-
PDF
- Split View
-
Views
-
Cite
Cite
Gang Xu, Tong Liu, Enzhao Liu, Lan Ye, Michael Shehata, Xunzhang Wang, Guangping Li, Radiofrequency catheter ablation at the non-coronary cusp for the treatment of para-hisian accessory pathways, EP Europace, Volume 17, Issue 6, June 2015, Pages 962–968, https://doi.org/10.1093/europace/euu271
Close - Share Icon Share
Abstract
Radiofrequency catheter ablation (RFCA) is well established as a definitive therapy of accessory pathways (APs). Successful RFCA of anteroseptal APs at the non-coronary cusp (NCC) have been reported in several case reports. We aimed to evaluate the prevalence, safety, efficacy, and long-term outcome of RFCA at the NCC for the treatment of para-hisian APs.
Our study included 17 patients (58.8% female, mean age 46.9 ± 15.9 years) with para-hisian APs. We performed two different ablation approaches which targeted at either the right anterior septum (RAS) (n = 10) or the NCC (n = 7) as the initial target. We compared safety, efficacy, and long-term outcome between these two approaches. The para-hisian APs were successfully ablated in 15 patients and damaged in 1 patient, for the remaining patients, the ablation was abandoned for the suspicion of no atrioventricular conduction. Considering all ablation sites of the para-hisian APs, radiofrequency (RF) delivered at the NCC had a higher success rate (11/12 vs. 5/12, P < 0.05) and a lower complication rate (0/12 vs. 4/12, P < 0.05) compared with the RAS. During a mean follow-up period of 22.4 ± 15.0 months, all the patients were free of arrhythmias without any anti-arrhythmic drugs.
Para-hisian APs can be safely and effectively ablated at the NCC. Compared with the ablation at the RAS, RF delivered at the NCC has a higher immediate success, lower complication rate, and good long-term outcome.
Two different ablation approaches which targeted at either the right anterior septum (RAS) (n = 10) or the non-coronary cusp (NCC) (n = 7) were performed in 17 patients with para-hisian accessory pathways (APs).
Para-hisian APs can be safely and effectively ablated at the NCC.
Compared with the ablation at RAS, radiofrequency delivered at the NCC has a higher immediate success, lower complication rate, and good long-term outcome.
Introduction
Radiofrequency catheter ablation (RFCA) is well established as a definitive therapy of accessory pathways (APs).1 However, para-hisian APs remain as a challenging task due to their anatomic proximity to the normal conduction system.2 Recurrence rate and risk of complete atrioventricular (AV) block are relatively higher in the patients with anterior-septal bypass fibres who were referred for RFCA. Our previous studies3,4 and other studies5 have suggested that atrial tachycardias can be successfully and safely ablated from the non-coronary cusp (NCC) or the left coronary cusp (LCC). However, catheter ablation of para-hisian APs at the NCC have been just reported in some case reports.6–11 Until now, larger studies investigating the safety and efficacy of catheter ablation at NCC for the treatment of para-hisian APs are still lacking. Therefore, we aimed to evaluate the prevalence, safety, and efficacy of RFCA at the NCC for the treatment of para-hisian APs.
Methods
Patient selection
From July 2009 to January 2013, a total of 285 consecutive patients underwent radiofrequency (RF) ablation of the AP in our laboratory. The study protocol was approved by the Institutional Review Board of Second Hospital of Tianjin Medical University. Seventeen patients who had an AP located at the para-hisian region were enrolled in the present study.
The 17 patients who initially enrolled in our study included 10 females, aged 14–70 years (mean age 46.9 ± 15.9 years). None of them had demonstrable structural heart disease. Fifteen patients presented with recurrent supraventricular tachycardia (SVT), and one patient with both SVT and paroxysmal atrial fibrillation (AF). The remaining one (No.3) presented with only paroxysmal AF with manifest para-hisian AP and without history of SVT.
