-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Goette, Winghan J Kwong, Michael D Ezekowitz, Maciej Banach, Soren P Hjortshoj, Dmitry Zamoryakhin, Gregory Y H Lip, Edoxaban therapy increases treatment satisfaction and reduces utilization of healthcare resources: an analysis from the EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) study, EP Europace, Volume 20, Issue 12, December 2018, Pages 1936–1943, https://doi.org/10.1093/europace/euy141
Close - Share Icon Share
Abstract
The EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) (NCT02072434) study was a multicentre prospective, randomized, open-label, blinded-endpoint evaluation (PROBE) trial comparing edoxaban with enoxaparin/warfarin followed by warfarin alone in 2199 non-valvular atrial fibrillation patients undergoing electrical cardioversion and showed comparable rates of bleeding and thromboembolism between treatments. This prespecified ancillary analysis investigated the impact of edoxaban therapy on treatment satisfaction and utilization of healthcare services.
The Perception of Anticoagulant Treatment Questionnaire (PACT-Q2) was completed by study patients on Day 28 post-cardioversion. Higher scores represent greater satisfaction. Healthcare resource utilizations were collected from randomization to Day 28 post-cardioversion. Data from patients who received at least one dose of study drugs were analysed. Patients treated with edoxaban were more satisfied than enoxaparin/warfarin in both PACT-Q treatment satisfaction and convenience scores (P < 0.001 for both). Differences in treatment satisfaction scores were greater in patients who underwent non-transoesophageal echocardiography (TOE)-guided cardioversion than in patients who underwent TOE-guided cardioversion. Edoxaban was associated with fewer clinic visits (4.75 visits vs. 7.60 visits; P < 0.001) and fewer hospital days (3.43 days vs. 5.41 days; P < 0.05). Rates of hospitalizations and emergency room visits were not significantly different. Overall, edoxaban therapy was estimated to reduce healthcare costs by €107.73, €437.92, €336.75, and $246.32 per patient in German, Spanish, Italian, and US settings, respectively.
The convenience of edoxaban therapy over warfarin in patients undergoing cardioversion may provide greater treatment satisfaction and cost savings to the healthcare system.
Edoxaban was associated with significantly higher ratings of convenience and treatment satisfaction than enoxaparin/warfarin in patients undergoing cardioversion.
The use of edoxaban as anticoagulation pericardioversion significantly reduced the number of days spent in the hospital and the number of clinic visits compared to enoxaparin/warfarin. These reductions in healthcare resource utilization could result in a saving of €107.73 per patient in Germany and €437.92 per patient in Spain.
Introduction
EdoxabaN vs. warfarin in subjectS UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) (NCT02072434) was a multicentre, prospective, randomized, open-label, blinded-endpoint evaluation clinical trial; it compared the efficacy and safety of once-daily edoxaban 60 mg with enoxaparin/warfarin in 2199 patients undergoing electrical cardioversion of non-valvular atrial fibrillation (NVAF).1 Electrical cardioversion [either transoesophageal echocardiography (TOE)-guided or non-TOE-guided] can be used in clinical practice to restore sinus rhythm in patients with AF.2–4 The ENSURE-AF study was the largest prospective randomized clinical trial of non-vitamin K antagonist oral anticoagulation (NOAC) in NVAF patients undergoing electrical cardioversion.1
Edoxaban, a NOAC, is a direct factor Xa inhibitor with linear and predictable pharmacokinetics indicated for the reduction of the risk of stroke or systemic embolic event (SEE) in patients with NVAF and for the treatment of venous thromboembolism.5,6 In the ENSURE-AF study, the primary endpoint—composite of stroke, SEE, myocardial infarction, and cardiovascular (CV) mortality—occurred in 5 (<1%) patients on edoxaban compared with 11 (1%) on enoxaparin/warfarin [odds ratio (OR) 0.46, 95% confidence interval (CI) 0.12–1.43].1 The primary safety endpoint—major and clinically relevant non-major bleeding—occurred in 16 (1%) patients on edoxaban and 11 (1%) patients on enoxaparin/warfarin (OR 1.48, 95% CI 0.64–3.55).1 It is unclear if NOAC therapy in electrical cardioversion of AF has an impact on patient convenience and overall healthcare costs. Due to the high number of cardioversions performed worldwide, this aspect of therapy potentially has a significant clinical importance.
The present prespecified subanalysis of ENSURE-AF assessed treatment convenience/satisfaction, impact of treatment on AF-related symptoms, healthcare service utilization, and costs associated with edoxaban vs. enoxaparin/warfarin treatment in NVAF patients undergoing planned electrical cardioversion.
Methods
Study design
The trial design and primary analyses of ENSURE-AF were previously reported.1,7 The ENSURE-AF trial was done in compliance with the Declaration of Helsinki, the International Conference on Harmonisation (ICH) consolidated Guideline E6 for Good Clinical Practice (CPMP/ICH/135/95), and applicable regulatory requirements. The protocol and its amendments were approved by ethics committees or institutional review boards. In the ENSURE-AF trial, patients with documented NVAF for ≥48 h but ≤12 months who were planned for electrical cardioversion and anticoagulation therapy were included. Patients with NVAF undergoing electrical cardioversion were randomized (1:1) to receive either once-daily edoxaban 60 mg or enoxaparin/warfarin within each stratum (i.e. TOE-guided or non-TOE-guided stratum). The dose of edoxaban was halved to 30 mg for patients who had creatinine clearance of 15–50 mL/min, low body weight (≤60 kg), or who used P-glycoprotein inhibitors concomitantly. At randomization, all patients were stratified based on cardioversion approach (TOE or non-TOE), prior experience in taking anticoagulants at the time of randomization (experienced or naïve), and whether they met the edoxaban dose reduction criteria.
Consent
All patients provided written informed consent prior to participation in the study.1
Convenience and treatment satisfaction
Anticoagulant treatment satisfaction was assessed at the end of active treatment (Day 28) or at follow-up/study discontinuation (up to Day 58) using the second module of the Perception of Anticoagulant Treatment Questionnaire (PACT-Q2). All of the items on the PACT-Q2 are answered on a 5-point Likert scale with higher score indicating greater convenience or treatment satisfaction.8 PACT-Q2 items are scored along two domains (convenience and anticoagulant treatment satisfaction) with a possible score of 100 in each domain (Supplementary material online, Table S1).8
Atrial fibrillation-related symptoms
Atrial fibrillation-related symptoms were assessed at randomization and at end of treatment (Day 28) using the European Heart Rhythm Association classification of AF-related Symptoms (EHRA). The EHRA assigns patients into four stages based on symptoms and their effects on daily activities: EHRA I (no symptoms); EHRA II (mild symptoms, normal daily activity not affected); EHRA III (severe symptoms, normal daily activity affected); EHRA IV (disabling symptoms, normal daily activity discontinued).
