NA | 0 | 0 |
| Kugler (1994) | 206 | 13.5 | NA | 0 | 2a |
| Xie (1994) | 49 | 0 | 35 ± 17 | 1 | 1 |
| Yeh (1994) | 14 | 7 ± 3 | NA | 1 | 0 |
| Epstein (1994) | 2 | 22.1 ± 12.9 | 72.7 ± 2.8 | 0 | 1 |
| Schaffer (1996) | 414 | 9.7 ± 9.9 | 10.9 ± 5.1 | 16 | 0 |
| Tai (1996) | 46 | 26 ± 14 | 13–77 | 2 | 0 |
| Lorga (1996) | 15 | NA | 31 ± 16 | 0 | 0 |
| Brugada (1998) | 97 | 27 ± 14 | NA | 2 | 0 |
| Lin (1998) | 19 | 17 ± 8 | 33.4 (12–69) | 1 | 0 |
| Calkins (1999) | 138 | 6.3 | NA | 4 | 0 |
| Benito (1999) | 3 | 32.3 ± 22.1 | 0.5 ± 0.4 | 0 | 1 |
| Benito (II) (1999) | 4 | 30.9 ± 16.4 | 0.5–18 | 0 | 0 |
| Álvarez (2002) | 59 | NA | 35 ± 12 | NA | NA |
| Pecht (2002) | 96 | 31.5 | 10.5 | 0 | 0 |
| Mandapati (2003) | 130 | NA | NA | 4 | 0 |
| Kimman (2003) | 15 | NA | NA | 0 | 1 |
| Wang (2003) | 40 | 36 | 38 ± 12 | 2 | 0 |
| Gatzoulis (2004) | 17 | 43.2 ± 48 | 37 ± 10 | 1 | 0 |
| Van Hare (2004) | 545 | 12 | 0–16 | 13 | 0 |
| Kobza (2005) | 95 | NA | 39 ± 18 | 2 | 0 |
| Chang (2005) | 38 | NA | 47 ± 17 | 1 | 0 |
| Kimman (2006) | 3 | 12 | NA | 0 | 1 |
| Nielsen (2006) | 36 | NA | 15 (1–18) | 1 | 0 |
| Belhassen (2007) | 174 | 85 ± 43 | NA | 2 | 0 |
| Spanish Reg. (2010) | 542 | NA | 34 ± 14 | 2 | 10 (a1) |
| Fiala (2012) | 25 | 56 ± 27 | 35 ± 13 | 0 | 0 |
| Adao (2011) | 287 | NA | 37.2 ± 19.8 | 4 | 2 |
| Buddhe (2012) | 27 | 41 (1–77) | 13.4 ± 3.7 | 0 | 0 |
| Kubus (2014) | 78 | 13.7 (5.7–21.5) | 14.9 (12.6–16.5) | 1 | 0 |
| Backhoff (2016) | 62 | NA | 11.6 (7.9–14.2) | 0 | 0 |
| RF studies . | Accessory pathways (number) . | Follow-up (months) . | Age (months) . | Complete AV block . | Other complications . |
|---|---|---|---|---|---|
| Jackman (1991) | 56 | 8 ± 5.4 | NA | 1 | 0 |
| Calkins (1992) | 59 | 10 ± 4 | NA | 2 | 0 |
| Kuck (1992) | 6 | 16.2 ± 2.9 | NA | 0 | 0 |
| Schlüter (1992) | 12 | 5.7 (0.5–27.8) | NA | 0 | 0 |
| Langberg (1992) | 26 | 4 ± 3 | 35 ± 15 | 1 | 0 |
| Swartz (1993) | 34 | 21.2 ± 4.6 | NA | 1 | 1 |
| Van Hare (1994) | 18 | 21.5(6–50) | 0.16–17 | 0 | 0 |
| Haissaguerre (1994) | 8 | 7 ± 6 | NA | 0 | 0 |
| Kugler (1994) | 206 | 13.5 | NA | 0 | 2a |
| Xie (1994) | 49 | 0 | 35 ± 17 | 1 | 1 |
| Yeh (1994) | 14 | 7 ± 3 | NA | 1 | 0 |
| Epstein (1994) | 2 | 22.1 ± 12.9 | 72.7 ± 2.8 | 0 | 1 |
| Schaffer (1996) | 414 | 9.7 ± 9.9 | 10.9 ± 5.1 | 16 | 0 |
| Tai (1996) | 46 | 26 ± 14 | 13–77 | 2 | 0 |
| Lorga (1996) | 15 | NA | 31 ± 16 | 0 | 0 |
| Brugada (1998) | 97 | 27 ± 14 | NA | 2 | 0 |
| Lin (1998) | 19 | 17 ± 8 | 33.4 (12–69) | 1 | 0 |
| Calkins (1999) | 138 | 6.3 | NA | 4 | 0 |
| Benito (1999) | 3 | 32.3 ± 22.1 | 0.5 ± 0.4 | 0 | 1 |
| Benito (II) (1999) | 4 | 30.9 ± 16.4 | 0.5–18 | 0 | 0 |
| Álvarez (2002) | 59 | NA | 35 ± 12 | NA | NA |
| Pecht (2002) | 96 | 31.5 | 10.5 | 0 | 0 |
| Mandapati (2003) | 130 | NA | NA | 4 | 0 |
| Kimman (2003) | 15 | NA | NA | 0 | 1 |
| Wang (2003) | 40 | 36 | 38 ± 12 | 2 | 0 |
| Gatzoulis (2004) | 17 | 43.2 ± 48 | 37 ± 10 | 1 | 0 |
| Van Hare (2004) | 545 | 12 | 0–16 | 13 | 0 |
| Kobza (2005) | 95 | NA | 39 ± 18 | 2 | 0 |
| Chang (2005) | 38 | NA | 47 ± 17 | 1 | 0 |
| Kimman (2006) | 3 | 12 | NA | 0 | 1 |
| Nielsen (2006) | 36 | NA | 15 (1–18) | 1 | 0 |
| Belhassen (2007) | 174 | 85 ± 43 | NA | 2 | 0 |
| Spanish Reg. (2010) | 542 | NA | 34 ± 14 | 2 | 10 (a1) |
| Fiala (2012) | 25 | 56 ± 27 | 35 ± 13 | 0 | 0 |
| Adao (2011) | 287 | NA | 37.2 ± 19.8 | 4 | 2 |
| Buddhe (2012) | 27 | 41 (1–77) | 13.4 ± 3.7 | 0 | 0 |
| Kubus (2014) | 78 | 13.7 (5.7–21.5) | 14.9 (12.6–16.5) | 1 | 0 |
| Backhoff (2016) | 62 | NA | 11.6 (7.9–14.2) | 0 | 0 |
RF, radiofrequency; NA, not available data; AV, atrioventricular.
Periprocedural deaths.
