-
PDF
- Split View
-
Views
-
Cite
Cite
Melissa E Middeldorp, Rajeev K Pathak, Megan Meredith, Abhinav B Mehta, Adrian D Elliott, Rajiv Mahajan, Darragh Twomey, Celine Gallagher, Jeroen M L Hendriks, Dominik Linz, R Doug McEvoy, Walter P Abhayaratna, Jonathan M Kalman, Dennis H Lau, Prashanthan Sanders, PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study, EP Europace, Volume 20, Issue 12, December 2018, Pages 1929–1935, https://doi.org/10.1093/europace/euy117
Close - Share Icon Share
Abstract
Atrial fibrillation (AF) is a progressive disease. Obesity is associated with progression of AF. This study evaluates the impact of weight and risk factor management (RFM) on progression of the AF.
As described in the Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up (LEGACY) Study, of 1415 consecutive AF patients, 825 had body mass index ≥ 27 kg/m2 and were offered weight and RFM. After exclusion, 355 were included for analysis. Weight loss was categorized as: Group 1 (<3%), Group 2 (3–9%), and Group 3 (≥10%). Change in AF type was determined by clinical review and 7-day Holter yearly. Atrial fibrillation type was categorized as per the Heart Rhythm Society consensus. There were no differences in baseline characteristic or follow-up duration between groups (P = NS). In Group 1, 41% progressed from paroxysmal to persistent and 26% from persistent to paroxysmal or no AF. In Group 2, 32% progressed from paroxysmal to persistent and 49% reversed from persistent to paroxysmal or no AF. In Group 3, 3% progressed to persistent and 88% reversed from persistent to paroxysmal or no AF (P < 0.001). Increased weight loss was significantly associated with greater AF freedom: 45 (39%) in Group 1, 69 (67%) in Group 2, and 116 (86%) in Group 3 (P ≤ 0.001).
Obesity is associated with progression of the AF disease. This study demonstrates the dynamic relationship between weight/risk factors and AF. Weight-loss management and RFM reverses the type and natural progression of AF.
Atrial fibrillation (AF) is a known progressive disease, weight loss of >10% with management of associated risk factors is associated with a reversal in AF type to more paroxysmal forms of the disease.
Progression of the AF disease is demonstrated to have a relationship between the degree of weight loss with less weight loss results in more progression of AF to more persistent forms of AF.
It has been shown that weight loss and risk factor management are an essential component in the management of patient’s symptoms and burden and is associated with a change in AF type and freedom of AF.
Introduction
Atrial fibrillation (AF) is a progressive disease. Over the course of time, many patients progress from paroxysmal AF (PAF) to persistent AF (PrsAF) and eventually more sustained forms of AF.1,2 Whilst this phenomenon was initially considered to be part of the arrhythmic process,2 more recent data suggest that both the type of AF and the likelihood of progression to more persistent forms of AF, are determined by the number of concomitant risk factors that are harboured.1,3 Indeed, progressive atrial remodelling has been documented in patients after successful AF ablation, implicating a detrimental role of persistent risk factors.4 Previous studies have looked at risk factors that contribute to the progression of AF to more persistent forms; however, none of these assessed the impact of treating risk factors to reverse AF disease.
In the Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up (LEGACY) study,5 we demonstrated that intensive weight and risk factor management had a dose-dependent effect on overall long-term freedom from AF. However, the impact of weight and risk factor modification on the progression of AF has not yet been characterized. Here, we hypothesize that weight-loss and concurrent risk factor management not only reduces patient AF symptoms and recurrence, but also has potential to ‘reverse’ the disease process.
Methods
Study population
This study comprised consecutive patients with symptomatic AF referred to the Centre for Heart Rhythm Disorders at the University of Adelaide, Australia. The details of the study registry have been presented in the LEGACY study,5 this study is a retrospective, sub-analysis of the LEGACY study. In brief, patients included in the analysis had a body mass index (BMI) ≥27 kg/m2. The study excluded those who had a history of myocardial infarction or cardiac surgery in the previous 12 months, significant cardiac valvulopathy or ventricular dysfunction, active malignancy, auto-immune or systemic inflammatory diseases, severe renal or hepatic failure, and <24 months of follow-up and/or from other states.
The study was approved by the Human Research Ethics Committee of the Royal Adelaide Hospital and the University of Adelaide, Adelaide, Australia. ANZCTR Clinical Trial Registration: ACTRN12614001123639.