Healthcare utilization outcomes and cost assessment
Healthcare resource utilization data—including hospital admissions, length of stay, and diagnosis; emergency department visits for CV reasons not resulting in hospitalizations; outpatient physician/nurse visits not associated with the study; and number of visits to the investigational site—were assessed throughout the trial.
Healthcare costs during 28 days of active treatment were compared between treatment groups. Published cost estimates were applied to each healthcare utilization outcome that was statistically different between treatment groups at P < 0.05 (Table 1). Due to the multinational nature of the study, cost estimates from Germany, Spain, Italy, and the USA were used to provide a broad perspective of cost differences under various healthcare systems. Cost estimates were derived from published sources, supplemented by an ad hoc analysis of a hospital database when published data were not available (see Table 1).
| Data input . | Cost estimate . | Source . |
|---|---|---|
| Germany | ||
| Cost of edoxaban (per pill) | €2.59 | Lauer-Taxe, February 2017 |
| Cost of warfarin (per pill) | €0.24 | Lauer-Taxe, February 2017 |
| Hospitalization cost (per diem) | €1412.00 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Cost of INR (per test) | €0.60 | AMNOG Dossier, Modul 3 (EBM Ziffer 32113) |
| Outpatient visit (per visit) | €28.86 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Spain | ||
| Cost of edoxaban (per pill) | €1.72 | 60 mg retail price (minus 7.5% Royal Decree Law 8/2010) Portalfarma Database |
| Cost of warfarin (per pill) | €0.06 | Acenocoumarol 2.75 mg/day––retail price (minus 15% Royal Decree Law 8/2010) Portalfarma Database |
| Per-diem hospitalization cost | €866.23 | Cardiology department—eSalud Database 2017 |
| Cost of INR (per test) | €25.12 | Spanish Hospital Pharmacy Society report (Informe GENESIS de la SEFH, 2012.) NOACS report (apixaban, dabigatran, rivaroxaban). Prevención de eventos tromboembólicos en pacientes con fibrilación auricular no valvular. Available at: http://gruposdetrabajo.sefh.es/genesis/ |
| Outpatient visit (per visit) | €84.32 | Specialist outpatient office—eSalud Spanish Costs Database 2017 |
| Italy | ||
| Cost of edoxaban (per pill) | €2.09 | Ex-factory price IHS Global Insight Database |
| Cost of warfarin (per pill) | €0.07 | Codifa.it (coumadin cost per day) |
| Per-diem hospitalization cost | €587.00 | Progr 122: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 1 |
| Cost of INR (per test) | €26.09 | Progr 655 Visita generale ‘Supplemento ordinario N° 8 alla Gazzetta Ufficiale, 28/01/2013, Allegato 3: Prestazioni di assistenza specialistica ambulatoriale’ + Prestazione91.49.2 (prelievo sangue venoso) + 90.75.4 (tempo di protrombina) |
| Outpatient visit (per visit) | €55.78 | Progr 627-629-630: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 3 |
| USA | ||
| Cost of edoxaban (per pill) | $9.24 per day | Wholesale acquisition cost (WAC), Magnuson et al.10 |
| Cost of warfarin (per pill) | $0.36 per day | WAC, Magnuson et al.10 |
| Per-diem hospitalization cost | $2800 per day | Ad hoc analysis of hospitalizations of adult AF patients in Premier database (January–September 2015) |
| Cost of INR (per test) | $20 per test | Magnuson et al.10 |
| Outpatient visit (per visit) | $60 per visit | Magnuson et al.10 |
| Data input . | Cost estimate . | Source . |
|---|---|---|
| Germany | ||
| Cost of edoxaban (per pill) | €2.59 | Lauer-Taxe, February 2017 |
| Cost of warfarin (per pill) | €0.24 | Lauer-Taxe, February 2017 |
| Hospitalization cost (per diem) | €1412.00 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Cost of INR (per test) | €0.60 | AMNOG Dossier, Modul 3 (EBM Ziffer 32113) |
| Outpatient visit (per visit) | €28.86 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Spain | ||
| Cost of edoxaban (per pill) | €1.72 | 60 mg retail price (minus 7.5% Royal Decree Law 8/2010) Portalfarma Database |
| Cost of warfarin (per pill) | €0.06 | Acenocoumarol 2.75 mg/day––retail price (minus 15% Royal Decree Law 8/2010) Portalfarma Database |
| Per-diem hospitalization cost | €866.23 | Cardiology department—eSalud Database 2017 |
| Cost of INR (per test) | €25.12 | Spanish Hospital Pharmacy Society report (Informe GENESIS de la SEFH, 2012.) NOACS report (apixaban, dabigatran, rivaroxaban). Prevención de eventos tromboembólicos en pacientes con fibrilación auricular no valvular. Available at: http://gruposdetrabajo.sefh.es/genesis/ |
| Outpatient visit (per visit) | €84.32 | Specialist outpatient office—eSalud Spanish Costs Database 2017 |
| Italy | ||
| Cost of edoxaban (per pill) | €2.09 | Ex-factory price IHS Global Insight Database |
| Cost of warfarin (per pill) | €0.07 | Codifa.it (coumadin cost per day) |
| Per-diem hospitalization cost | €587.00 | Progr 122: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 1 |
| Cost of INR (per test) | €26.09 | Progr 655 Visita generale ‘Supplemento ordinario N° 8 alla Gazzetta Ufficiale, 28/01/2013, Allegato 3: Prestazioni di assistenza specialistica ambulatoriale’ + Prestazione91.49.2 (prelievo sangue venoso) + 90.75.4 (tempo di protrombina) |
| Outpatient visit (per visit) | €55.78 | Progr 627-629-630: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 3 |
| USA | ||
| Cost of edoxaban (per pill) | $9.24 per day | Wholesale acquisition cost (WAC), Magnuson et al.10 |
| Cost of warfarin (per pill) | $0.36 per day | WAC, Magnuson et al.10 |
| Per-diem hospitalization cost | $2800 per day | Ad hoc analysis of hospitalizations of adult AF patients in Premier database (January–September 2015) |
| Cost of INR (per test) | $20 per test | Magnuson et al.10 |
| Outpatient visit (per visit) | $60 per visit | Magnuson et al.10 |
AF, atrial fibrillation; INR, international normalization ratio.