Procedural and patients characteristics of radiofrequency ablation of septal accessory pathways studies
| RF studies . | Accessory pathways (number) . | Follow-up (months) . | Age (months) . | Complete AV block . | Other complications . |
|---|---|---|---|---|---|
| Jackman (1991) | 56 | 8 ± 5.4 | NA | 1 | 0 |
| Calkins (1992) | 59 | 10 ± 4 | NA | 2 | 0 |
| Kuck (1992) | 6 | 16.2 ± 2.9 | NA | 0 | 0 |
| Schlüter (1992) | 12 | 5.7 (0.5–27.8) | NA | 0 | 0 |
| Langberg (1992) | 26 | 4 ± 3 | 35 ± 15 | 1 | 0 |
| Swartz (1993) | 34 | 21.2 ± 4.6 | NA | 1 | 1 |
| Van Hare (1994) | 18 | 21.5(6–50) | 0.16–17 | 0 | 0 |
| Haissaguerre (1994) | 8 | 7 ± 6 | NA | 0 | 0 |
| Kugler (1994) | 206 | 13.5 | NA | 0 | 2a |
| Xie (1994) | 49 | 0 | 35 ± 17 | 1 | 1 |
| Yeh (1994) | 14 | 7 ± 3 | NA | 1 | 0 |
| Epstein (1994) | 2 | 22.1 ± 12.9 | 72.7 ± 2.8 | 0 | 1 |
| Schaffer (1996) | 414 | 9.7 ± 9.9 | 10.9 ± 5.1 | 16 | 0 |
| Tai (1996) | 46 | 26 ± 14 | 13–77 | 2 | 0 |
| Lorga (1996) | 15 | NA | 31 ± 16 | 0 | 0 |
| Brugada (1998) | 97 | 27 ± 14 | NA | 2 | 0 |
| Lin (1998) | 19 | 17 ± 8 | 33.4 (12–69) | 1 | 0 |
| Calkins (1999) | 138 | 6.3 | NA | 4 | 0 |
| Benito (1999) | 3 | 32.3 ± 22.1 | 0.5 ± 0.4 | 0 | 1 |
| Benito (II) (1999) | 4 | 30.9 ± 16.4 | 0.5–18 | 0 | 0 |
| Álvarez (2002) | 59 | NA | 35 ± 12 | NA | NA |
| Pecht (2002) | 96 | 31.5 | 10.5 | 0 | 0 |
| Mandapati (2003) | 130 | NA | NA | 4 | 0 |
| Kimman (2003) | 15 | NA | NA | 0 | 1 |
| Wang (2003) | 40 | 36 | 38 ± 12 | 2 | 0 |
| Gatzoulis (2004) | 17 | 43.2 ± 48 | 37 ± 10 | 1 | 0 |
| Van Hare (2004) | 545 | 12 | 0–16 | 13 | 0 |
| Kobza (2005) | 95 | NA | 39 ± 18 | 2 | 0 |
| Chang (2005) | 38 | NA | 47 ± 17 | 1 | 0 |
| Kimman (2006) | 3 | 12 | NA | 0 | 1 |
| Nielsen (2006) | 36 | NA | 15 (1–18) | 1 | 0 |
| Belhassen (2007) | 174 | 85 ± 43 | NA | 2 | 0 |
| Spanish Reg. (2010) | 542 | NA | 34 ± 14 | 2 | 10 (a1) |
| Fiala (2012) | 25 | 56 ± 27 | 35 ± 13 | 0 | 0 |
| Adao (2011) | 287 | NA | 37.2 ± 19.8 | 4 | 2 |
| Buddhe (2012) | 27 | 41 (1–77) | 13.4 ± 3.7 | 0 | 0 |
| Kubus (2014) | 78 | 13.7 (5.7–21.5) | 14.9 (12.6–16.5) | 1 | 0 |
| Backhoff (2016) | 62 | NA | 11.6 (7.9–14.2) | 0 | 0 |
| RF studies . | Accessory pathways (number) . | Follow-up (months) . | Age (months) . | Complete AV block . | Other complications . |
|---|---|---|---|---|---|
| Jackman (1991) | 56 | 8 ± 5.4 | NA | 1 | 0 |
| Calkins (1992) | 59 | 10 ± 4 | NA | 2 | 0 |
| Kuck (1992) | 6 | 16.2 ± 2.9 | NA | 0 | 0 |
| Schlüter (1992) | 12 | 5.7 (0.5–27.8) | NA | 0 | 0 |
| Langberg (1992) | 26 | 4 ± 3 | 35 ± 15 | 1 | 0 |
| Swartz (1993) | 34 | 21.2 ± 4.6 | NA | 1 | 1 |
| Van Hare (1994) | 18 | 21.5(6–50) | 0.16–17 | 0 | 0 |
| Haissaguerre (1994) | 8 | 7 ± 6 | NA | 0 | 0 |
| Kugler (1994) | 206 | 13.5 | NA | 0 | 2a |
| Xie (1994) | 49 | 0 | 35 ± 17 | 1 | 1 |
| Yeh (1994) | 14 | 7 ± 3 | NA | 1 | 0 |
| Epstein (1994) | 2 | 22.1 ± 12.9 | 72.7 ± 2.8 | 0 | 1 |
| Schaffer (1996) | 414 | 9.7 ± 9.9 | 10.9 ± 5.1 | 16 | 0 |
| Tai (1996) | 46 | 26 ± 14 | 13–77 | 2 | 0 |
| Lorga (1996) | 15 | NA | 31 ± 16 | 0 | 0 |
| Brugada (1998) | 97 | 27 ± 14 | NA | 2 | 0 |
| Lin (1998) | 19 | 17 ± 8 | 33.4 (12–69) | 1 | 0 |
| Calkins (1999) | 138 | 6.3 | NA | 4 | 0 |
| Benito (1999) | 3 | 32.3 ± 22.1 | 0.5 ± 0.4 | 0 | 1 |
| Benito (II) (1999) | 4 | 30.9 ± 16.4 | 0.5–18 | 0 | 0 |
| Álvarez (2002) | 59 | NA | 35 ± 12 | NA | NA |
| Pecht (2002) | 96 | 31.5 | 10.5 | 0 | 0 |
| Mandapati (2003) | 130 | NA | NA | 4 | 0 |
| Kimman (2003) | 15 | NA | NA | 0 | 1 |
| Wang (2003) | 40 | 36 | 38 ± 12 | 2 | 0 |
| Gatzoulis (2004) | 17 | 43.2 ± 48 | 37 ± 10 | 1 | 0 |
| Van Hare (2004) | 545 | 12 | 0–16 | 13 | 0 |
| Kobza (2005) | 95 | NA | 39 ± 18 | 2 | 0 |
| Chang (2005) | 38 | NA | 47 ± 17 | 1 | 0 |
| Kimman (2006) | 3 | 12 | NA | 0 | 1 |
| Nielsen (2006) | 36 | NA | 15 (1–18) | 1 | 0 |
| Belhassen (2007) | 174 | 85 ± 43 | NA | 2 | 0 |
| Spanish Reg. (2010) | 542 | NA | 34 ± 14 | 2 | 10 (a1) |
| Fiala (2012) | 25 | 56 ± 27 | 35 ± 13 | 0 | 0 |
| Adao (2011) | 287 | NA | 37.2 ± 19.8 | 4 | 2 |
| Buddhe (2012) | 27 | 41 (1–77) | 13.4 ± 3.7 | 0 | 0 |
| Kubus (2014) | 78 | 13.7 (5.7–21.5) | 14.9 (12.6–16.5) | 1 | 0 |
| Backhoff (2016) | 62 | NA | 11.6 (7.9–14.2) | 0 | 0 |
RF, radiofrequency; NA, not available data; AV, atrioventricular.