Weight-loss and risk factor management
All patients were offered participation in a dedicated physician-led clinic focused on weight and risk factors. The weight and risk factor management programme used by our clinic has been previously presented.5,6 This is a goal-directed, motivational, structured program where patients received one on one individualized counselling. Weight was initially managed by providing a tailored programme. Patients maintained a lifestyle journal and were provided with a meal plan with an initial target of >10% weight loss. Meal replacement was prescribed if patients lost <3% at 3 months. Patients were advised to undertake 30 min of exercise 3–4 times a week with the aim to increase this to 200 min per week. Hypertension was managed with salt restriction and pharmacotherapy as required. Patients were encouraged to monitor blood pressure 2–3 times a day. We aimed for 80% of home blood pressure readings to be <130/80 mmHg, with the blood pressure during rest and exercise consistently <130/80 mmHg and <200/100 mmHg, respectively. This was corroborated by in office blood pressure readings, exercise stress testing screening for exercise-induced hypertension, 24-h ambulatory monitoring, and echocardiography to ensure the resolution of left ventricular hypertrophy. Cholesterol and glucose intolerance was managed initially with lifestyle measures; however, if this was not achieved pharmacotherapy was prescribed. Patients underwent sleep study with continuous positive airway pressure therapy prescribed if their apnoea–hypopnoea index ≥30/h. Smoking cessation was encouraged along with alcohol reduction to ≤30 g/week is advised.
Weight-loss definition
As per LEGACY study,5 weight loss groups were divided as follows: <3% weight loss or weight gain (Group 1); 3–9% weight loss (Group 2); and ≥10% weight loss (Group 3).
Categorization of atrial fibrillation
Type of atrial fibrillation
Based on the patients’ clinical history and 7-day Holter monitoring results, patients were divided into the following groups as defined by the Heart Rhythm Society Consensus Statement7:
Paroxysmal AF: AF episodes that are self-terminating and last less than 1 week;
Persistent AF: AF episodes either lasting >7 days or requiring termination by cardioversion, either with pharmacotherapy or by direct current cardioversion (DCC) after this time.
To assess the change in AF type, AF type was taken according to the clinical status over the preceding 12 months.
Burden of atrial fibrillation
To further categorize type of AF based on AF burden, episodes were divided per duration at baseline and in the last 12 months of follow-up:
Paroxysmal AF: Episodes lasting ≤48 h (short PAF) and episodes lasting >48 h but <1 week but spontaneously reverted to sinus rhythm (long PAF).
Persistent AF: Episodes lasting ≥1 week (short PrsAF) and <3 months and episodes lasting ≥3 months (long PrsAF).
Arrhythmia management
Management of AF was undertaken in a separate dedicated AF clinic independent of the weight management clinic. Usage of rate and rhythm control strategies was at the discretion of the treating physician. Sotalol and Flecainide were preferred anti-arrhythmic drugs (AAD). Amiodarone was not routinely used. Ablation was advocated in patients who remained symptomatic despite use of AAD and risk factor management. The ablation technique utilized at our institution has been previously described.8 Atrial fibrillation type and burden was determined by at least annual clinical review, 12-lead electrocardiogram (ECG), device interrogation, and 7-day Holter monitoring. In patients undergoing ablation, procedural success was determined after a 3-month blanking period. Recurrent arrhythmia was defined as any atrial arrhythmia ≥30 s. The earliest date with documented AF was set as the date of arrhythmia recurrence. Follow-up was standard for our clinic with a follow-up duration for each group 48.4 ± 18.2, 46.0 ± 16.7, and 48.3 ± 18.4 months respectively (P = 0.3).
Study outcomes
Primary outcome was change in AF category from baseline to the last year of review. Atrial fibrillation status was determined by patient symptoms, 7-day Holter monitoring, ECG, or implantable device. Secondary outcomes included AF episode burden as assessed by 7-day Holter, need for single or multiple AF ablation procedures, AV node ablation and pacemaker implantation.
Statistical analysis
Categorical variables are represented as frequencies and percentages. Continuous variables are summarized as mean ± SD. Differences between the weight-loss groups were assessed using ANOVA procedures for baseline characteristics. A repeated measure ANOVA was used to assess change over time. For categorical variables, change in status at follow-up was compared between groups using a χ2 test. Two-tailed P < 0.05 was considered statistically significant. Statistically significant predictor of progression of AF was assessed using a logistic regression model. Candidate variables with P < 0.1 in univariate analyses were considered in multivariate regression models. Statistical analysis was performed with SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
As described in LEGACY,5 of the 1415 consecutive patients with symptomatic AF, 825 patients had a BMI ≥27 kg/m2. After screening for exclusion criteria, the final cohort consisted of 355 patients (Figure 1): 116 in Group 1 (<3% weight loss), 104 in Group 2 (3–9% weight loss), and 135 in Group 3 (≥10% weight loss). Baseline characteristics and follow-up duration (48.3 ± 18.4, 46.0 ± 16.7, and 48.4 ± 18.2 months respectively, P = 0.3) were similar for all groups (Table 1).