| Data input . | Cost estimate . | Source . |
|---|---|---|
| Germany | ||
| Cost of edoxaban (per pill) | €2.59 | Lauer-Taxe, February 2017 |
| Cost of warfarin (per pill) | €0.24 | Lauer-Taxe, February 2017 |
| Hospitalization cost (per diem) | €1412.00 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Cost of INR (per test) | €0.60 | AMNOG Dossier, Modul 3 (EBM Ziffer 32113) |
| Outpatient visit (per visit) | €28.86 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Spain | ||
| Cost of edoxaban (per pill) | €1.72 | 60 mg retail price (minus 7.5% Royal Decree Law 8/2010) Portalfarma Database |
| Cost of warfarin (per pill) | €0.06 | Acenocoumarol 2.75 mg/day––retail price (minus 15% Royal Decree Law 8/2010) Portalfarma Database |
| Per-diem hospitalization cost | €866.23 | Cardiology department—eSalud Database 2017 |
| Cost of INR (per test) | €25.12 | Spanish Hospital Pharmacy Society report (Informe GENESIS de la SEFH, 2012.) NOACS report (apixaban, dabigatran, rivaroxaban). Prevención de eventos tromboembólicos en pacientes con fibrilación auricular no valvular. Available at: http://gruposdetrabajo.sefh.es/genesis/ |
| Outpatient visit (per visit) | €84.32 | Specialist outpatient office—eSalud Spanish Costs Database 2017 |
| Italy | ||
| Cost of edoxaban (per pill) | €2.09 | Ex-factory price IHS Global Insight Database |
| Cost of warfarin (per pill) | €0.07 | Codifa.it (coumadin cost per day) |
| Per-diem hospitalization cost | €587.00 | Progr 122: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 1 |
| Cost of INR (per test) | €26.09 | Progr 655 Visita generale ‘Supplemento ordinario N° 8 alla Gazzetta Ufficiale, 28/01/2013, Allegato 3: Prestazioni di assistenza specialistica ambulatoriale’ + Prestazione91.49.2 (prelievo sangue venoso) + 90.75.4 (tempo di protrombina) |
| Outpatient visit (per visit) | €55.78 | Progr 627-629-630: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 3 |
| USA | ||
| Cost of edoxaban (per pill) | $9.24 per day | Wholesale acquisition cost (WAC), Magnuson et al.10 |
| Cost of warfarin (per pill) | $0.36 per day | WAC, Magnuson et al.10 |
| Per-diem hospitalization cost | $2800 per day | Ad hoc analysis of hospitalizations of adult AF patients in Premier database (January–September 2015) |
| Cost of INR (per test) | $20 per test | Magnuson et al.10 |
| Outpatient visit (per visit) | $60 per visit | Magnuson et al.10 |
| Data input . | Cost estimate . | Source . |
|---|---|---|
| Germany | ||
| Cost of edoxaban (per pill) | €2.59 | Lauer-Taxe, February 2017 |
| Cost of warfarin (per pill) | €0.24 | Lauer-Taxe, February 2017 |
| Hospitalization cost (per diem) | €1412.00 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Cost of INR (per test) | €0.60 | AMNOG Dossier, Modul 3 (EBM Ziffer 32113) |
| Outpatient visit (per visit) | €28.86 | Bruggenjurgen et al.9; Supplementary material online, Table S1 |
| Spain | ||
| Cost of edoxaban (per pill) | €1.72 | 60 mg retail price (minus 7.5% Royal Decree Law 8/2010) Portalfarma Database |
| Cost of warfarin (per pill) | €0.06 | Acenocoumarol 2.75 mg/day––retail price (minus 15% Royal Decree Law 8/2010) Portalfarma Database |
| Per-diem hospitalization cost | €866.23 | Cardiology department—eSalud Database 2017 |
| Cost of INR (per test) | €25.12 | Spanish Hospital Pharmacy Society report (Informe GENESIS de la SEFH, 2012.) NOACS report (apixaban, dabigatran, rivaroxaban). Prevención de eventos tromboembólicos en pacientes con fibrilación auricular no valvular. Available at: http://gruposdetrabajo.sefh.es/genesis/ |
| Outpatient visit (per visit) | €84.32 | Specialist outpatient office—eSalud Spanish Costs Database 2017 |
| Italy | ||
| Cost of edoxaban (per pill) | €2.09 | Ex-factory price IHS Global Insight Database |
| Cost of warfarin (per pill) | €0.07 | Codifa.it (coumadin cost per day) |
| Per-diem hospitalization cost | €587.00 | Progr 122: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 1 |
| Cost of INR (per test) | €26.09 | Progr 655 Visita generale ‘Supplemento ordinario N° 8 alla Gazzetta Ufficiale, 28/01/2013, Allegato 3: Prestazioni di assistenza specialistica ambulatoriale’ + Prestazione91.49.2 (prelievo sangue venoso) + 90.75.4 (tempo di protrombina) |
| Outpatient visit (per visit) | €55.78 | Progr 627-629-630: Supplemento ordinario N° 8 alla Gazzetta ufficiale 28/1/2013: allegato 3 |
| USA | ||
| Cost of edoxaban (per pill) | $9.24 per day | Wholesale acquisition cost (WAC), Magnuson et al.10 |
| Cost of warfarin (per pill) | $0.36 per day | WAC, Magnuson et al.10 |
| Per-diem hospitalization cost | $2800 per day | Ad hoc analysis of hospitalizations of adult AF patients in Premier database (January–September 2015) |
| Cost of INR (per test) | $20 per test | Magnuson et al.10 |
| Outpatient visit (per visit) | $60 per visit | Magnuson et al.10 |
AF, atrial fibrillation; INR, international normalization ratio.
Statistical analysis
All analyses were performed using the Safety Analysis Set (all randomized patients who received ≥1 dose of study medication). The two domain scores for PACT-Q2 were compared between the treatment regimens using least squares mean difference scores controlling for cardioversion approach and anticoagulant experience. The proportion of patients in the various EHRA classes by treatment regimen at randomization and end of treatment (Day 28) were compared using the Cochran–Mantel–Haenszel (CMH) test. Healthcare resource utilization was compared between the treatment regimens during the active treatment period (from randomization to Day 28 or study discontinuation visit, whichever was earlier).