Periprocedural deaths.
Procedural and patients characteristics of cryoablation of septal accessory pathways studies studies
| Cryoablation studies . | APs location (number) . | Follow-up (months) . | Age (years) . | Tip catheter (mm) . | Applications (number) . | Duration of cryoapplications (s) . | Temp (°C) . | Procedure duration (h) . |
|---|---|---|---|---|---|---|---|---|
| Hospital Gregorio Marañón Registry (2002–2012) | 119 | 11.8 (0.4–60) | 25.8 ± 12.6 (4–62) | 4, 6 | 4.2 ± 4 (1–18) | 603 ± 459 (20–2324) | −75 | 2.44 ± 1.15 (0.5–6.1) |
| Lanzotti (2002) | 7 | NA | 38 | 4 | 1 | 180 (120–240) | −75 | NA |
| Kimman (2003) | 7 | NA | NA | NA | 2 (1–21) | 240 | −70 | NA |
| Gaita (2003) | 20 | 15 ± 6 | NA | 4 | 1.2 ± 0.4 | 240 | −75 | NA |
| Lowe (2003) | 8 | 3 | 42 ± 12 | 4, 6 | 3.1 ± 1.9 | 240 | −80 | 1.5 ± 0.5 |
| Atienza (2004) | 22 | 9.5 (1–14) | 28 ± 12 (11–55) | 4 | NA | 240 | −70 | 3 ± 1 |
| Friedman (2004) | 25 | 6 | 39 ± 13 | 4 | NA | 240 | −70 | 4.3 ± 1.9 |
| Wong (2004) | 2 | NA | NA | 4 | 3.2 ± 3.9 | NA | −70 | NA |
| Drago (2005) | 13 | 1–22 | 13.2 ± 3.6 | 4 | 3 ± 2 | 360 ± 120 (120–480) | −75 | 4 ± 1.1 |
| Kirsch (2005) | 16 | 3 | 13 ± 4 | 4, 6 | 2.9 ± 3.3 | 240 | −75 | 3.5 ± 1.1 |
| Kriebel (2005) | 6 | 8.9 (1–15) | 10.1 ± 3.5 | 4, 6 | 2 (1–10) | 240 | −70 | 4 ± 1 |
| Miyazaki (2005) | 8 | 8.2 (0.8–14.4) | 11.1 ± 2.9 | 4, 6 | 2.8 ± 1.3 | 120–480 | −70 | NA |
| Drago (2006v | 22 | 13 (6–30) | 12 ± 3 (5–20) | 4 | NA | 240–480 | −75 | 1.6 ± 0.9 |
| Bar−Cohen (2006) | 37 | 6.9 (0–23.1) | 15.6 (4.3–40.9) | 4, 6 | NA | 240 | −75 | NA |
| Papez (2006) | 13 | 8.1 ± 7 (2–30) | 13.4 ± 3.9 (4–21) | 4, 6 | NA | NA | −70 | 4.5 |
| Kimman (2006) | 5 | 12 | NA | 4 | 3 (1–8) | 240 | −70 | NA |
| Gaita (2006) | 39 | 27 ± 13 | 36 ± 17 | 4 | 1.2 ± 0.8 | 240 | −75 | NA |
| Drago (2009) | 27 | 20 (4–25) | 12.6 ± 2.9 | 4 | 2.5 ± 1 | 240–480 | −75 | NA |
| Bastani (2010) | 27 | 36 | 29 (15–65) | 6 | NA | 240 | −80 | 2.7 |
| Buddhe (2012) | 3 | 41 (1–77) | 13.4 ± 3.7 | 4 | NA | 240 | −70 | NA |
| Ergul (2013) | 24 | 14.2 ± 7 | 11.9 ± 4.3 | 6, 8 | NA | NA | NA | 2.8 ± 1 |
| Yildirim (2013) | 25 | 17.5 (6–34) | 13 (8–18) | 6 | 4 (3–6) | 240–300 | −80 | 2 (1–5) |
| Drago (2013) | 50 | 18.6 ± 6.6 | 12 ± 3(5–19) | 4, 6 | NA | 240–480 | −75 | NA |
| Insulander (2014) | 100 | 24 (6–96) | 52 (4–65) | 6 | NA | 240 | −80 | 3 ± 0.7 |
| Liberman (2014) | 6 | 13 (3–37) | 15.9 (7.2–18.2) | 4, 6, 8 | NA | NA | −70 | NA |
| Karadeniz (2014) | 43 | 8.8 ± 4.8 | 13.2 ± 5.5 | 6, 8 | 6.1 ± 3.1 | 240–360 | −75 | 3 ± 1 |
| Swissa (2015) | 50 | 59.7 (6–102) | 16.5 (5.3–20) | 6 | 2.6 ± 1.2 | 240 | −80 | NA |
| Tanidir (2016) | 25 | 32 ± 15 | 13.1 ± 3.7 | 8 | 4.5 (1–20) | 240–360 | −75 | 3.2 ± 1 |
| Cryoablation studies . | APs location (number) . | Follow-up (months) . | Age (years) . | Tip catheter (mm) . | Applications (number) . | Duration of cryoapplications (s) . | Temp (°C) . | Procedure duration (h) . |
|---|---|---|---|---|---|---|---|---|
| Hospital Gregorio Marañón Registry (2002–2012) | 119 | 11.8 (0.4–60) | 25.8 ± 12.6 (4–62) | 4, 6 | 4.2 ± 4 (1–18) | 603 ± 459 (20–2324) | −75 | 2.44 ± 1.15 (0.5–6.1) |
| Lanzotti (2002) | 7 | NA | 38 | 4 | 1 | 180 (120–240) | −75 | NA |
| Kimman (2003) | 7 | NA | NA | NA | 2 (1–21) | 240 | −70 | NA |
| Gaita (2003) | 20 | 15 ± 6 | NA | 4 | 1.2 ± 0.4 | 240 | −75 | NA |
| Lowe (2003) | 8 | 3 | 42 ± 12 | 4, 6 | 3.1 ± 1.9 | 240 | −80 | 1.5 ± 0.5 |
| Atienza (2004) | 22 | 9.5 (1–14) | 28 ± 12 (11–55) | 4 | NA | 240 | −70 | 3 ± 1 |
| Friedman (2004) | 25 | 6 | 39 ± 13 | 4 | NA | 240 | −70 | 4.3 ± 1.9 |
| Wong (2004) | 2 | NA | NA | 4 | 3.2 ± 3.9 | NA | −70 | NA |
| Drago (2005) | 13 | 1–22 | 13.2 ± 3.6 | 4 | 3 ± 2 | 360 ± 120 (120–480) | −75 | 4 ± 1.1 |
| Kirsch (2005) | 16 | 3 | 13 ± 4 | 4, 6 | 2.9 ± 3.3 | 240 | −75 | 3.5 ± 1.1 |