| . | <3% WL Group 1 n = 116 . | 3–9% WL Group 2 n = 104 . | ≥10% WL Group 3 n = 135 . | P-value . |
|---|---|---|---|---|
| Age (years) | 61 ± 11 | 63 ± 11 | 65 ± 11 | 0.06 |
| Male gender, n (%) | 83 (71) | 65 (63) | 86 (64) | 0.37 |
| Anthropometric measures and blood pressure | ||||
| BMI (kg/m2) | 32.9 ± 4.8 | 32.7 ± 4.4 | 33.6 ± 4.7 | 0.24 |
| SBP (mmHg) | 146 ± 17 | 144 ± 17 | 147 ± 17 | 0.33 |
| Atrial fibrillation type | ||||
| Paroxysmal AF, n (%) | 61 (53) | 62 (60) | 73 (54) | 0.55 |
| Non-paroxysmal, n (%) | 55 (47) | 42 (40) | 62 (46) | |
| Atrial episode burden, n (%) | ||||
| 48 h | 29 (25) | 13 (13) | 24 (18) | 0.39 |
| <1 week with no DCCV | 48 (41) | 49 (47) | 56 (42) | |
| >1 week OR DCCV | 35 (30) | 37 (36) | 49 (36) | |
| 3 months | 4 (3) | 5 (5) | 6 (4) | |
| Metabolic risk factors | ||||
| Hypertension, n (%) | 90 (78) | 75 (73) | 109 (81) | 0.30 |
| DM, n (%) | 34 (29) | 28 (27) | 41 (30) | 0.35 |
| IGT, n (%) | 8 (7) | 8 (8) | 18 (13) | |
| Hyperlipidaemia, n (%) | 56 (48) | 45 (44) | 66 (49) | 0.70 |
| Coronary artery disease, n (%) | 11 (9) | 12 (12) | 21 (16) | 0.31 |
| AHI > 30, n (%) | 61 (52) | 52 (50) | 69 (51) | 0.97 |
| Alcohol excess (>30 g/week), n (%) | 34 (29) | 35 (34) | 42 (31) | 0.73 |
| Smoker, n (%) | 47 (40) | 41 (40) | 50 (37) | 0.86 |
| Medication use | ||||
| Mean no. of anti-arrhythmic (±SD) | 0.9 ± 0.8 | 1.0 ± 0.7 | 1.1 ± 0.7 | 0.10 |
| Mean no. of anti-hypertensive (±SD) | 1.1 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.9 | 0.08 |
| . | <3% WL Group 1 n = 116 . | 3–9% WL Group 2 n = 104 . | ≥10% WL Group 3 n = 135 . | P-value . |
|---|---|---|---|---|
| Age (years) | 61 ± 11 | 63 ± 11 | 65 ± 11 | 0.06 |
| Male gender, n (%) | 83 (71) | 65 (63) | 86 (64) | 0.37 |
| Anthropometric measures and blood pressure | ||||
| BMI (kg/m2) | 32.9 ± 4.8 | 32.7 ± 4.4 | 33.6 ± 4.7 | 0.24 |
| SBP (mmHg) | 146 ± 17 | 144 ± 17 | 147 ± 17 | 0.33 |
| Atrial fibrillation type | ||||
| Paroxysmal AF, n (%) | 61 (53) | 62 (60) | 73 (54) | 0.55 |
| Non-paroxysmal, n (%) | 55 (47) | 42 (40) | 62 (46) | |
| Atrial episode burden, n (%) | ||||
| 48 h | 29 (25) | 13 (13) | 24 (18) | 0.39 |
| <1 week with no DCCV | 48 (41) | 49 (47) | 56 (42) | |
| >1 week OR DCCV | 35 (30) | 37 (36) | 49 (36) | |
| 3 months | 4 (3) | 5 (5) | 6 (4) | |
| Metabolic risk factors | ||||
| Hypertension, n (%) | 90 (78) | 75 (73) | 109 (81) | 0.30 |
| DM, n (%) | 34 (29) | 28 (27) | 41 (30) | 0.35 |
| IGT, n (%) | 8 (7) | 8 (8) | 18 (13) | |
| Hyperlipidaemia, n (%) | 56 (48) | 45 (44) | 66 (49) | 0.70 |
| Coronary artery disease, n (%) | 11 (9) | 12 (12) | 21 (16) | 0.31 |
| AHI > 30, n (%) | 61 (52) | 52 (50) | 69 (51) | 0.97 |
| Alcohol excess (>30 g/week), n (%) | 34 (29) | 35 (34) | 42 (31) | 0.73 |
| Smoker, n (%) | 47 (40) | 41 (40) | 50 (37) | 0.86 |
| Medication use | ||||
| Mean no. of anti-arrhythmic (±SD) | 0.9 ± 0.8 | 1.0 ± 0.7 | 1.1 ± 0.7 | 0.10 |
| Mean no. of anti-hypertensive (±SD) | 1.1 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.9 | 0.08 |
AF, atrial fibrillation; AHI, apnea–hypopnea index; BMI, body mass index; DCCV, direct current cardioversion; DM, diabetes mellitus; IGT, impaired glucose tolerance; SBP, systolic blood pressure; WL, weight loss.