Electrophysiological study
All anti-arrhythmic therapies were discontinued for five half-lives before the study. Written informed consent was obtained from all the patients before the procedure. Under fluoroscopic visualization, two or three quadripolar catheters were advanced to the high atrial atrium, His bundle, and/or right ventricular apex. A decapolar catheter was placed in the coronary sinus. The most proximal bipole of this catheter was positioned at the coronary sinus ostium. Intra-cardiac electrograms were simultaneously displayed with electrocardiographic leads I, aVF, and V1 on a multi-channel recording system at a paper speed of 150–200 mm/s (EP MED Systems). The bipolar signals were filtered at 30–500 Hz. We induced tachycardia by programmed electrical stimulation. Following documenting narrow QRS tachycardias, differential diagnosis was performed by atrial or ventricular extra-stimulations, atrial or ventricular entrainment pacing, or para-hisian pacing. Conduction intervals and refractory periods were measured and defined as previously described.12 The diagnosis of various forms of atrioventricular reentrant tachycardias (AVRTs) incorporating APs was made and defined according to the previously described criteria.1 Localization and identification of the APs were achieved by careful mapping of the atrial or ventricular activation pattern, or both, using unipolar and bipolar electrograms recorded from regular electrode catheters as well as a steerable ablation catheter.
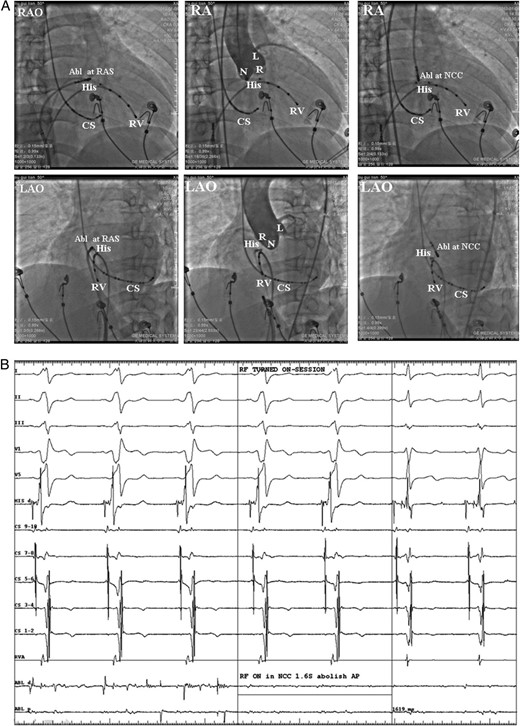
(A) Fluoroscopic images from the case No. 6 who was firstly got failed ablation from the right atrial septum (RAS), and then the ablation target were changed to the NCC and successfully ablated the AP. Left: catheter location at the RAS ablation site in the right anterior oblique (RAO) and left anterior oblique (LAO) views. Middle: aortic root angiograms taken from a pigtail catheter at the NCC at the same fluoroscopic angles before ablation at the NCC. Right: the successful ablation site was located at the NCC. (B) Intra-cardiac electrograms of the successful ablation target at the NCC. Radiofrequency catheter ablation at the NCC successfully ablated the AP. Abl, ablation catheter; RAS, right anterior septum; CS, coronary sinus; RV, right ventricle; RVA, RV apex. L, left coronary cusp; N, non-coronary cusp; R, right coronary cusp; RF, radiofrequency; AP, accessory pathway.
The insertion sites of APs were considered to be para-hisian when a discernible His bundle potential was recorded (at the site of earliest atrial activation) during AVRT or following ablation of a manifest AP with disappearance of ventricular pre-excitation, or the successful ablation target located within 5 mm in distance (measured from the X-ray image) to the His electrode which manifest significant His bundle potential for concealed AP and intermittent pre-excited AP or after successful elimination of a manifest AP. The possibility of atrioventricular node reentry tachycardia (AVNRT) was excluded by noting earlier atrial excitation without changing the activation sequence by delivery of a ventricular premature beat during tachycardia at a time when the His bundle was refractory. The possibility that a reciprocating tachycardia might incorporate a retrogradely conducting concealed nodo-ventricular pathway was evaluated by isolating the atria from the tachycardia circuit by delivery of an atrial or ventricular extra-stimulus during tachycardia.