| Kriebel (2005) | 6 | 8.9 (1–15) | 10.1 ± 3.5 | 4, 6 | 2 (1–10) | 240 | −70 | 4 ± 1 |
| Miyazaki (2005) | 8 | 8.2 (0.8–14.4) | 11.1 ± 2.9 | 4, 6 | 2.8 ± 1.3 | 120–480 | −70 | NA |
| Drago (2006v | 22 | 13 (6–30) | 12 ± 3 (5–20) | 4 | NA | 240–480 | −75 | 1.6 ± 0.9 |
| Bar−Cohen (2006) | 37 | 6.9 (0–23.1) | 15.6 (4.3–40.9) | 4, 6 | NA | 240 | −75 | NA |
| Papez (2006) | 13 | 8.1 ± 7 (2–30) | 13.4 ± 3.9 (4–21) | 4, 6 | NA | NA | −70 | 4.5 |
| Kimman (2006) | 5 | 12 | NA | 4 | 3 (1–8) | 240 | −70 | NA |
| Gaita (2006) | 39 | 27 ± 13 | 36 ± 17 | 4 | 1.2 ± 0.8 | 240 | −75 | NA |
| Drago (2009) | 27 | 20 (4–25) | 12.6 ± 2.9 | 4 | 2.5 ± 1 | 240–480 | −75 | NA |
| Bastani (2010) | 27 | 36 | 29 (15–65) | 6 | NA | 240 | −80 | 2.7 |
| Buddhe (2012) | 3 | 41 (1–77) | 13.4 ± 3.7 | 4 | NA | 240 | −70 | NA |
| Ergul (2013) | 24 | 14.2 ± 7 | 11.9 ± 4.3 | 6, 8 | NA | NA | NA | 2.8 ± 1 |
| Yildirim (2013) | 25 | 17.5 (6–34) | 13 (8–18) | 6 | 4 (3–6) | 240–300 | −80 | 2 (1–5) |
| Drago (2013) | 50 | 18.6 ± 6.6 | 12 ± 3(5–19) | 4, 6 | NA | 240–480 | −75 | NA |
| Insulander (2014) | 100 | 24 (6–96) | 52 (4–65) | 6 | NA | 240 | −80 | 3 ± 0.7 |
| Liberman (2014) | 6 | 13 (3–37) | 15.9 (7.2–18.2) | 4, 6, 8 | NA | NA | −70 | NA |
| Karadeniz (2014) | 43 | 8.8 ± 4.8 | 13.2 ± 5.5 | 6, 8 | 6.1 ± 3.1 | 240–360 | −75 | 3 ± 1 |
| Swissa (2015) | 50 | 59.7 (6–102) | 16.5 (5.3–20) | 6 | 2.6 ± 1.2 | 240 | −80 | NA |
| Tanidir (2016) | 25 | 32 ± 15 | 13.1 ± 3.7 | 8 | 4.5 (1–20) | 240–360 | −75 | 3.2 ± 1 |
APs, accessory pathways; NA, not available data; TEMP, temperature.
Procedural and patients characteristics of cryoablation of septal accessory pathways studies studies
| Cryoablation studies . | APs location (number) . | Follow-up (months) . | Age (years) . | Tip catheter (mm) . | Applications (number) . | Duration of cryoapplications (s) . | Temp (°C) . | Procedure duration (h) . |
|---|---|---|---|---|---|---|---|---|
| Hospital Gregorio Marañón Registry (2002–2012) | 119 | 11.8 (0.4–60) | 25.8 ± 12.6 (4–62) | 4, 6 | 4.2 ± 4 (1–18) | 603 ± 459 (20–2324) | −75 | 2.44 ± 1.15 (0.5–6.1) |
| Lanzotti (2002) | 7 | NA | 38 | 4 | 1 | 180 (120–240) | −75 | NA |
| Kimman (2003) | 7 | NA | NA | NA | 2 (1–21) | 240 | −70 | NA |
| Gaita (2003) | 20 | 15 ± 6 | NA | 4 | 1.2 ± 0.4 | 240 | −75 | NA |
| Lowe (2003) | 8 | 3 | 42 ± 12 | 4, 6 | 3.1 ± 1.9 | 240 | −80 | 1.5 ± 0.5 |
| Atienza (2004) | 22 | 9.5 (1–14) | 28 ± 12 (11–55) | 4 | NA | 240 | −70 | 3 ± 1 |
| Friedman (2004) | 25 | 6 | 39 ± 13 | 4 | NA | 240 | −70 | 4.3 ± 1.9 |
| Wong (2004) | 2 | NA | NA | 4 | 3.2 ± 3.9 | NA | −70 | NA |
| Drago (2005) | 13 | 1–22 | 13.2 ± 3.6 | 4 | 3 ± 2 | 360 ± 120 (120–480) | −75 | 4 ± 1.1 |
| Kirsch (2005) | 16 | 3 | 13 ± 4 | 4, 6 | 2.9 ± 3.3 | 240 | −75 | 3.5 ± 1.1 |
| Kriebel (2005) | 6 | 8.9 (1–15) | 10.1 ± 3.5 | 4, 6 | 2 (1–10) | 240 | −70 | 4 ± 1 |
| Miyazaki (2005) | 8 | 8.2 (0.8–14.4) | 11.1 ± 2.9 | 4, 6 | 2.8 ± 1.3 | 120–480 | −70 | NA |
| Drago (2006v | 22 | 13 (6–30) | 12 ± 3 (5–20) | 4 | NA | 240–480 | −75 | 1.6 ± 0.9 |
| Bar−Cohen (2006) | 37 | 6.9 (0–23.1) | 15.6 (4.3–40.9) | 4, 6 | NA | 240 | −75 | NA |
| Papez (2006) | 13 | 8.1 ± 7 (2–30) | 13.4 ± 3.9 (4–21) | 4, 6 | NA | NA | −70 | 4.5 |
| Kimman (2006) | 5 | 12 | NA | 4 | 3 (1–8) | 240 | −70 | NA |
| Gaita (2006) | 39 | 27 ± 13 | 36 ± 17 | 4 | 1.2 ± 0.8 | 240 | −75 | NA |
| Drago (2009) | 27 | 20 (4–25) | 12.6 ± 2.9 | 4 | 2.5 ± 1 | 240–480 | −75 | NA |
| Bastani (2010) | 27 | 36 | 29 (15–65) | 6 | NA | 240 | −80 | 2.7 |
| Buddhe (2012) | 3 | 41 (1–77) | 13.4 ± 3.7 | 4 | NA | 240 | −70 | NA |
| Ergul (2013) | 24 | 14.2 ± 7 | 11.9 ± 4.3 | 6, 8 | NA | NA | NA | 2.8 ± 1 |
| Yildirim (2013) | 25 | 17.5 (6–34) | 13 (8–18) | 6 | 4 (3–6) | 240–300 | −80 | 2 (1–5) |
| Drago (2013) | 50 | 18.6 ± 6.6 | 12 ± 3(5–19) | 4, 6 | NA | 240–480 | −75 | NA |
| Insulander (2014) | 100 | 24 (6–96) | 52 (4–65) | 6 | NA | 240 | −80 | 3 ± 0.7 |