| . | <3% WL Group 1 n = 116 . | 3–9% WL Group 2 n = 104 . | ≥10% WL Group 3 n = 135 . | P-value . |
|---|---|---|---|---|
| Age (years) | 61 ± 11 | 63 ± 11 | 65 ± 11 | 0.06 |
| Male gender, n (%) | 83 (71) | 65 (63) | 86 (64) | 0.37 |
| Anthropometric measures and blood pressure | ||||
| BMI (kg/m2) | 32.9 ± 4.8 | 32.7 ± 4.4 | 33.6 ± 4.7 | 0.24 |
| SBP (mmHg) | 146 ± 17 | 144 ± 17 | 147 ± 17 | 0.33 |
| Atrial fibrillation type | ||||
| Paroxysmal AF, n (%) | 61 (53) | 62 (60) | 73 (54) | 0.55 |
| Non-paroxysmal, n (%) | 55 (47) | 42 (40) | 62 (46) | |
| Atrial episode burden, n (%) | ||||
| 48 h | 29 (25) | 13 (13) | 24 (18) | 0.39 |
| <1 week with no DCCV | 48 (41) | 49 (47) | 56 (42) | |
| >1 week OR DCCV | 35 (30) | 37 (36) | 49 (36) | |
| 3 months | 4 (3) | 5 (5) | 6 (4) | |
| Metabolic risk factors | ||||
| Hypertension, n (%) | 90 (78) | 75 (73) | 109 (81) | 0.30 |
| DM, n (%) | 34 (29) | 28 (27) | 41 (30) | 0.35 |
| IGT, n (%) | 8 (7) | 8 (8) | 18 (13) | |
| Hyperlipidaemia, n (%) | 56 (48) | 45 (44) | 66 (49) | 0.70 |
| Coronary artery disease, n (%) | 11 (9) | 12 (12) | 21 (16) | 0.31 |
| AHI > 30, n (%) | 61 (52) | 52 (50) | 69 (51) | 0.97 |
| Alcohol excess (>30 g/week), n (%) | 34 (29) | 35 (34) | 42 (31) | 0.73 |
| Smoker, n (%) | 47 (40) | 41 (40) | 50 (37) | 0.86 |
| Medication use | ||||
| Mean no. of anti-arrhythmic (±SD) | 0.9 ± 0.8 | 1.0 ± 0.7 | 1.1 ± 0.7 | 0.10 |
| Mean no. of anti-hypertensive (±SD) | 1.1 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.9 | 0.08 |
| . | <3% WL Group 1 n = 116 . | 3–9% WL Group 2 n = 104 . | ≥10% WL Group 3 n = 135 . | P-value . |
|---|---|---|---|---|
| Age (years) | 61 ± 11 | 63 ± 11 | 65 ± 11 | 0.06 |
| Male gender, n (%) | 83 (71) | 65 (63) | 86 (64) | 0.37 |
| Anthropometric measures and blood pressure | ||||
| BMI (kg/m2) | 32.9 ± 4.8 | 32.7 ± 4.4 | 33.6 ± 4.7 | 0.24 |
| SBP (mmHg) | 146 ± 17 | 144 ± 17 | 147 ± 17 | 0.33 |
| Atrial fibrillation type | ||||
| Paroxysmal AF, n (%) | 61 (53) | 62 (60) | 73 (54) | 0.55 |
| Non-paroxysmal, n (%) | 55 (47) | 42 (40) | 62 (46) | |
| Atrial episode burden, n (%) | ||||
| 48 h | 29 (25) | 13 (13) | 24 (18) | 0.39 |
| <1 week with no DCCV | 48 (41) | 49 (47) | 56 (42) | |
| >1 week OR DCCV | 35 (30) | 37 (36) | 49 (36) | |
| 3 months | 4 (3) | 5 (5) | 6 (4) | |
| Metabolic risk factors | ||||
| Hypertension, n (%) | 90 (78) | 75 (73) | 109 (81) | 0.30 |
| DM, n (%) | 34 (29) | 28 (27) | 41 (30) | 0.35 |
| IGT, n (%) | 8 (7) | 8 (8) | 18 (13) | |
| Hyperlipidaemia, n (%) | 56 (48) | 45 (44) | 66 (49) | 0.70 |
| Coronary artery disease, n (%) | 11 (9) | 12 (12) | 21 (16) | 0.31 |
| AHI > 30, n (%) | 61 (52) | 52 (50) | 69 (51) | 0.97 |
| Alcohol excess (>30 g/week), n (%) | 34 (29) | 35 (34) | 42 (31) | 0.73 |
| Smoker, n (%) | 47 (40) | 41 (40) | 50 (37) | 0.86 |
| Medication use | ||||
| Mean no. of anti-arrhythmic (±SD) | 0.9 ± 0.8 | 1.0 ± 0.7 | 1.1 ± 0.7 | 0.10 |
| Mean no. of anti-hypertensive (±SD) | 1.1 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.9 | 0.08 |
AF, atrial fibrillation; AHI, apnea–hypopnea index; BMI, body mass index; DCCV, direct current cardioversion; DM, diabetes mellitus; IGT, impaired glucose tolerance; SBP, systolic blood pressure; WL, weight loss.