Mapping and catheter ablation
A 7 Fr quadripolar deflectable catheter with a 4 mm distal electrode and a 2 mm inter-electrode distance between the distal two electrodes (Mansfield-Webster) was introduced percutaneously into a femoral vein, and advanced to the right atrium. Using the His bundle and the coronary sinus catheters for reference, we positioned the tip of the electrode catheter at various sites in the AV junctional area to map the para-hisian APs.
When the presence of the para-hisian APs were confirmed, two different ablation approaches which targeted at either the right anterior septum (RAS) near the para-hisian region or the NCC as the initial target. From July 2009 to July 2011, we targeted at the RAS as the initial choice. If the initial ablation failed then we shifted to map and target at NCC. From August 2011 to January 2013, we targeted at the NCC first, if the initial ablation failed then we shifted to map and target at the para-hisian region of the right atrium. The success rate and the incidence of complications were compared between the two ablation strategies. Aortic angiography was performed to establish the location of coronary arteries and to delineate the anatomical features of the coronary cusps before the NCC was mapped.
Radiofrequency catheter ablation was performed under continuous digital monitoring of the power and the impedance. The ablation procedure was discontinued immediately if the rise of impedance, AV block, chest pain, hypotension, or severe bradycardia were noted. A power of 15–20 W for 5–10 s was delivered initially. If it was effective without AV block, a booster current was delivered one to three more times at the same location. Anterograde and retrograde conduction properties were evaluated immediately after each current application. If it did not work and without heart block for a certain current delivered at least 30 s, a more aggressive power (5–10 W more) was delivered for at least 30 s. The max power delivered at the RAS was limited to 30 W, and that at the NCC was limited to 40 W. If it failed to abolish the APs after at least twice by the maximal ablation current or manifest rapid junctional reaction or transient AV block or bundle block, then we shift the other ablation strategy (from right atrium to NCC or from NCC to right atrium).
Patients follow-up
Following catheter ablation, the electrophysiological study was repeated during intravenous isoproterenol infusion (1–4 µg/min to achieve a 20% increase in sinus rate) to ensure the success of ablation. The patient was then observed in the hospital for 3 days with measurements of serum creatinine phosphokinase, ECG, a two-dimensional echocardiographic examination, and a 24 h Holter recording. Patients were arranged to visit the outpatient clinic at 1, 3, 6, and 12 months following RFCA. Electrocardiogram was recorded at each visit, and 24 h Holter was evaluated at anytime whenever the patients experienced symptoms.
Statistical analysis
The data are expressed as mean ± SD and were analysed using Student's t test and χ2 analysis. At a P value of <0.05, differences were considered statistically significant.
Results
Electrophysiological characteristics
There were altogether seven patients with the manifest pre-excitation: six have persistent pre-excitation and one patient had intermittent pre-excitation (No. 6) (Table 1). Among them, one patient (No. 7) had multiple APs: a manifest left posterior AP in addition to the concealed para-hisian AP. One patient (No. 3) with persistent ventricular pre-excitation had no history of paroxysmal SVT but paroxysmal AF with rapid ventricular response. The electrophysiology study manifested right anterior-septal AP, and could not induce SVT. When the RF current was delivered at the RAS (20 W for 20 s) and subsequently at the NCC (30 W for 5 s) both showed transient AV block and the current ceased immediately. During decremental atrial pacing it presented with fixed pre-excitation so it was suspected that the patient had no AV node conduction, therefore the ablation procedure was abandoned. Therefore, this case was excluded from the final statistical analysis of the present study.