Results
Patient demographic and clinical characteristics
Demographic and clinical characteristics of 2149 patients in the Safety Analysis Set were similar in the edoxaban and enoxaparin/warfarin treatment arms (Table 2). The majority of patients were male (65.2%) and white (97.7%); mean [standard deviation (SD)] age was 64.2 (10.53) years and mean (SD) CHA2DS2-VASc score was 2.6 (1.44). Most of the patients randomized to edoxaban (91.5%) did not receive a dose reduction.
| . | Edoxaban . | Enoxaparin/Warfarin . | ||||
|---|---|---|---|---|---|---|
| . | TOE (n = 570) . | Non-TOE (n = 497) . | Total (n = 1067) . | TOE (n = 577) . | Non-TOE (n = 505) . | Total (n = 1082) . |
| Age (years), mean (SD) | 64.7 (10.5) | 63.6 (10.2) | 64.2 (10.4) | 64.4 (11.1) | 63.7 (10.2) | 64.1 (10.7) |
| Male, n (%) | 376 (66.0) | 329 (66.2) | 705 (66.1) | 376 (65.2) | 329 (65.1) | 705 (65.2) |
| Race, n (%) | ||||||
| White | 551 (96.7) | 483 (97.2) | 1034 (96.9) | 565 (97.9) | 500 (99.0) | 1065 (98.4) |
| Black/African–American | 1 (0.2) | 4 (0.8) | 5 (0.5) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Asian | 0 | 2 (0.4) | 2 (0.2) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Not indicated | 18 (3.2) | 8 (1.6) | 26 (2.4) | 10 (1.7) | 2 (0.4) | 12 (1.1) |
| Weight (kg), mean (SD) | 90.3 (18.58) | 92.6 (18.15) | 90.9 (18.38) | 90.2 (17.21) | 92.3 (20.88) | 91.2 (19.03) |
| CrCl (mL/min), mean (SD) | 92.1 (35.83) | 96.3 (35.70) | 94.1 (35.81) | 91.9 (31.98) | 96.9 (37.40) | 94.3 (34.72) |
| Dose reduced, n (%)a | 48 (8.4) | 43 (8.7) | 91 (8.5) | 49 (8.5) | 40 (7.9) | 89 (8.2) |
| CHA2DS2-VASc, mean (SD) | 2.7 (1.52) | 2.5 (1.45) | 2.6 (1.49) | 2.7 (1.45) | 2.5 (1.33) | 2.6 (1.40) |
| HAS-BLED, mean (SD) | 0.9 (0.78) | 0.9 (0.77) | 0.9 (0.78) | 0.9 (0.81) | 0.9 (0.76) | 0.9 (0.76) |
| . | Edoxaban . | Enoxaparin/Warfarin . | ||||
|---|---|---|---|---|---|---|
| . | TOE (n = 570) . | Non-TOE (n = 497) . | Total (n = 1067) . | TOE (n = 577) . | Non-TOE (n = 505) . | Total (n = 1082) . |
| Age (years), mean (SD) | 64.7 (10.5) | 63.6 (10.2) | 64.2 (10.4) | 64.4 (11.1) | 63.7 (10.2) | 64.1 (10.7) |
| Male, n (%) | 376 (66.0) | 329 (66.2) | 705 (66.1) | 376 (65.2) | 329 (65.1) | 705 (65.2) |
| Race, n (%) | ||||||
| White | 551 (96.7) | 483 (97.2) | 1034 (96.9) | 565 (97.9) | 500 (99.0) | 1065 (98.4) |
| Black/African–American | 1 (0.2) | 4 (0.8) | 5 (0.5) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Asian | 0 | 2 (0.4) | 2 (0.2) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Not indicated | 18 (3.2) | 8 (1.6) | 26 (2.4) | 10 (1.7) | 2 (0.4) | 12 (1.1) |
| Weight (kg), mean (SD) | 90.3 (18.58) | 92.6 (18.15) | 90.9 (18.38) | 90.2 (17.21) | 92.3 (20.88) | 91.2 (19.03) |
| CrCl (mL/min), mean (SD) | 92.1 (35.83) | 96.3 (35.70) | 94.1 (35.81) | 91.9 (31.98) | 96.9 (37.40) | 94.3 (34.72) |
| Dose reduced, n (%)a | 48 (8.4) | 43 (8.7) | 91 (8.5) | 49 (8.5) | 40 (7.9) | 89 (8.2) |
| CHA2DS2-VASc, mean (SD) | 2.7 (1.52) | 2.5 (1.45) | 2.6 (1.49) | 2.7 (1.45) | 2.5 (1.33) | 2.6 (1.40) |
| HAS-BLED, mean (SD) | 0.9 (0.78) | 0.9 (0.77) | 0.9 (0.78) | 0.9 (0.81) | 0.9 (0.76) | 0.9 (0.76) |
CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, and prior Stroke or transient ischaemic attack or thromboembolism, Vascular disease, Age 65–74 years, Sex category; CrCl, creatinine clearance; HAS-BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding history or disposition, Labile INR, Elderly, Drugs or alcohol; INR, international normalized ratio; SD, standard deviation; TOE, transoesophageal echocardiography.
Patients meeting ≥1 of the following criteria: CrCl ≥15–≤50 mL/min, low body weight (≤60 kg), or concomitant use of P-gp inhibitors (with the exception of amiodarone).