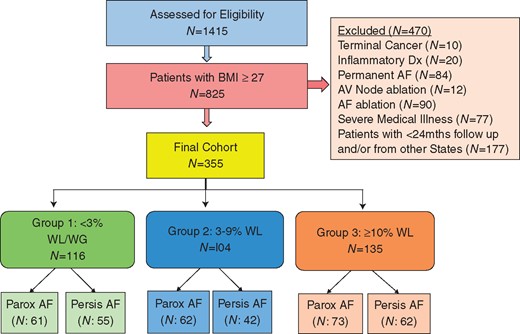
Patient selection. Consort diagram of the patient selection. AF, atrial fibrillation; AV, atrioventricular; BMI, body mass index; mths, months; Parox, paroxysmal; Persis, persistent; WG, weight gain; WL, weight loss.
Effect of weight loss of atrial fibrillation type
Table 2 shows the effect of weight loss on AF type. At baseline, Group 1: paroxysmal 61 (53%) and persistent 55 (47%); Group 2: paroxysmal 62 (60%) and persistent 42 (40%); Group 3: paroxysmal 73 (54%) and persistent 62 (46%). At final follow-up, Group 1: 48 (41%) progressed AF disease from paroxysmal to persistent; whereas only 1 (1%) patient went from PrsAF to PAF, 37 (32%) had no change in AF type, and 30 (25%) were free from AF at final follow-up. Group 2: 33 (32%) progressed from paroxysmal to persistent, 18 (17%) who reversed from PrsAF to PAF and 20 (19%) had no change in AF type, while 33 (32%) were free from AF at final follow-up. Group 3: 4 (3%) patients progressed from PAF to PrsAF, while 49 (36%) reversed AF disease from PrsAF to PAF, 12 (9%) had no change in AF type, with 70 (52%) patients free from AF over the final year of follow-up (P = 0.001) (Figures 2 and 3).
| Risk factors . | <3% WL Group 1; n = 116 . | 3–9% WL Group 2; n = 104 . | ≥10% WL Group 3; n = 135 . | P-value† . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | . | |
| BMI (kg/m2) | 33.0 ± 4.9 | 33.5 ± 5.3 | <0.001 | 32.7 ± 4.4 | 30.8 ± 4.2 | <0.001 | 33.7 ± 4.7 | 28.4 ± 4.0 | <0.001 | <0.001 | |
| Mean SBP (mmHg) | 146 ± 17 | 139 ± 15 | <0.001 | 144 ± 17 | 134 ± 14 | <0.001 | 147 ± 17 | 129 ± 12 | <0.001 | <0.001 | |
| Medication use | |||||||||||
| Mean no. of anti-HTN, n | 0.8 ± 1.0 | 1.0 ± 0.7 | 0.01 | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.74 | 1.0 ± 0.9 | 0.5 ± 0.6 | <0.001 | <0.001 | |
| Mean no. of AAD, n | 0.8 ± 0.8 | 0.4 ± 0.6 | <0.001 | 1.0 ± 0.7 | 0.5 ± 0.6 | <0.001 | 1.1 ± 0.7 | 0.1 ± 0.4 | <0.001 | <0.001 | |
| AF type, n (%) | |||||||||||
| Paroxysmal AF | 61 (53) | – | 62 (60) | – | 73 (54) | – | 0.55 | ||||
| Persistent AF | 55 (47) | – | 42 (40) | – | 62 (46) | – | |||||
| Paroxysmal to persistent AF | – | 48 (41) | – | 33 (32) | – | 4 (3) | <0.001 | ||||
| Persistent to paroxysmal AF | – | 1 (1) | – | 18 (17) | – | 49 (36) | |||||
| No change in AF type | – | 37 (32) | – | 20 (19) | – | 12 (9) | |||||
| No AF | – | 30 (25) | – | 33 (32) | – | 70 (52) | |||||
| Last AF episode duration | |||||||||||
| Paroxysmal AF | 48 h, n (%) | 20 (17) | 1 (1) | 13 (14) | 5 (5) | 21 (15) | 21 (15) | <0.001 | |||
| Δ to <1 week | 19 (16) | 8 (8) | – | 0 (0) | |||||||
| <1 week, n (%) | 41 (35) | 38 (33) | 49 (47) | 15 (14) | 52 (39) | 8 (6) | |||||
| Δ to 48 h, n (%) | 3 (3) | 34 (33) | – | 44 (33) | |||||||
| Persistent AF | >1 week, n (%) | 40 (34) | 21 (18) | 36 (34) | 15 (14) | 49 (36) | 39 (29) | ||||
| Δ to >3 months, n (%) | 19 (16) | 21 (20) | – | 10 (7) | |||||||
| >3 months, n (%) | 15 (13) | 12 (10) | 5 (5) | 1 (1) | 13 (10) | 0 (0) | |||||