| Case # . | Sex . | Age (years) . | Arrhythmia . | Procedure . | Final target . | Success power . | Time to success (S) . | Complication . | Fluoroscopy duration (min) . | Final result . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | CBT + AVRT | RA to NCC | NCC | 40 | 35 | – | 43.10 | Success |
| 2 | M | 60 | WPW | RA to NCC | NCC | 30 | 18 | – | 42.18 | Success |
| 3a | M | 47 | AF + AP | RA to NCC | Abortion | AVD (RA) | 35.25 | Abandoned | ||
| 4 | F | 61 | CBT + AVRT | RA to NCC | NCC | 35 | 20 | AVD (RA) | 31.41 | Success |
| 5 | F | 50 | CBT + AVRT | RA | RA | 20 | 60 | – | 20.14 | Success |
| 6 | M | 50 | Intermittent AP | RA to NCC | NCC | 40 | 33 | – | 16.85 | Success |
| 7 | F | 44 | Multiple AP | RA | RA | 30 | 30 | cAVB(RA) | 12.32 | cAVB no SVT |
| 8 | F | 44 | WPW | RA | RA | 30 | 2.3 | – | 2.82 | Success |
| 9 | F | 70 | CBT + AVRT | RA | RA | 15 | 30 | AVD(RA) | 2.36 | Success |
| 10 | F | 50 | WPW | RA to NCC | NCC | 30 | 1.6 | – | 1.81 | Success |
| 11 | M | 51 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 21.6 | Success |
| 12 | F | 15 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 42.87 | Success |
| 13 | M | 34 | CBT + AVRT | NCC | NCC | 30 | 15 | – | 33.51 | Success |
| 14 | F | 69 | CBT + AVRT | NCC | NCC | 40 | 3 | – | 27.40 | Success |
| 15 | F | 60 | CBT + AVRT | NCC | NCC | 30 | 20 | – | 23.06 | Success |
| 16 | M | 45 | WPW | 1st: NCC | NCC | 40 | 30 | – | 31.31 | AP damaged |
| 2nd: RA | RA | 30 | 20 | – | ||||||
| 17 | M | 35 | WPW | NCC to RA | RA | 20 | 10 | – | 12.78 | Success |
| Mean | 47.0 | 22.4 |
| Case # . | Sex . | Age (years) . | Arrhythmia . | Procedure . | Final target . | Success power . | Time to success (S) . | Complication . | Fluoroscopy duration (min) . | Final result . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | CBT + AVRT | RA to NCC | NCC | 40 | 35 | – | 43.10 | Success |
| 2 | M | 60 | WPW | RA to NCC | NCC | 30 | 18 | – | 42.18 | Success |
| 3a | M | 47 | AF + AP | RA to NCC | Abortion | AVD (RA) | 35.25 | Abandoned | ||
| 4 | F | 61 | CBT + AVRT | RA to NCC | NCC | 35 | 20 | AVD (RA) | 31.41 | Success |
| 5 | F | 50 | CBT + AVRT | RA | RA | 20 | 60 | – | 20.14 | Success |
| 6 | M | 50 | Intermittent AP | RA to NCC | NCC | 40 | 33 | – | 16.85 | Success |
| 7 | F | 44 | Multiple AP | RA | RA | 30 | 30 | cAVB(RA) | 12.32 | cAVB no SVT |
| 8 | F | 44 | WPW | RA | RA | 30 | 2.3 | – | 2.82 | Success |
| 9 | F | 70 | CBT + AVRT | RA | RA | 15 | 30 | AVD(RA) | 2.36 | Success |
| 10 | F | 50 | WPW | RA to NCC | NCC | 30 | 1.6 | – | 1.81 | Success |
| 11 | M | 51 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 21.6 | Success |
| 12 | F | 15 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 42.87 | Success |
| 13 | M | 34 | CBT + AVRT | NCC | NCC | 30 | 15 | – | 33.51 | Success |
| 14 | F | 69 | CBT + AVRT | NCC | NCC | 40 | 3 | – | 27.40 | Success |
| 15 | F | 60 | CBT + AVRT | NCC | NCC | 30 | 20 | – | 23.06 | Success |
| 16 | M | 45 | WPW | 1st: NCC | NCC | 40 | 30 | – | 31.31 | AP damaged |
| 2nd: RA | RA | 30 | 20 | – | ||||||
| 17 | M | 35 | WPW | NCC to RA | RA | 20 | 10 | – | 12.78 | Success |
| Mean | 47.0 | 22.4 |
CBT, concealed bypass tract; AVRT, atrioventricular reciprocating tachycardia; RA, right atrium; NCC, non-coronary cusp; AVD, atrioventricular conduction delay; WPW, Wolf–Parkinson–White syndrome; cAVB, complete atrioventricular block; SVT, supraventricular tachycardia; CRBBB, complete right bundle branch block; AP, accessory pathway.