| . | Edoxaban . | Enoxaparin/Warfarin . | ||||
|---|---|---|---|---|---|---|
| . | TOE (n = 570) . | Non-TOE (n = 497) . | Total (n = 1067) . | TOE (n = 577) . | Non-TOE (n = 505) . | Total (n = 1082) . |
| Age (years), mean (SD) | 64.7 (10.5) | 63.6 (10.2) | 64.2 (10.4) | 64.4 (11.1) | 63.7 (10.2) | 64.1 (10.7) |
| Male, n (%) | 376 (66.0) | 329 (66.2) | 705 (66.1) | 376 (65.2) | 329 (65.1) | 705 (65.2) |
| Race, n (%) | ||||||
| White | 551 (96.7) | 483 (97.2) | 1034 (96.9) | 565 (97.9) | 500 (99.0) | 1065 (98.4) |
| Black/African–American | 1 (0.2) | 4 (0.8) | 5 (0.5) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Asian | 0 | 2 (0.4) | 2 (0.2) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Not indicated | 18 (3.2) | 8 (1.6) | 26 (2.4) | 10 (1.7) | 2 (0.4) | 12 (1.1) |
| Weight (kg), mean (SD) | 90.3 (18.58) | 92.6 (18.15) | 90.9 (18.38) | 90.2 (17.21) | 92.3 (20.88) | 91.2 (19.03) |
| CrCl (mL/min), mean (SD) | 92.1 (35.83) | 96.3 (35.70) | 94.1 (35.81) | 91.9 (31.98) | 96.9 (37.40) | 94.3 (34.72) |
| Dose reduced, n (%)a | 48 (8.4) | 43 (8.7) | 91 (8.5) | 49 (8.5) | 40 (7.9) | 89 (8.2) |
| CHA2DS2-VASc, mean (SD) | 2.7 (1.52) | 2.5 (1.45) | 2.6 (1.49) | 2.7 (1.45) | 2.5 (1.33) | 2.6 (1.40) |
| HAS-BLED, mean (SD) | 0.9 (0.78) | 0.9 (0.77) | 0.9 (0.78) | 0.9 (0.81) | 0.9 (0.76) | 0.9 (0.76) |
| . | Edoxaban . | Enoxaparin/Warfarin . | ||||
|---|---|---|---|---|---|---|
| . | TOE (n = 570) . | Non-TOE (n = 497) . | Total (n = 1067) . | TOE (n = 577) . | Non-TOE (n = 505) . | Total (n = 1082) . |
| Age (years), mean (SD) | 64.7 (10.5) | 63.6 (10.2) | 64.2 (10.4) | 64.4 (11.1) | 63.7 (10.2) | 64.1 (10.7) |
| Male, n (%) | 376 (66.0) | 329 (66.2) | 705 (66.1) | 376 (65.2) | 329 (65.1) | 705 (65.2) |
| Race, n (%) | ||||||
| White | 551 (96.7) | 483 (97.2) | 1034 (96.9) | 565 (97.9) | 500 (99.0) | 1065 (98.4) |
| Black/African–American | 1 (0.2) | 4 (0.8) | 5 (0.5) | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Asian | 0 | 2 (0.4) | 2 (0.2) | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Not indicated | 18 (3.2) | 8 (1.6) | 26 (2.4) | 10 (1.7) | 2 (0.4) | 12 (1.1) |
| Weight (kg), mean (SD) | 90.3 (18.58) | 92.6 (18.15) | 90.9 (18.38) | 90.2 (17.21) | 92.3 (20.88) | 91.2 (19.03) |
| CrCl (mL/min), mean (SD) | 92.1 (35.83) | 96.3 (35.70) | 94.1 (35.81) | 91.9 (31.98) | 96.9 (37.40) | 94.3 (34.72) |
| Dose reduced, n (%)a | 48 (8.4) | 43 (8.7) | 91 (8.5) | 49 (8.5) | 40 (7.9) | 89 (8.2) |
| CHA2DS2-VASc, mean (SD) | 2.7 (1.52) | 2.5 (1.45) | 2.6 (1.49) | 2.7 (1.45) | 2.5 (1.33) | 2.6 (1.40) |
| HAS-BLED, mean (SD) | 0.9 (0.78) | 0.9 (0.77) | 0.9 (0.78) | 0.9 (0.81) | 0.9 (0.76) | 0.9 (0.76) |
CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75, Diabetes mellitus, and prior Stroke or transient ischaemic attack or thromboembolism, Vascular disease, Age 65–74 years, Sex category; CrCl, creatinine clearance; HAS-BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding history or disposition, Labile INR, Elderly, Drugs or alcohol; INR, international normalized ratio; SD, standard deviation; TOE, transoesophageal echocardiography.
Patients meeting ≥1 of the following criteria: CrCl ≥15–≤50 mL/min, low body weight (≤60 kg), or concomitant use of P-gp inhibitors (with the exception of amiodarone).
Convenience and treatment satisfaction
Treatment convenience and treatment satisfaction were significantly different between the two treatment arms in the overall study sample. Overall, patients in the edoxaban group reported significantly greater treatment convenience and satisfaction vs. enoxaparin/warfarin group as assessed by the PACT-Q2 domain scores (P < 0.001 for both) (Figure 1). In both TOE and non-TOE subgroups, patients receiving edoxaban had consistently and significantly higher satisfaction ratings on both convenience and treatment satisfaction than those receiving enoxaparin/warfarin. When the analysis was repeated by including only patients who did not have any protocol deviations and completed at least 28 days of study treatment, mean [standard error (SE)] treatment convenience score [86.31 (0.53) vs. 82.78 (0.533), P < 0.001] and treatment satisfaction score [70.58 (0.547) vs. 65.99 (0.549), P < 0.001) remained higher for the edoxaban group than the enoxaparin/warfarin group.
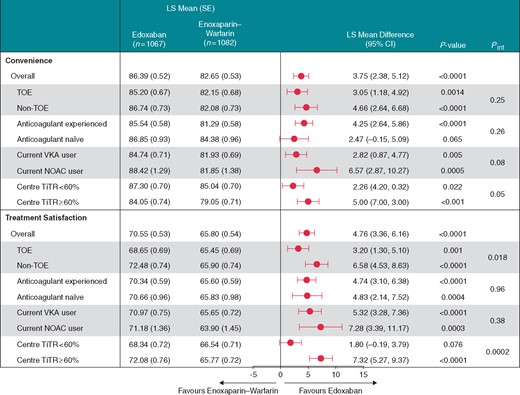
PACT-Q2 scores for the convenience and treatment satisfaction domains. CI, confidence interval; LS, least squares; NOAC, non-vitamin K antagonist oral anticoagulant; SE, standard error; TiTR, time in therapeutic range; TOE, transoesophageal echocardiography; VKA, vitamin K antagonists.
Edoxaban patients reported higher satisfaction ratings on both convenience and treatment satisfaction than enoxaparin/warfarin patients among those with prior experience with anticoagulation therapy (P < 0.001 for both). Both convenience and treatment satisfaction ratings were in favour of edoxaban in both patients who were using vitamin K antagonist (VKA) therapy or NOAC therapy prior to the study (P ≤ 0.005 for all comparisons). Among patients new to anticoagulant therapy, favourable rating for edoxaban vs. enoxaparin/warfarin was significant for treatment satisfaction (P = 0.004), but not for convenience (P = 0.065). Edoxaban patients also reported higher ratings for treatment convenience and treatment satisfaction vs. enoxaparin/warfarin in centres with time in therapeutic range (TiTR) ≥60% (P < 0.001 for both). For patients who had 2.0 ≤ international normalized ratio (INR) ≤ 3.0 during the on-treatment period, TiTR was defined as the percent of time in therapeutic range (2.0 ≤ INR ≤ 3.0) from the first date of 2.0 ≤ INR ≤ 3.0. Among patients from centres with TiTR <60%, favourable ratings for edoxaban vs. enoxaparin/warfarin was significant for treatment convenience (P = 0.022) but not for treatment satisfaction (P = 0.076).
Atrial fibrillation-related symptoms
The proportions of patients in each EHRA class at baseline were similar between the edoxaban group and enoxaparin/warfarin group (Figure 2). More patients reported no AF symptoms (EHRA I) at end of treatment (Day 28) than at baseline, irrespective of treatment group. The proportion of patients in each EHRA class was not significantly different between the treatment group overall (P = 0.88) or between treatment groups in the TOE stratum (P = 0.89) and the non-TOE stratum (P = 0.86; data not shown).