| Liberman (2014) | 6 | 13 (3–37) | 15.9 (7.2–18.2) | 4, 6, 8 | NA | NA | −70 | NA |
| Karadeniz (2014) | 43 | 8.8 ± 4.8 | 13.2 ± 5.5 | 6, 8 | 6.1 ± 3.1 | 240–360 | −75 | 3 ± 1 |
| Swissa (2015) | 50 | 59.7 (6–102) | 16.5 (5.3–20) | 6 | 2.6 ± 1.2 | 240 | −80 | NA |
| Tanidir (2016) | 25 | 32 ± 15 | 13.1 ± 3.7 | 8 | 4.5 (1–20) | 240–360 | −75 | 3.2 ± 1 |
| Cryoablation studies . | APs location (number) . | Follow-up (months) . | Age (years) . | Tip catheter (mm) . | Applications (number) . | Duration of cryoapplications (s) . | Temp (°C) . | Procedure duration (h) . |
|---|---|---|---|---|---|---|---|---|
| Hospital Gregorio Marañón Registry (2002–2012) | 119 | 11.8 (0.4–60) | 25.8 ± 12.6 (4–62) | 4, 6 | 4.2 ± 4 (1–18) | 603 ± 459 (20–2324) | −75 | 2.44 ± 1.15 (0.5–6.1) |
| Lanzotti (2002) | 7 | NA | 38 | 4 | 1 | 180 (120–240) | −75 | NA |
| Kimman (2003) | 7 | NA | NA | NA | 2 (1–21) | 240 | −70 | NA |
| Gaita (2003) | 20 | 15 ± 6 | NA | 4 | 1.2 ± 0.4 | 240 | −75 | NA |
| Lowe (2003) | 8 | 3 | 42 ± 12 | 4, 6 | 3.1 ± 1.9 | 240 | −80 | 1.5 ± 0.5 |
| Atienza (2004) | 22 | 9.5 (1–14) | 28 ± 12 (11–55) | 4 | NA | 240 | −70 | 3 ± 1 |
| Friedman (2004) | 25 | 6 | 39 ± 13 | 4 | NA | 240 | −70 | 4.3 ± 1.9 |
| Wong (2004) | 2 | NA | NA | 4 | 3.2 ± 3.9 | NA | −70 | NA |
| Drago (2005) | 13 | 1–22 | 13.2 ± 3.6 | 4 | 3 ± 2 | 360 ± 120 (120–480) | −75 | 4 ± 1.1 |
| Kirsch (2005) | 16 | 3 | 13 ± 4 | 4, 6 | 2.9 ± 3.3 | 240 | −75 | 3.5 ± 1.1 |
| Kriebel (2005) | 6 | 8.9 (1–15) | 10.1 ± 3.5 | 4, 6 | 2 (1–10) | 240 | −70 | 4 ± 1 |
| Miyazaki (2005) | 8 | 8.2 (0.8–14.4) | 11.1 ± 2.9 | 4, 6 | 2.8 ± 1.3 | 120–480 | −70 | NA |
| Drago (2006v | 22 | 13 (6–30) | 12 ± 3 (5–20) | 4 | NA | 240–480 | −75 | 1.6 ± 0.9 |
| Bar−Cohen (2006) | 37 | 6.9 (0–23.1) | 15.6 (4.3–40.9) | 4, 6 | NA | 240 | −75 | NA |
| Papez (2006) | 13 | 8.1 ± 7 (2–30) | 13.4 ± 3.9 (4–21) | 4, 6 | NA | NA | −70 | 4.5 |
| Kimman (2006) | 5 | 12 | NA | 4 | 3 (1–8) | 240 | −70 | NA |
| Gaita (2006) | 39 | 27 ± 13 | 36 ± 17 | 4 | 1.2 ± 0.8 | 240 | −75 | NA |
| Drago (2009) | 27 | 20 (4–25) | 12.6 ± 2.9 | 4 | 2.5 ± 1 | 240–480 | −75 | NA |
| Bastani (2010) | 27 | 36 | 29 (15–65) | 6 | NA | 240 | −80 | 2.7 |
| Buddhe (2012) | 3 | 41 (1–77) | 13.4 ± 3.7 | 4 | NA | 240 | −70 | NA |
| Ergul (2013) | 24 | 14.2 ± 7 | 11.9 ± 4.3 | 6, 8 | NA | NA | NA | 2.8 ± 1 |
| Yildirim (2013) | 25 | 17.5 (6–34) | 13 (8–18) | 6 | 4 (3–6) | 240–300 | −80 | 2 (1–5) |
| Drago (2013) | 50 | 18.6 ± 6.6 | 12 ± 3(5–19) | 4, 6 | NA | 240–480 | −75 | NA |
| Insulander (2014) | 100 | 24 (6–96) | 52 (4–65) | 6 | NA | 240 | −80 | 3 ± 0.7 |
| Liberman (2014) | 6 | 13 (3–37) | 15.9 (7.2–18.2) | 4, 6, 8 | NA | NA | −70 | NA |
| Karadeniz (2014) | 43 | 8.8 ± 4.8 | 13.2 ± 5.5 | 6, 8 | 6.1 ± 3.1 | 240–360 | −75 | 3 ± 1 |
| Swissa (2015) | 50 | 59.7 (6–102) | 16.5 (5.3–20) | 6 | 2.6 ± 1.2 | 240 | −80 | NA |
| Tanidir (2016) | 25 | 32 ± 15 | 13.1 ± 3.7 | 8 | 4.5 (1–20) | 240–360 | −75 | 3.2 ± 1 |
APs, accessory pathways; NA, not available data; TEMP, temperature.

Selection flowchart of the studies included in the meta-analyses. aOne study comprises cryoablation and radiofrequency-treated patients. bThe final analysis comprises 28 cryoablation studies, since we include our Centre previously unpublished cryoablation registry (see Table 2).
Overall, 26% RFA and 71% cryoablation studies included totally or partially paediatric patients. The predominant comorbidity among paediatric patients was congenital heart disease. Four-millimetre catheter tip size was used in half of the cryoablation studies, whereas the remaining used either 4 or 6 mm. Other procedural characteristics such as cryoablation applications duration, number of cryoapplications or procedural duration were inconsistently reported, precluding meaningful stratification of outcomes by procedural characteristics.