aPatient No. 3 was excluded from the final analysis.
| Case # . | Sex . | Age (years) . | Arrhythmia . | Procedure . | Final target . | Success power . | Time to success (S) . | Complication . | Fluoroscopy duration (min) . | Final result . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | CBT + AVRT | RA to NCC | NCC | 40 | 35 | – | 43.10 | Success |
| 2 | M | 60 | WPW | RA to NCC | NCC | 30 | 18 | – | 42.18 | Success |
| 3a | M | 47 | AF + AP | RA to NCC | Abortion | AVD (RA) | 35.25 | Abandoned | ||
| 4 | F | 61 | CBT + AVRT | RA to NCC | NCC | 35 | 20 | AVD (RA) | 31.41 | Success |
| 5 | F | 50 | CBT + AVRT | RA | RA | 20 | 60 | – | 20.14 | Success |
| 6 | M | 50 | Intermittent AP | RA to NCC | NCC | 40 | 33 | – | 16.85 | Success |
| 7 | F | 44 | Multiple AP | RA | RA | 30 | 30 | cAVB(RA) | 12.32 | cAVB no SVT |
| 8 | F | 44 | WPW | RA | RA | 30 | 2.3 | – | 2.82 | Success |
| 9 | F | 70 | CBT + AVRT | RA | RA | 15 | 30 | AVD(RA) | 2.36 | Success |
| 10 | F | 50 | WPW | RA to NCC | NCC | 30 | 1.6 | – | 1.81 | Success |
| 11 | M | 51 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 21.6 | Success |
| 12 | F | 15 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 42.87 | Success |
| 13 | M | 34 | CBT + AVRT | NCC | NCC | 30 | 15 | – | 33.51 | Success |
| 14 | F | 69 | CBT + AVRT | NCC | NCC | 40 | 3 | – | 27.40 | Success |
| 15 | F | 60 | CBT + AVRT | NCC | NCC | 30 | 20 | – | 23.06 | Success |
| 16 | M | 45 | WPW | 1st: NCC | NCC | 40 | 30 | – | 31.31 | AP damaged |
| 2nd: RA | RA | 30 | 20 | – | ||||||
| 17 | M | 35 | WPW | NCC to RA | RA | 20 | 10 | – | 12.78 | Success |
| Mean | 47.0 | 22.4 |
| Case # . | Sex . | Age (years) . | Arrhythmia . | Procedure . | Final target . | Success power . | Time to success (S) . | Complication . | Fluoroscopy duration (min) . | Final result . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | CBT + AVRT | RA to NCC | NCC | 40 | 35 | – | 43.10 | Success |
| 2 | M | 60 | WPW | RA to NCC | NCC | 30 | 18 | – | 42.18 | Success |
| 3a | M | 47 | AF + AP | RA to NCC | Abortion | AVD (RA) | 35.25 | Abandoned | ||
| 4 | F | 61 | CBT + AVRT | RA to NCC | NCC | 35 | 20 | AVD (RA) | 31.41 | Success |
| 5 | F | 50 | CBT + AVRT | RA | RA | 20 | 60 | – | 20.14 | Success |
| 6 | M | 50 | Intermittent AP | RA to NCC | NCC | 40 | 33 | – | 16.85 | Success |
| 7 | F | 44 | Multiple AP | RA | RA | 30 | 30 | cAVB(RA) | 12.32 | cAVB no SVT |
| 8 | F | 44 | WPW | RA | RA | 30 | 2.3 | – | 2.82 | Success |
| 9 | F | 70 | CBT + AVRT | RA | RA | 15 | 30 | AVD(RA) | 2.36 | Success |
| 10 | F | 50 | WPW | RA to NCC | NCC | 30 | 1.6 | – | 1.81 | Success |
| 11 | M | 51 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 21.6 | Success |