| Δ to >1 week, n (%) | 3 (3) | 4 (4) | – | 13 (10) | |||||||
| Total AF freedom and ablation | |||||||||||
| Total freedom from AF | – | 45 (39) | – | 69 (67) | – | 116 (86) | <0.001 | ||||
| No AF ablation | – | 5 (13) | – | 15 (22) | – | 53 (45.5 | 0.001 | ||||
| Single AF ablation | – | 15 (34 | – | 32 (46 | – | 44 (37.5) | 0.8 | ||||
| Multiple AF ablation | – | 25 (53) | – | 22 (32) | – | 19 (17) | 0.007 | ||||
| Risk factors . | <3% WL Group 1; n = 116 . | 3–9% WL Group 2; n = 104 . | ≥10% WL Group 3; n = 135 . | P-value† . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | . | |
| BMI (kg/m2) | 33.0 ± 4.9 | 33.5 ± 5.3 | <0.001 | 32.7 ± 4.4 | 30.8 ± 4.2 | <0.001 | 33.7 ± 4.7 | 28.4 ± 4.0 | <0.001 | <0.001 | |
| Mean SBP (mmHg) | 146 ± 17 | 139 ± 15 | <0.001 | 144 ± 17 | 134 ± 14 | <0.001 | 147 ± 17 | 129 ± 12 | <0.001 | <0.001 | |
| Medication use | |||||||||||
| Mean no. of anti-HTN, n | 0.8 ± 1.0 | 1.0 ± 0.7 | 0.01 | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.74 | 1.0 ± 0.9 | 0.5 ± 0.6 | <0.001 | <0.001 | |
| Mean no. of AAD, n | 0.8 ± 0.8 | 0.4 ± 0.6 | <0.001 | 1.0 ± 0.7 | 0.5 ± 0.6 | <0.001 | 1.1 ± 0.7 | 0.1 ± 0.4 | <0.001 | <0.001 | |
| AF type, n (%) | |||||||||||
| Paroxysmal AF | 61 (53) | – | 62 (60) | – | 73 (54) | – | 0.55 | ||||
| Persistent AF | 55 (47) | – | 42 (40) | – | 62 (46) | – | |||||
| Paroxysmal to persistent AF | – | 48 (41) | – | 33 (32) | – | 4 (3) | <0.001 | ||||
| Persistent to paroxysmal AF | – | 1 (1) | – | 18 (17) | – | 49 (36) | |||||
| No change in AF type | – | 37 (32) | – | 20 (19) | – | 12 (9) | |||||
| No AF | – | 30 (25) | – | 33 (32) | – | 70 (52) | |||||
| Last AF episode duration | |||||||||||
| Paroxysmal AF | 48 h, n (%) | 20 (17) | 1 (1) | 13 (14) | 5 (5) | 21 (15) | 21 (15) | <0.001 | |||
| Δ to <1 week | 19 (16) | 8 (8) | – | 0 (0) | |||||||
| <1 week, n (%) | 41 (35) | 38 (33) | 49 (47) | 15 (14) | 52 (39) | 8 (6) | |||||
| Δ to 48 h, n (%) | 3 (3) | 34 (33) | – | 44 (33) | |||||||
| Persistent AF | >1 week, n (%) | 40 (34) | 21 (18) | 36 (34) | 15 (14) | 49 (36) | 39 (29) | ||||
| Δ to >3 months, n (%) | 19 (16) | 21 (20) | – | 10 (7) | |||||||
| >3 months, n (%) | 15 (13) | 12 (10) | 5 (5) | 1 (1) | 13 (10) | 0 (0) | |||||
| Δ to >1 week, n (%) | 3 (3) | 4 (4) | – | 13 (10) | |||||||
| Total AF freedom and ablation | |||||||||||
| Total freedom from AF | – | 45 (39) | – | 69 (67) | – | 116 (86) | <0.001 | ||||
| No AF ablation | – | 5 (13) | – | 15 (22) | – | 53 (45.5 | 0.001 | ||||
| Single AF ablation | – | 15 (34 | – | 32 (46 | – | 44 (37.5) | 0.8 | ||||
| Multiple AF ablation | – | 25 (53) | – | 22 (32) | – | 19 (17) | 0.007 | ||||
AAD, anti-arrhythmic drug; AF, atrial fibrillation; BMI, body mass index; HTN, hypertension; SBP, systolic blood pressure; WL, weight loss.
Median follow-up 48.3 ± 18.4, 46.0 ± 16.7, and 48.4 ± 18.2 months, respectively.
P-value refers to within group differences (baseline to follow-up).
P-value refers to difference between groups over time (group–time interaction).