Efficacy outcomes of ablation of septal accessory pathways
As shown in Figure 2, 28 studies reported the procedural success of cryoablation and 33 studies did for RFA. Acute procedural success achieved with cryoablation was 86.0% (95% CI 81.4–89.4%) and RFA 89.0% (95% CI 86.8–91.0%). Twenty-eight cryoablation studies and 29 RFA studies reported the recurrence rate after the first ablation procedure. The recurrence rate for the cryoablation group was 18.1% (95% CI 14.8–21.8%) and RFA 9.9% (95% CI 8.2–12.0%). Eligible data regarding freedom from long-term recurrent AP conduction were available in 27 cryoablation studies and 27 RFA, after an average follow-up time of 13.5 (0–60) and 37.5 (0–50) months, respectively. Long-term success after multiple or uncertain number of procedures achieved with cryoablation was 75.9% (95% CI 68.2–82.3%) and RFA 88.4% (95% CI 84.7–91.3%) (Figure 3). These data include the efficacy of repeated procedures, which was similar to the acute success rate when reported (only five cryoablation studies and four RFA provided acute outcomes of redo procedures).
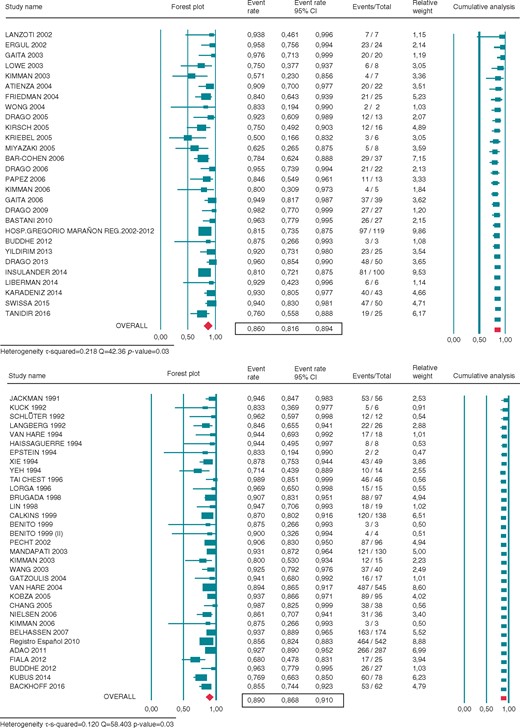
Forest plot showing the individual and pooled rate of acute procedural success of cryoenergy (CRYO) (upper panel) and radiofrequency (RF) (lower panel) for septal APs ablation. Square boxes denote event rate; horizontal lines represent 95% confidence interval (CI).
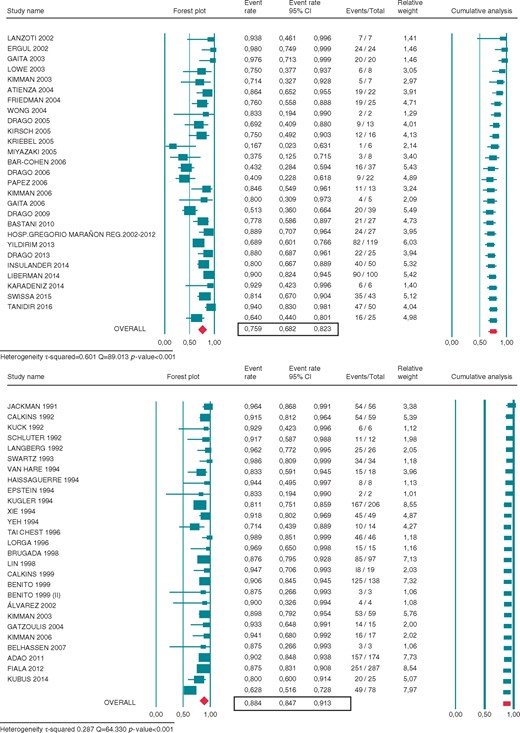
Forest plot showing the individual and pooled rate of long-term efficacy of cryoenergy (CRYO) (upper panel) and radiofrequency (RF) (lower panel) for septal APs ablation. Square boxes denote event rate; horizontal lines represent 95% confidence interval (CI).
Safety outcomes
Nineteen cryoablation studies and 34 RFA studies reported adverse events rates, the most relevant being persistent AV block (Tables 1 and 2). No case of persistent AV block was reported using cryoablation, whereas RFA accounts for a 2.7% rate (95% CI 2.2–3.4%).
Other procedural complications, mainly derived from vascular access and pericardial effusion, were extremely rare in both groups. Three deaths are reported in the RFA cohort, two of them affecting patients with underlying congenital heart conditions. No deaths have been reported in the cryoablation group.
Efficacy and safety outcomes of ablation of septal accessory pathways according to accessory pathway location
Table 3 shows the results of septal AP ablation according to the specific location of the AP and the energy source. Cryoablation acute procedure success rate was highest for para-hisian APs (90.8 [85.1–94.5]), with a slight to moderately lower efficacy for mid-septal (87.4% [79–92.3%]) and anteroseptal APs (80.2% [68.9–88.1%]), and a lowest acute efficacy rate for posteroseptal APs (70.8% [59.7–79.9%]) location. Recurrence rate of cryoablation for all different septal locations ranged 21–22%. Long-term efficacy rate after multiple or uncertain number of procedures was highest for cryoablation of para-hisian APs (85.9% [76.9–91.8%]), with a decrease for mid-septal and anteroseptal locations, and a lowest long-term efficacy rate for posteroseptal APs locations (57.4% [44.3–69.5%]). There were no reported cases of persistent AV block during the cryoablation procedure for any AP septal location.
Septal accessory pathways ablation results according to the specific location and the energy source
| . | Ablation energy source . | |
|---|---|---|
| . | cryoablation . | radiofrequency . |
| Para-hisian APs | ||
| Acute procedural success | 90.8 (85.1–94.5) | 80.5 (59.4–92.1) |
| Recurrence rate | 21.1 (15.8–27.7) | 7.1 (3.2–15.0) |
| Long-term efficacy | 85.9 (76.9–91.8) | 83.1 (72.1–90.3) |
| Persistent AV block | 0 | 5.4 (2.2–12.9) |
| Mid-septal APs | ||
| Acute procedural success | 87.4 (79.0–92.3) | 90.1 (83.5–94.3) |
| Recurrence rate | 21.4 (15.2–29.2) | 11.5 (7.5–17.3) |
| Long-term efficacy | 76.3 (60.5–87.1) | 87.0 (73.6–94.1) |
| Persistent AV Block | 0 | 7.2 (4.9–10.5) |
| Anteroseptal APs | ||
| Acute procedural success | 80.2 (68.9–88.1) | 91.1 (86.6–94.2) |
| Recurrence rate | 21.0 (15.0–28.5) | 13.3 (9.3–18.9) |
| Long-term efficacy | 73.6 (60.5–83.5) | 84.0 (76.5–89.5) |
| Persistent AV block | 0 | 5.5 (3.6–8.3) |
| Posteroseptal APs | ||
| Acute procedural success | 70.8 (59.7–79.9) | 90.4 (87.4–92.7) |
| Recurrence rate | 22.3 (14.0–33.7) | 8.9 (6.9–11.4) |
| Long-term efficacy | 57.4 (44.3–69.5) | 88.7 (86.1–90.9) |
| Persistent AV block | 0 | 2.2 (1.5–3.3) |
| . | Ablation energy source . | |
|---|---|---|
| . | cryoablation . | radiofrequency . |
| Para-hisian APs | ||
| Acute procedural success | 90.8 (85.1–94.5) | 80.5 (59.4–92.1) |
| Recurrence rate | 21.1 (15.8–27.7) | 7.1 (3.2–15.0) |
| Long-term efficacy | 85.9 (76.9–91.8) | 83.1 (72.1–90.3) |
| Persistent AV block | 0 | 5.4 (2.2–12.9) |
| Mid-septal APs | ||
| Acute procedural success | 87.4 (79.0–92.3) | 90.1 (83.5–94.3) |
| Recurrence rate | 21.4 (15.2–29.2) | 11.5 (7.5–17.3) |
| Long-term efficacy | 76.3 (60.5–87.1) | 87.0 (73.6–94.1) |
| Persistent AV Block | 0 | 7.2 (4.9–10.5) |
| Anteroseptal APs | ||
| Acute procedural success | 80.2 (68.9–88.1) | 91.1 (86.6–94.2) |
| Recurrence rate | 21.0 (15.0–28.5) | 13.3 (9.3–18.9) |
| Long-term efficacy | 73.6 (60.5–83.5) | 84.0 (76.5–89.5) |
| Persistent AV block | 0 | 5.5 (3.6–8.3) |
| Posteroseptal APs | ||
| Acute procedural success | 70.8 (59.7–79.9) | 90.4 (87.4–92.7) |
| Recurrence rate | 22.3 (14.0–33.7) | 8.9 (6.9–11.4) |
| Long-term efficacy | 57.4 (44.3–69.5) | 88.7 (86.1–90.9) |
| Persistent AV block | 0 | 2.2 (1.5–3.3) |
Values expressed in %.