| 12 | F | 15 | CBT + AVRT | NCC | NCC | 30 | 30 | – | 42.87 | Success |
| 13 | M | 34 | CBT + AVRT | NCC | NCC | 30 | 15 | – | 33.51 | Success |
| 14 | F | 69 | CBT + AVRT | NCC | NCC | 40 | 3 | – | 27.40 | Success |
| 15 | F | 60 | CBT + AVRT | NCC | NCC | 30 | 20 | – | 23.06 | Success |
| 16 | M | 45 | WPW | 1st: NCC | NCC | 40 | 30 | – | 31.31 | AP damaged |
| 2nd: RA | RA | 30 | 20 | – | ||||||
| 17 | M | 35 | WPW | NCC to RA | RA | 20 | 10 | – | 12.78 | Success |
| Mean | 47.0 | 22.4 |
CBT, concealed bypass tract; AVRT, atrioventricular reciprocating tachycardia; RA, right atrium; NCC, non-coronary cusp; AVD, atrioventricular conduction delay; WPW, Wolf–Parkinson–White syndrome; cAVB, complete atrioventricular block; SVT, supraventricular tachycardia; CRBBB, complete right bundle branch block; AP, accessory pathway.
aPatient No. 3 was excluded from the final analysis.
All of the 16 patients who were included in the final analysis had induced sustained orthodromic AVRT. The cycle length of tachycardia was 346 ± 54 ms (ranged from 290 to 445 ms); the AH interval was 207 ± 57 ms (ranged from 100 to 310 ms); and the HA interval was 139 ± 29 ms (ranged from 90 to 200 ms). In these 16 patients, the atrium could be reset during tachycardia when a ventricular extra-stimulus was delivered while the His bundle was still refractory. However, the atrium could not be isolated in any of these patients when an atrial extra-stimulus was delivered during tachycardia.
In the six patients with persistent ventricular pre-excitation, the longest atrial pacing cycle length that induced conduction block in the APs was 360 ± 131 ms (ranged from 260 to 640 ms); the effective refractory period of the APs was 309 ± 62 ms (ranged from 260 to 410 ms). In all the 12 patients, the longest ventricular-paced cycle length that induced retrograde block in the APs was measured in 12 patients, and was ≤295 ± 58 ms (ranged from 260 to 460 ms), and in the remaining 4 patients, the tachycardia were induced when the ventricle was paced at shorter cycle length, and therefore it could not be measured.
Catheter ablation
Anterograde mapping for the AP insertions in the seven patients with ventricular pre-excitation was performed by positioning the ablation catheter at the RAS and different sites of Koch's triangle with recording of bipolar electrograms. In six of these seven patients who had an inducible orthodromic AVRT, the insertion site of the AP was also indicated by retrograde mapping during AVRT, and was concordant with that during anterograde mapping. Of the remaining nine patients without ventricular pre-excitation, the insertion sites of the APs were localized by mapping during incremental ventricular pacing or induced orthodromic AVRT.