| Risk factors . | <3% WL Group 1; n = 116 . | 3–9% WL Group 2; n = 104 . | ≥10% WL Group 3; n = 135 . | P-value† . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | . | |
| BMI (kg/m2) | 33.0 ± 4.9 | 33.5 ± 5.3 | <0.001 | 32.7 ± 4.4 | 30.8 ± 4.2 | <0.001 | 33.7 ± 4.7 | 28.4 ± 4.0 | <0.001 | <0.001 | |
| Mean SBP (mmHg) | 146 ± 17 | 139 ± 15 | <0.001 | 144 ± 17 | 134 ± 14 | <0.001 | 147 ± 17 | 129 ± 12 | <0.001 | <0.001 | |
| Medication use | |||||||||||
| Mean no. of anti-HTN, n | 0.8 ± 1.0 | 1.0 ± 0.7 | 0.01 | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.74 | 1.0 ± 0.9 | 0.5 ± 0.6 | <0.001 | <0.001 | |
| Mean no. of AAD, n | 0.8 ± 0.8 | 0.4 ± 0.6 | <0.001 | 1.0 ± 0.7 | 0.5 ± 0.6 | <0.001 | 1.1 ± 0.7 | 0.1 ± 0.4 | <0.001 | <0.001 | |
| AF type, n (%) | |||||||||||
| Paroxysmal AF | 61 (53) | – | 62 (60) | – | 73 (54) | – | 0.55 | ||||
| Persistent AF | 55 (47) | – | 42 (40) | – | 62 (46) | – | |||||
| Paroxysmal to persistent AF | – | 48 (41) | – | 33 (32) | – | 4 (3) | <0.001 | ||||
| Persistent to paroxysmal AF | – | 1 (1) | – | 18 (17) | – | 49 (36) | |||||
| No change in AF type | – | 37 (32) | – | 20 (19) | – | 12 (9) | |||||
| No AF | – | 30 (25) | – | 33 (32) | – | 70 (52) | |||||
| Last AF episode duration | |||||||||||
| Paroxysmal AF | 48 h, n (%) | 20 (17) | 1 (1) | 13 (14) | 5 (5) | 21 (15) | 21 (15) | <0.001 | |||
| Δ to <1 week | 19 (16) | 8 (8) | – | 0 (0) | |||||||
| <1 week, n (%) | 41 (35) | 38 (33) | 49 (47) | 15 (14) | 52 (39) | 8 (6) | |||||
| Δ to 48 h, n (%) | 3 (3) | 34 (33) | – | 44 (33) | |||||||
| Persistent AF | >1 week, n (%) | 40 (34) | 21 (18) | 36 (34) | 15 (14) | 49 (36) | 39 (29) | ||||
| Δ to >3 months, n (%) | 19 (16) | 21 (20) | – | 10 (7) | |||||||
| >3 months, n (%) | 15 (13) | 12 (10) | 5 (5) | 1 (1) | 13 (10) | 0 (0) | |||||
| Δ to >1 week, n (%) | 3 (3) | 4 (4) | – | 13 (10) | |||||||
| Total AF freedom and ablation | |||||||||||
| Total freedom from AF | – | 45 (39) | – | 69 (67) | – | 116 (86) | <0.001 | ||||
| No AF ablation | – | 5 (13) | – | 15 (22) | – | 53 (45.5 | 0.001 | ||||
| Single AF ablation | – | 15 (34 | – | 32 (46 | – | 44 (37.5) | 0.8 | ||||
| Multiple AF ablation | – | 25 (53) | – | 22 (32) | – | 19 (17) | 0.007 | ||||
| Risk factors . | <3% WL Group 1; n = 116 . | 3–9% WL Group 2; n = 104 . | ≥10% WL Group 3; n = 135 . | P-value† . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | Baseline . | Follow-upa . | P-value* . | . | |
| BMI (kg/m2) | 33.0 ± 4.9 | 33.5 ± 5.3 | <0.001 | 32.7 ± 4.4 | 30.8 ± 4.2 | <0.001 | 33.7 ± 4.7 | 28.4 ± 4.0 | <0.001 | <0.001 | |
| Mean SBP (mmHg) | 146 ± 17 | 139 ± 15 | <0.001 | 144 ± 17 | 134 ± 14 | <0.001 | 147 ± 17 | 129 ± 12 | <0.001 | <0.001 | |
| Medication use | |||||||||||
| Mean no. of anti-HTN, n | 0.8 ± 1.0 | 1.0 ± 0.7 | 0.01 | 0.7 ± 0.8 | 0.7 ± 0.6 | 0.74 | 1.0 ± 0.9 | 0.5 ± 0.6 | <0.001 | <0.001 | |
| Mean no. of AAD, n | 0.8 ± 0.8 | 0.4 ± 0.6 | <0.001 | 1.0 ± 0.7 | 0.5 ± 0.6 | <0.001 | 1.1 ± 0.7 | 0.1 ± 0.4 | <0.001 | <0.001 | |
| AF type, n (%) | |||||||||||
| Paroxysmal AF | 61 (53) | – | 62 (60) | – | 73 (54) | – | 0.55 | ||||
| Persistent AF | 55 (47) | – | 42 (40) | – | 62 (46) | – | |||||
| Paroxysmal to persistent AF | – | 48 (41) | – | 33 (32) | – | 4 (3) | <0.001 | ||||
| Persistent to paroxysmal AF | – | 1 (1) | – | 18 (17) | – | 49 (36) | |||||
| No change in AF type | – | 37 (32) | – | 20 (19) | – | 12 (9) | |||||
| No AF | – | 30 (25) | – | 33 (32) | – | 70 (52) | |||||
| Last AF episode duration | |||||||||||
| Paroxysmal AF | 48 h, n (%) | 20 (17) | 1 (1) | 13 (14) | 5 (5) | 21 (15) | 21 (15) | <0.001 | |||
| Δ to <1 week | 19 (16) | 8 (8) | – | 0 (0) | |||||||
| <1 week, n (%) | 41 (35) | 38 (33) | 49 (47) | 15 (14) | 52 (39) | 8 (6) | |||||
| Δ to 48 h, n (%) | 3 (3) | 34 (33) | – | 44 (33) | |||||||
| Persistent AF | >1 week, n (%) | 40 (34) | 21 (18) | 36 (34) | 15 (14) | 49 (36) | 39 (29) | ||||
| Δ to >3 months, n (%) | 19 (16) | 21 (20) | – | 10 (7) | |||||||
| >3 months, n (%) | 15 (13) | 12 (10) | 5 (5) | 1 (1) | 13 (10) | 0 (0) | |||||
| Δ to >1 week, n (%) | 3 (3) | 4 (4) | – | 13 (10) | |||||||
| Total AF freedom and ablation | |||||||||||
| Total freedom from AF | – | 45 (39) | – | 69 (67) | – | 116 (86) | <0.001 | ||||
| No AF ablation | – | 5 (13) | – | 15 (22) | – | 53 (45.5 | 0.001 | ||||
| Single AF ablation | – | 15 (34 | – | 32 (46 | – | 44 (37.5) | 0.8 | ||||
| Multiple AF ablation | – | 25 (53) | – | 22 (32) | – | 19 (17) | 0.007 | ||||
AAD, anti-arrhythmic drug; AF, atrial fibrillation; BMI, body mass index; HTN, hypertension; SBP, systolic blood pressure; WL, weight loss.