APs, accessory pathways; AV, atrioventricular.
Confidence interval: 95%.
Septal accessory pathways ablation results according to the specific location and the energy source
| . | Ablation energy source . | |
|---|---|---|
| . | cryoablation . | radiofrequency . |
| Para-hisian APs | ||
| Acute procedural success | 90.8 (85.1–94.5) | 80.5 (59.4–92.1) |
| Recurrence rate | 21.1 (15.8–27.7) | 7.1 (3.2–15.0) |
| Long-term efficacy | 85.9 (76.9–91.8) | 83.1 (72.1–90.3) |
| Persistent AV block | 0 | 5.4 (2.2–12.9) |
| Mid-septal APs | ||
| Acute procedural success | 87.4 (79.0–92.3) | 90.1 (83.5–94.3) |
| Recurrence rate | 21.4 (15.2–29.2) | 11.5 (7.5–17.3) |
| Long-term efficacy | 76.3 (60.5–87.1) | 87.0 (73.6–94.1) |
| Persistent AV Block | 0 | 7.2 (4.9–10.5) |
| Anteroseptal APs | ||
| Acute procedural success | 80.2 (68.9–88.1) | 91.1 (86.6–94.2) |
| Recurrence rate | 21.0 (15.0–28.5) | 13.3 (9.3–18.9) |
| Long-term efficacy | 73.6 (60.5–83.5) | 84.0 (76.5–89.5) |
| Persistent AV block | 0 | 5.5 (3.6–8.3) |
| Posteroseptal APs | ||
| Acute procedural success | 70.8 (59.7–79.9) | 90.4 (87.4–92.7) |
| Recurrence rate | 22.3 (14.0–33.7) | 8.9 (6.9–11.4) |
| Long-term efficacy | 57.4 (44.3–69.5) | 88.7 (86.1–90.9) |
| Persistent AV block | 0 | 2.2 (1.5–3.3) |
| . | Ablation energy source . | |
|---|---|---|
| . | cryoablation . | radiofrequency . |
| Para-hisian APs | ||
| Acute procedural success | 90.8 (85.1–94.5) | 80.5 (59.4–92.1) |
| Recurrence rate | 21.1 (15.8–27.7) | 7.1 (3.2–15.0) |
| Long-term efficacy | 85.9 (76.9–91.8) | 83.1 (72.1–90.3) |
| Persistent AV block | 0 | 5.4 (2.2–12.9) |
| Mid-septal APs | ||
| Acute procedural success | 87.4 (79.0–92.3) | 90.1 (83.5–94.3) |
| Recurrence rate | 21.4 (15.2–29.2) | 11.5 (7.5–17.3) |
| Long-term efficacy | 76.3 (60.5–87.1) | 87.0 (73.6–94.1) |
| Persistent AV Block | 0 | 7.2 (4.9–10.5) |
| Anteroseptal APs | ||
| Acute procedural success | 80.2 (68.9–88.1) | 91.1 (86.6–94.2) |
| Recurrence rate | 21.0 (15.0–28.5) | 13.3 (9.3–18.9) |
| Long-term efficacy | 73.6 (60.5–83.5) | 84.0 (76.5–89.5) |
| Persistent AV block | 0 | 5.5 (3.6–8.3) |
| Posteroseptal APs | ||
| Acute procedural success | 70.8 (59.7–79.9) | 90.4 (87.4–92.7) |
| Recurrence rate | 22.3 (14.0–33.7) | 8.9 (6.9–11.4) |
| Long-term efficacy | 57.4 (44.3–69.5) | 88.7 (86.1–90.9) |
| Persistent AV block | 0 | 2.2 (1.5–3.3) |
Values expressed in %.
APs, accessory pathways; AV, atrioventricular.
Confidence interval: 95%.
On the other hand, RFA acute procedure success rate was ranged 90–91% for all septal locations, but diminished to (80.5 [59.4–92.1]) for para-hisian APs. Recurrence rate of RFA ranged from 7.1 to 13.3%. RFA for long-term efficacy rate after multiple or uncertain number of procedures was highest for posteroseptal (88.7 [86.1–90.9]) and mid-septal (87.0 [73.6–94.1]) APs locations and lowest for para-hisian APs (83.1 [72.1–90.3]). Finally, AV block rate after RFA ablation was highest for mid-septal APs (7.2 [4.9–10.5]), intermediate for para-hisian and anteroseptal APs and lowest for posteroseptal APs (2.2 [1.5–3.3]) location.
Meta-regression, heterogeneity, and publication time influence
We conducted meta-regression analyses to analyse the influence of age on the outcome of both cryoablation and RFA, and found lower long-term efficacy in paediatric patients, especially in the RFA group (see Supplementary material online, Figure S1).
Potential publication bias was detected regarding acute success of cryoablation (Egger test, P = 0.01) and long-term success of RFA (P = 0.009) (see Supplementary material online, Figure S2), attributable in part to the presence of small size studies, as well as for long-term success results with cryoablation (P < 0.001) (Figures 2 and 3).
Significant heterogeneity could be also explained by the tendency to lower success rates reported in recent cryoablation series. This publication time influence on success could be due to a progressively more widespread use of cryoablation outside the para-hisian and mid-septal regions. Finally, another relevant source of heterogeneity was the differential acute and long-term efficacy rates between groups according to different septal AP locations (Table 3).