The APs were successfully ablated in 15 patients and damaged in 1 patient (No. 16) (Table 1). Nine patients (Group A) were ablated from the RAS initially, four patients were successfully ablated from the RAS, and five patients got failed ablation from the RAS or developed AV conduction delay during current delivery from the RAS, and then the ablation target were changed to NCC and successfully ablated the AP (Figure 1). Seven patients (Group B) were ablated at the NCC initially, five patients were successfully ablated at the NCC (Figure 2), the AP in one patient (No. 16) was damaged following ablation at the NCC, and with second ablation at the RAS did not completely eliminate the AP. However, AVRT was not induced during intravenous isoproterenol infusion. Finally, the other patient (No. 17) got failed ablation at the NCC and was successfully ablated at the RAS where slightly inferior to level of His bundle. The procedure time was 164 ± 87 min, the fluoroscopic exposure time was 45 ± 32 min, the number of current applications was 15 ± 17 times, the power of the RF was 30 ± 8 W, and the application duration was 11 ± 6 s.
Complications
There were four patients who developed complications in the present study, all of them developed complications during or following RFCA in the RAS (Table 1). Three patients (Nos. 3, 4, 9) developed transient AV conduction delay when delivering current from the para-hisian target in the RAS and subsequently the target was changed to NCC according to the study protocol and successfully ablated the APs and no further AV block developed during the follow-up period. In the remaining one patient (No. 7) with multiple AP, We mapped the para-hisian AP just inferior to the level of His bundle, and during the RF application a transient complete AV block developed and the RF ceased immediately. Two minutes later the AV conduction recovered without signs of the para-hisian AP resumption and the ablation procedure was discontinued. Unfortunately, complete AV block developed 3 days later and persisted; a permanent pacemaker implantation was required. None of the seven patients in whom the APs were ablated at the NCC developed any complications.
Success and complication rate in the right anterior septum vs. non-coronary cusp ablation
Considering all ablation sites of the para-hisian APs, RF delivered at the NCC has a higher success rate (11/12 vs. 5/12, P < 0.05) and a lower complications rate (0/12 vs. 4/12, P < 0.05) compared with the RAS. The RFCA energy that was delivered for successful ablation of the APs at NCC seems higher than that at the RAS (35.00 ± 5.00 W, n = 10 vs. 23.00 ± 6.71 W, n = 6, P = 0.001). The duration of RF application was similar between Groups A and B (21.42 ± 11.53 s, n = 10 vs. 16.46 ± 12.75 s, n = 6, P = 0.452).
Follow-up
During a mean follow-up period of 22.4 ± 15.0 months (ranged from 1.7 to 43.1 months), all patients were free of arrhythmias without anti-arrhythmic drugs. Among the six patients who were successfully ablated at the NCC, there was no resumption of ventricular pre-excitation in the five of six patients with a previous ventricular pre-excitation. The remaining one (No. 16) with damaged AP had intermittent pre-excitation without arrhythmias during the 31 months follow-up period.
Discussion
In this case series of para-hisian APs, we observed the following: (i) para-hisian APs can be safely and effectively ablated within the NCC, and with good long-term outcome; (ii) Compared with the ablation at the RAS, RF delivered at the NCC for the para-hisian APs has a higher immediate success with lower complication rate.
Ablation of anteroseptal and mid-septal AP remains challenging because of its close proximity to the normal conduction system and the potential risk of heart block. Immediate success rate for catheter ablation of anteroseptal AP is 96% in the current era. However, the recurrence rate and the risk of atrial-ventricular (AV) block are higher in RFCA of the anteroseptal AP. Cryoablation can be applied to septal AP to avoid injury to the normal conduction system, but its success rate is also suboptimal.13 Anteroseptal APs have been successfully eliminated by RFCA and cryoablation in the NCC on rare occasions.6–11 In patients with previously failed ablation, careful mapping of an anteroseptal AP at the NCC has been suggested as an alternative approach.10,14
The NCC has immediate anatomic relationship with the interatrial septum and the septal portions of the right and left atrium. Possible electrically active myocardial connections, constituting an AP, can occur in the region of the NCC between ventricular myocardium (just below and extending above the NCC) and atrial myocardium (adjacent to the interatrial septum).15 Therefore, the NCC can be an alternative target for catheter ablation for the para-hisian APs if there was failed previous ablation in RAS.