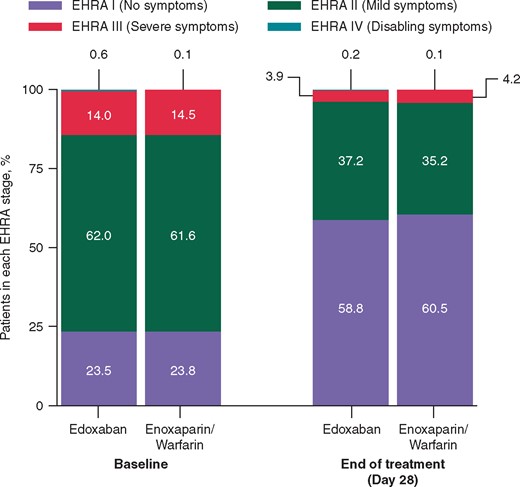
Comparison of EHRA stage change results at baseline and end of treatment. EHRA, European Heart Rhythm Association.
Healthcare utilization outcomes
The proportion of patients with ≥1 hospital admission was similar between the treatment groups during the 28-day active treatment period (Table 3). Patients in the edoxaban group had significantly shorter hospital stay vs. enoxaparin/warfarin. Most hospital admissions were categorized as CV-related in both arms. The incidence of bleeding-related hospitalizations, emergency room visits for CV-related reasons, and the number of outpatient visits did not differ between treatment groups.
| . | Edoxaban (n = 1067) . | Enoxaparin/ warfarin (n = 1082) . | P-value . |
|---|---|---|---|
| All-cause hospitalization | |||
| Number of admissions | 41 | 41 | |
| Patients with ≥1 hospitalizations, n (%) | 40 (3.7) | 38 (3.5) | 0.7692 |
| Hospital days, mean (SD) | 3.43 (2.69) | 5.41 (4.76) | 0.0260 |
| Reasons for hospital admissions, n | |||
| Bleeding-related | 3 | 2 | |
| CV-related | 32 | 31 | |
| Arrhythmia | 27 | 25 | |
| CHF | 2 | 3 | |
| Other CV-related | 3 | 3 | |
| Non-CV/bleeding-related | 10 | 8 | |
| CV-related hospitalization | |||
| Patients with ≥1 hospitalization, n (%) | 31 (2.9) | 29 (2.7) | 0.7515 |
| Hospital days, mean (SD) | 3.06 (2.46) | 5.07 (4.06) | 0.0234 |
| CV-related ER visitsa | |||
| Patients with ≥1 ER visits, n (%) | 11 (1.0) | 5 (0.5) | 0.125 |
| . | Edoxaban (n = 1067) . | Enoxaparin/ warfarin (n = 1082) . | P-value . |
|---|---|---|---|
| All-cause hospitalization | |||
| Number of admissions | 41 | 41 | |
| Patients with ≥1 hospitalizations, n (%) | 40 (3.7) | 38 (3.5) | 0.7692 |
| Hospital days, mean (SD) | 3.43 (2.69) | 5.41 (4.76) | 0.0260 |
| Reasons for hospital admissions, n | |||
| Bleeding-related | 3 | 2 | |
| CV-related | 32 | 31 | |
| Arrhythmia | 27 | 25 | |
| CHF | 2 | 3 | |
| Other CV-related | 3 | 3 | |
| Non-CV/bleeding-related | 10 | 8 | |
| CV-related hospitalization | |||
| Patients with ≥1 hospitalization, n (%) | 31 (2.9) | 29 (2.7) | 0.7515 |
| Hospital days, mean (SD) | 3.06 (2.46) | 5.07 (4.06) | 0.0234 |
| CV-related ER visitsa | |||
| Patients with ≥1 ER visits, n (%) | 11 (1.0) | 5 (0.5) | 0.125 |
Treatment period from randomization to Day 28 or study discontinuation, whichever came first.
CHF, congestive heart failure; CV, cardiovascular; ER, emergency room visit; SD, standard deviation.
ER visits not resulting in hospitalizations or outpatient visits for CV-related causes not associated with the study protocol.
| . | Edoxaban (n = 1067) . | Enoxaparin/ warfarin (n = 1082) . | P-value . |
|---|---|---|---|
| All-cause hospitalization | |||
| Number of admissions | 41 | 41 | |
| Patients with ≥1 hospitalizations, n (%) | 40 (3.7) | 38 (3.5) | 0.7692 |
| Hospital days, mean (SD) | 3.43 (2.69) | 5.41 (4.76) | 0.0260 |
| Reasons for hospital admissions, n | |||
| Bleeding-related | 3 | 2 | |
| CV-related | 32 | 31 | |
| Arrhythmia | 27 | 25 | |
| CHF | 2 | 3 | |
| Other CV-related | 3 | 3 | |
| Non-CV/bleeding-related | 10 | 8 | |
| CV-related hospitalization | |||
| Patients with ≥1 hospitalization, n (%) | 31 (2.9) | 29 (2.7) | 0.7515 |
| Hospital days, mean (SD) | 3.06 (2.46) | 5.07 (4.06) | 0.0234 |
| CV-related ER visitsa | |||
| Patients with ≥1 ER visits, n (%) | 11 (1.0) | 5 (0.5) | 0.125 |
| . | Edoxaban (n = 1067) . | Enoxaparin/ warfarin (n = 1082) . | P-value . |
|---|---|---|---|
| All-cause hospitalization | |||
| Number of admissions | 41 | 41 | |
| Patients with ≥1 hospitalizations, n (%) | 40 (3.7) | 38 (3.5) | 0.7692 |
| Hospital days, mean (SD) | 3.43 (2.69) | 5.41 (4.76) | 0.0260 |
| Reasons for hospital admissions, n | |||
| Bleeding-related | 3 | 2 | |
| CV-related | 32 | 31 | |
| Arrhythmia | 27 | 25 | |
| CHF | 2 | 3 | |
| Other CV-related | 3 | 3 | |
| Non-CV/bleeding-related | 10 | 8 | |
| CV-related hospitalization | |||
| Patients with ≥1 hospitalization, n (%) | 31 (2.9) | 29 (2.7) | 0.7515 |
| Hospital days, mean (SD) | 3.06 (2.46) | 5.07 (4.06) | 0.0234 |
| CV-related ER visitsa | |||
| Patients with ≥1 ER visits, n (%) | 11 (1.0) | 5 (0.5) | 0.125 |
Treatment period from randomization to Day 28 or study discontinuation, whichever came first.
CHF, congestive heart failure; CV, cardiovascular; ER, emergency room visit; SD, standard deviation.