Discussion
Main findings
To the best of our knowledge, this is the first systematic literature review and meta-analysis of studies of septal APs ablation using either radiofrequency or cryoenergy. Here we show that studies of RFA for treatment of septal AP report higher overall acute and long-term efficacy rates than do studies using cryoenergy, mainly due to the higher recurrence rate after cryoablation. In contrast, RFA studies report a higher incidence of permanent AV block as compared with cryoablation studies. Nevertheless, efficacy is highly dependent on the AP location. Acutely, cryoablation of para-hisian APs had a significantly higher efficacy than RFA, indirectly indicating the possible trend to reschedule difficult AP to cryoablation after a failed RFA procedure. In contrast, cryoablation of posteroseptal APs showed significantly lower acute efficacy than RFA. With regards to the long-term efficacy rate, cryoablation of para-hisian APs achieves similar success rates to those obtained by RFA, but efficacy is progressively lower for mid-septal and anteroseptal APs locations, being poorest for posteroseptal APs locations.
Septal accessory pathways ablation energy sources
Radiofrequency catheter ablation is the treatment of choice for eliminating accessory pathways, but septal APs ablation remains a challenge as compared with other locations.1–4 The anatomical proximity to structures such as the AV conduction system and the right coronary artery makes the elimination of septal APs a challenging task, especially in paediatric patients.5 Moreover, the long-term clinical implications of a serious complication such as permanent AV block is of special concern in this specific population. Consequently, a significant proportion of patients are subject to high-risk ablations or left untreated because of safety issues.
Cryoablation has recently emerged as an alternative energy source, with a good safety and efficacy profile in the perinodal region.6 The unique characteristics of cryoenergy, such as cryoadherence and cryomapping and the ability to create well circumscribed lesions, lead to use it on substrates previously discarded due to safety concerns.6–8
The results of the present meta-analyses of cryoablation and RFA of septal APs, including the largest consecutive series of patients reported to date from our own centre, demonstrate the superiority of RFA over cryoablation regarding long-term efficacy outcomes. However, when analysing the results according to APs location, cryoablation efficacy outcomes for para-hisian APs are comparable with those achieved by RFA. In contrast, the acute and long-term efficacy of cryoablation at other septal locations was significantly lower.
Not surprisingly, para-hisian and mid-septal APs are the most commonly treated locations in our registry as well as in our cryoablation review, due to the higher AV block risk at these sites.2–4 Of note, the number of para-hisian APs treated with cryoablation exceeds that of para-hisian APs treated with RFA in the literature, despite the superiority of RFA in number of studies and years of experience, indirectly indicating the tendency to avoid RFA at high-risk locations. On the contrary, the number of posteroseptal and anteroseptal APs is rare in cryoablation studies but constitutes the most frequent locations treated by RFA according to our review (see Supplementary material online, Tables S1 and S2). It is in these settings (i.e. posteroseptal APs) where cryoablation shows significantly poorer results.
Recurrence rate
Despite their similar acute efficacy rate, the higher recurrence rate following cryoablation is responsible for the lower efficacy in the long-term. Several circumstances may explain this outcome. Cryoablation gives rise to sharp and well demarcated lesions, yielding a smaller volume of ablated tissue as compared with RF ablation lesions.7,8 Moreover, cryoadhesion of the catheter to the endocardium during ablation produce more ‘focused’ lesions as compared with the ‘brushing effect’ that occurs during RF delivery.7 Additionally, cryoablation delivery at (−80 °C) creates a gradient of temperature whereby deeper layers may only reach between (0 to −30 °C), temperature at which lesions are reversible (cryomapping).8 On the other hand, recurrence rate of cryoablation tends to be greater for studies that selectively included younger patients, due to the difficulties imposed by the reduced heart size and the limited catheter sizes availability.12,13 Finally, some studies included exceptionally difficult accessory pathways after prior failures using RFA (high risk of AV block subgroup).9,10,13 This is in agreement with studies that reported recurrence rates in the range of 10–22% after cryoablation of other perinodal substrates (i.e. AVNRT), suggesting that cryoablation biophysical properties might be responsible for these results.17,18 Although recent reports indicate that larger catheter tip size and the freeze–thaw–freeze protocols may generate more efficient cryoablation lesions, these variables could not be systematically analysed in this review.
Safety
Despite technological improvements, extensive RFA experience and cautious radiofrequency applications, the iatrogenic incidence of undesired AVB during septal ablations is still non-negligible (ranging from 3 to 8%).2–5 In contrast, no cases of undesired persistent AVB during cryoablation of right septal arrhythmic substrates have been reported to date.6,9–13,17–19 This favourable safety profile is due to the ability of cryotechnology to create reversible lesions during applications.7,8 Thus, although reversible tissue injury might be a relative weakness of cryoablation in terms of recurrence risk, this feature is also a major strength of this technology when ablating near normal conduction tissues. Even though small numbers do not allow meaningful statistical comparisons of these outcomes, the present review adds to multiple publications involving both paediatric and adult patients that attest to the general safety of cryoablation.8–13
Study limitations
The present study shares the limitations inherent to most systematic reviews and meta-analyses, with no access to the primary data of the studies. Variations in study methodologies, patient and procedural characteristics and follow-up duration limit direct comparisons of the various studies. Moreover, because most of the studies were single-centre case series, it is possible that efficacy outcomes and adverse events were reported with less consistency than in typical randomized controlled trials. Furthermore, some variables, such as the low resolution and number of cryoablation applications, catheter tip size and incremental use of RFA irrigated catheters were not consistently reported in all studies, precluding the assessment of the impact of such confounders on the results. Finally, the significant heterogeneity could be explained not only by the effect of small population size studies and large differences in follow-up time between the two techniques but also by the tendency to lower success rates reported in recent and larger cryoablation series. Nevertheless, results tended to stabilize in studies published from 2006 onwards and are in line with the results of our prospective registry.
Clinical implications
The results of the present study indicate that RF ablation will continue to be the energy of choice for ablation of septal APs, especially for posteroseptal APs locations where cryoablation long-term efficacy is considerably low. However, the improved safety associated with cryoablation makes it a reasonable alternative to RF ablation of perinodal APs, particularly in paediatric patients and young individuals in whom AV block would be a disastrous outcome.20 In this population, cryoablation could be considered the ablation energy of first choice, since the slightly lower long-term success rate will be counterbalanced by the zero-risk of inadvertent AV block. Thus, the improved safety associated with cryoablation makes it a reasonable alternative to RF ablation of anatomically difficult septal APs, with previously failed or discarded for RFA. Whether methodologic or technologic improvements in cryoablation devices might preserve this safety benefit and further reducing the likelihood of recurrence remains to be proven.
Conclusions
Studies of RFA for treatment of septal APs report higher efficacy rates than do studies using cryoablation, but a higher rate of AVB.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: F.A. has served on the advisory board of Medtronic and Livanova.
Funding
This work was supported in part by grants from the Ministerio de Economía y Competitividad, Instituto Carlos III, CIBER Cardiovascular; and Instituto Carlos III, (PI14/00857, DTS16/160) to F.A.