Non-coronary cusp ablation has been reported in the young adolescents and adults as well as a 4-month-old infant with incessant orthodromic AVRT.6–11 Huang et al.7 reported the use of irrigated RF ablation in the NCC for ablating anteroseptal AP, which could not be ablated despite three ablation procedures in the right and left atrium, and that recurred following successful ablation in the NCC with conventional RF energy. Irrigated RF ablation at the NCC may also be an alternative approach in patients with failed conventional energy. Recently, Park et al.14 reported successful ablation of seven patients with para-hisian AP at NCC (n = 2) or RCC (n = 5) from 27 mid- to anteroseptal AP. However, one patient developed complete heart block 2 days following the procedure in five patients ablated at the RCC. In the present work, we observed a lower risk of AV block when we ablated para-hisian AP at the NCC. And we have shown good safety and efficacy for catheter ablation of para-hisian AP at the NCC, with high success and low complication rates (especially compared with the traditional ablation approach which targeted the para-hisian region of right atrium). The electrogram of our ablation target at NCC has small His potential or without His potential (Figure 2B). The approach with the NCC as the initial target for the catheter ablation of para-hisian APs needs further investigation.
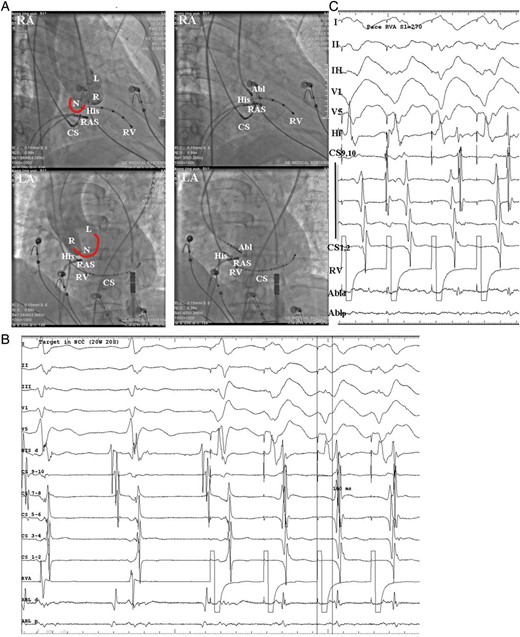
(A) Fluoroscopic images from the case No. 11 who was firstly ablated at the NCC and successfully ablated the AP. Left: aortic root angiograms taken from a pigtail catheter at the same fluoroscopic angles before ablation at the NCC in the RAO and LAO views. Right: the successful ablation site was located at the NCC. (B) Intra-cardiac electrograms of the successful ablation target at the NCC, ventricular pacing showed the retrograde A was at the same line with the His bundle A. (C) Following ablation, ventricular pacing showed retrograde VA Wenckebach block, confirmed the successful ablation of retrograde AP. And the last paced V is conducted to A using an atrioventricular node which was suggested by the longer VA interval and change in the sequence of retrograde A activation. Abl, ablation catheter; CS, coronary sinus; RV, right ventricle; RVA, RV apex. L, left coronary cusp; N, non-coronary cusp; R, right coronary cusp; AP, accessory pathway.
Limitations
The major limitation of our study is the fact that this was not a prospective, randomized study comparing different ablation approaches. During the first years, the RAS approach was an initial one, and later on—an NCC approach was always initially attempted, thus gaining experience of operators might have also influenced the results. Secondly, some anteroseptal pathways could also be successfully ablated using a jugular or subclavian vein approach. However, we did not use this approach in the current study.
Conclusion
Para-hisian APs can be safely and effectively ablated at the NCC. Compared with the ablation at the RA anterior septum, RF delivered at the NCC has a higher immediate success, lower complication rate, and good long-term outcome. The approach with the NCC as the initial target for the catheter ablation of the para-hisian APs needs further investigation.
Conflict of interest: none declared.