ER visits not resulting in hospitalizations or outpatient visits for CV-related causes not associated with the study protocol.
The patients in the enoxaparin/warfarin group visited the investigational site more often than patients in the edoxaban group by Days 8 and 28 (P < 0.0001 for both; Figure 3). The number of investigational site visits did not significantly differ between patients who had a centre-level TiTR of the INR <60% vs. those with a centre-level TiTR ≥60% on Day 8 (P = 0.187) and Day 28 (P = 0.202), for the total sample (data not shown).
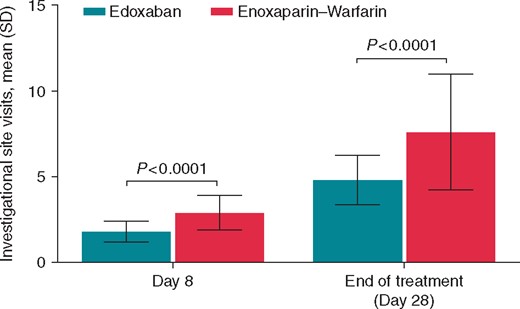
Visits to the investigational site per patient by Day 8 and during 28-day active treatment period. SD, standard deviation.
Healthcare costs
Cost estimates for per-diem hospitalization, INR monitoring, outpatient office visits, and unit drug cost for edoxaban and warfarin were applied to assess healthcare cost during the 28 days of active treatment.
Based on cost data from the German healthcare setting, using edoxaban in place of warfarin resulted in a saving of €107.73 per patient during the 28 days of treatment. Difference in drug acquisition cost between edoxaban and warfarin was completely offset by reductions in hospital days, outpatient visits, and INR monitoring. Similar cost savings were noted in Spanish (−€437.92), Italian (−€336.75), and US (−$246.32) healthcare settings (Table 4).
| . | Germany . | Spain . | Italy . | USA . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . |
| (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | |
| Anticoagulant cost (28-day treatment) | €77 378.84 | €7271.04 | €51 386.72 | €1802.61 | €62 440.84 | €2120.72 | $276 054.24 | $10 906.56 |
| Hospitalization cost | €193 726.40 | €290 278.96 | €118 815.20 | €178 032.28 | €80 536.40 | €120 675.46 | $384 160.00 | $575 624.00 |
| Office visit + INR monitoring | €146 269.70 | €242 255.47 | €427 354.84 | €899 947.01 | €282 706.99 | €673 233.38 | $304 095.00 | $657 856.00 |
| Total cost in study cohort | €417 374.94 | €539 805.47 | €597 556.76 | €1 079 781.90 | €425 684.23 | €796 029.56 | $964 309.24 | $1 244 386.56 |
| Total cost per patient | €391.17 | €498.90 | €560.03 | €997.95 | €398.95 | €735.70 | $903.76 | $1150.08 |
| Difference per patient | −€107.73 | −€437.92 | −€336.75 | −$246.32 | ||||
| . | Germany . | Spain . | Italy . | USA . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . |
| (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | |
| Anticoagulant cost (28-day treatment) | €77 378.84 | €7271.04 | €51 386.72 | €1802.61 | €62 440.84 | €2120.72 | $276 054.24 | $10 906.56 |
| Hospitalization cost | €193 726.40 | €290 278.96 | €118 815.20 | €178 032.28 | €80 536.40 | €120 675.46 | $384 160.00 | $575 624.00 |
| Office visit + INR monitoring | €146 269.70 | €242 255.47 | €427 354.84 | €899 947.01 | €282 706.99 | €673 233.38 | $304 095.00 | $657 856.00 |
| Total cost in study cohort | €417 374.94 | €539 805.47 | €597 556.76 | €1 079 781.90 | €425 684.23 | €796 029.56 | $964 309.24 | $1 244 386.56 |
| Total cost per patient | €391.17 | €498.90 | €560.03 | €997.95 | €398.95 | €735.70 | $903.76 | $1150.08 |
| Difference per patient | −€107.73 | −€437.92 | −€336.75 | −$246.32 | ||||
INR, international normalization ratio.
| . | Germany . | Spain . | Italy . | USA . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . |
| (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | |
| Anticoagulant cost (28-day treatment) | €77 378.84 | €7271.04 | €51 386.72 | €1802.61 | €62 440.84 | €2120.72 | $276 054.24 | $10 906.56 |
| Hospitalization cost | €193 726.40 | €290 278.96 | €118 815.20 | €178 032.28 | €80 536.40 | €120 675.46 | $384 160.00 | $575 624.00 |
| Office visit + INR monitoring | €146 269.70 | €242 255.47 | €427 354.84 | €899 947.01 | €282 706.99 | €673 233.38 | $304 095.00 | $657 856.00 |
| Total cost in study cohort | €417 374.94 | €539 805.47 | €597 556.76 | €1 079 781.90 | €425 684.23 | €796 029.56 | $964 309.24 | $1 244 386.56 |
| Total cost per patient | €391.17 | €498.90 | €560.03 | €997.95 | €398.95 | €735.70 | $903.76 | $1150.08 |
| Difference per patient | −€107.73 | −€437.92 | −€336.75 | −$246.32 | ||||
| . | Germany . | Spain . | Italy . | USA . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . | Edoxaban . | Warfarin . |
| (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | (n = 1067) . | (n = 1082) . | |
| Anticoagulant cost (28-day treatment) | €77 378.84 | €7271.04 | €51 386.72 | €1802.61 | €62 440.84 | €2120.72 | $276 054.24 | $10 906.56 |
| Hospitalization cost | €193 726.40 | €290 278.96 | €118 815.20 | €178 032.28 | €80 536.40 | €120 675.46 | $384 160.00 | $575 624.00 |
| Office visit + INR monitoring | €146 269.70 | €242 255.47 | €427 354.84 | €899 947.01 | €282 706.99 | €673 233.38 | $304 095.00 | $657 856.00 |
| Total cost in study cohort | €417 374.94 | €539 805.47 | €597 556.76 | €1 079 781.90 | €425 684.23 | €796 029.56 | $964 309.24 | $1 244 386.56 |
| Total cost per patient | €391.17 | €498.90 | €560.03 | €997.95 | €398.95 | €735.70 | $903.76 | $1150.08 |
| Difference per patient | −€107.73 | −€437.92 | −€336.75 | −$246.32 | ||||
INR, international normalization ratio.
Discussion
In this prespecified ancillary analysis of the largest randomized trial for anticoagulation pericardioversion, patients receiving edoxaban consistently reported higher ratings of convenience and treatment satisfaction with study treatment than patients receiving enoxaparin/warfarin. The difference in satisfaction ratings is unlikely to be related to clinical outcomes because AF-related symptom improvement post-cardioversion and rates of hospitalization were similar between the edoxaban and enoxaparin/warfarin treatment arms. However, the duration of hospitalization was significantly shorter in patients receiving edoxaban vs. enoxaparin/warfarin during the active treatment period, and edoxaban patients also had fewer visits to the investigational sites than patients on enoxaparin/warfarin. These differences in healthcare resource use also suggest that edoxaban could reduce the overall healthcare cost in patients undergoing electrical cardioversion of NVAF.
Data assessing treatment convenience/satisfaction and healthcare cost in NVAF patients undergoing cardioversion are limited. The ENSURE-AF trial is the largest electrical cardioversion trial in NVAF, which assessed differences in treatment convenience, treatment satisfaction, and healthcare utilization in patients treated with edoxaban vs. warfarin/enoxaparin therapy.
Patient preference for NOAC therapy over VKA have been shown in the PREvention oF thromboembolic events—European Registry in Atrial Fibrillation (PREFER in AF) previously.11 In the PREFER in AF registry, patients receiving NOAC therapy reported higher ratings of convenience and treatment satisfaction than patients receiving VKA therapy. Considering edoxaban does not require routine laboratory monitoring, patients are expected not to have to visit healthcare facilities as frequently as those on VKA therapy; this is especially the case during the first few weeks of therapy when dose adjustment of VKA necessitates closer monitoring. Our findings corroborate this expectation; we found that edoxaban patients in ENSURE-AF incurred significantly fewer clinic visits during the first week of treatment initiation, and statistically significant reduction was maintained over the 28-day treatment period.
The assigned strategy of cardioversion (TOE- vs. non-TOE-guided approach) appears to have some impact on treatment satisfaction. Edoxaban patients undergoing a non-TOE-guided approach show most pronounced differences in treatment satisfaction ratings relative to standard enoxaparin/warfarin therapy than patients undergoing TOE -guided cardioversion. This might be explained by the longer exposure to enoxaparin/warfarin in this strategy (23 vs. 3 days in TOE-guided patients). This finding may also suggest that inconvenience might be even more pronounced if therapy with warfarin is prolonged. Our study also shows that prior anticoagulant treatment experience may play an important role in perceived treatment convenience. Although anticoagulation-naïve patients did not perceive significant differences between edoxaban and enoxaparin/warfarin in terms of treatment convenience, patients who used NOAC therapy before the study perceived larger difference in convenience favouring edoxaban vs. enoxaparin/warfarin therapy, with a non-significant trend (Pint = 0.08). Similarly, differences in intensity of INR monitoring between study centres with TiTR <60% and study centres with TiTR ≥60% may explain the observed difference in results of treatment satisfaction ratings between edoxaban and standard therapy in these two settings.
The present study shows that edoxaban regimens reduced utilization of healthcare services and costs. The reduction of healthcare service use and cost are independent of severity of symptoms and outcome of cardioversion as these outcomes were not significantly different between edoxaban and enoxaparin/warfarin group. Using published cost estimates, our analysis estimated that healthcare cost during the 28 days of oral anticoagulant treatment in patients undergoing electrocardioversion was lower for edoxaban compared with enoxaparin/warfarin, with a cost savings ranging from €107.73 to €437.92 per patient depending on healthcare settings. The cost difference was driven by reduction in the length of hospitalization and the reduced number of outpatient visits in edoxaban-treated patients. As the incidence and prevalence of AF are projected to rise over time, this cost difference due to the reduced length of hospitalization with edoxaban vs. warfarin may contribute to even greater future resource savings from the perspective of the overall healthcare system.12
The cost of NOAC therapy vs. warfarin in cardioversion has previously been examined in the X-VeRT study.13 Considering the costs for drug therapy, monitoring of VKAs, and the cost of cardioversion procedure and rescheduling of procedure, the X-VeRT study estimated the use of rivaroxaban will result in a savings of £421 and €360 per patient in UK and Italian settings, respectively. Of note, our cost analysis methodology and objective was different from the X-VeRT study in that the latter focused on the impact of postponed cardioversions and rescheduling of the procedure. Unfortunately, data on changes of cardioversion scheduling were not captured in the ENSURE-AF and were not included in our cost analysis. Therefore, our results may have underestimated the potential cost savings to healthcare systems.
Limitations
The study has several limitations. First, the study used an open-label study design. Although clinical endpoint evaluation was blinded to study investigators in ENSURE-AF, patient-reported outcomes evaluation could not be blinded. Knowledge of treatment may introduce biases to study results by affecting expectations and may distort the subjectivity of outcome assessment. In addition, VKA-experienced patients who were not satisfied with VKA previously may be more interested in participating in the study.14 Investigators also may be more willing to select patients who are good candidates for NOACs to participate in the trial. As a result, patient selection bias can limit the generalizability of study results. Furthermore, the study was a multinational clinical trial enrolling patients receiving clinical care under different healthcare systems. We applied unit cost from a single country to resource use data collected from all patients in ENSURE-AF. This approach assumes healthcare resource use findings are similar and transferable from one country to another.15 While clinical findings may be transferable from one country to another, economic endpoints such as hospitalizations may be affected by local clinical practice and resource availability and not generalizable from one country to another.
Conclusion
In conclusion, patients in ENSURE-AF consistently favoured edoxaban over enoxaparin/warfarin in terms of convenience and treatment satisfaction. Atrial fibrillation-related symptom improvement post-cardioversion and rates of hospitalization were similar between the edoxaban and enoxaparin/warfarin treatment arms. The convenience of edoxaban therapy over warfarin in patients undergoing cardioversion may provide greater treatment satisfaction and healthcare cost savings.
Acknowledgements
The editorial support was provided by Narender Dhingra, MBBS, PhD, of AlphaBioCom, LLC, and funded by Daiichi Sankyo, Inc. The authors would like to thank Mark Atkinson, PhD, for assistance in statistical analysis and Jose M. Rodriguez Barrios, PhD, MPH, for providing data on European cost estimates.
Funding
Daiichi Sankyo, Inc.
Conflict of interest: A.G. has served as a consultant for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer; and as a speaker for AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi. W.J.K. is employed by Daiichi Sankyo. D.Z. was employed by Daiichi Sankyo at the time of the conduction of the study. M.D.E. has served as a consultant for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Medtronic, Merck, Pfizer, Portola, and Sanofi; and as a speaker for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, and Pfizer. M.B. and S.P.H. have nothing to disclose. G.Y.H.L. has served on consultant/conferences/advisory board for AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Meda, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.