Median follow-up 48.3 ± 18.4, 46.0 ± 16.7, and 48.4 ± 18.2 months, respectively.
P-value refers to within group differences (baseline to follow-up).
P-value refers to difference between groups over time (group–time interaction).
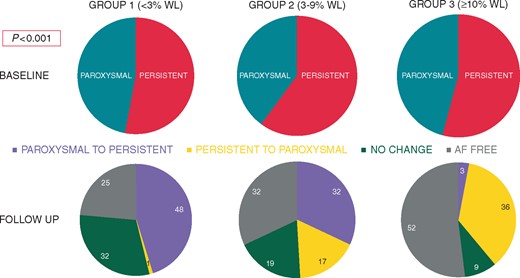
Change in AF type between groups. Pie graphs demonstrating baseline and follow-up of patients change in AF type. AF, atrial fibrillation; WL, weight loss.
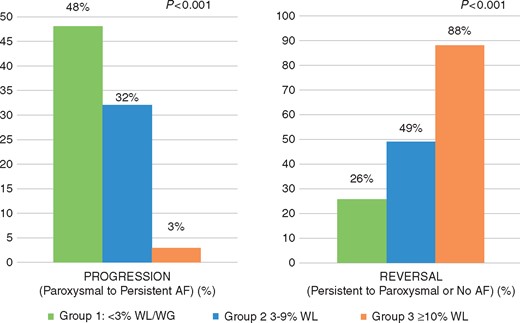
Atrial fibrillation disease progression and reversal. Bar charts showing change in AF type at following weight loss. With green representing Group 1, blue representing Group 2, and orange representing Group 3. AF, atrial fibrillation; WL, weight loss.
Weight loss was a significant univariate and multivariate predictor of AF regression (P = 0.001). On multivariate analysis, >10% weight loss with accompanied risk factor modification was associated with significantly greater likelihood of transition from PrsAF to PAF (odds ratio 4.3, 95% confidence interval 2.7–6.8; P < 0.001). At baseline, there was no difference in mean number of AAD between the three groups: Group 1 0.8 ± 1.0, Group 2 0.7 ± 0.8, and Group 3 1.0 ± 0.9 (P = 0.1). At final follow-up, while all the groups had reduced AAD use, Group 1 0.4 ± 0.6 (P ≤ 0.001), Group 2 0.5 ± 0.6 (P ≤ 0.001), this was significantly greater in Group 3 0.1 ± 0.4 (P ≤ 0.001) (Table 2).
Effect of weight loss on AF burden
In Group 1 with PAF, 95% (19/20 patients) had progression from short PAF to long PAF and only 7% (3/41 patients) went from long to short PAF group (P < 0.001). Those with PrsAF, 47% (19/40 patients) of short PrsAF had an increase in episode duration to long PrsAF and only 20% (3/15 patients) long PrsAF went to short PrsAF (P < 0.001).
Patients in Group 2 with PAF, 61% (8/13 patients) had progression from short PAF to long PAF and 69% (34/49 patients) went from long to short PAF group (P < 0.001). Those with PrsAF, 58% (21/36 patients) of short PrsAF had an increase in episode duration to long PrsAF and 80% (4/5 patients) long PrsAF went to short PrsAF (P < 0.001).
In Group 3, none of the 21 patients progressed from short PAF group to long PAF group and large number of patients had reduction in AF burden with 85% (44/52 patients) who previously had long PAF reducing to short PAF group (P < 0.001). All 13 patients with long PrsAF at baseline, had a reduction in burden and went into short PrsAF group and only 20% (10/49 patients) who previously had short PrsAF went into long PrsAF (P < 0.001).
Pacemaker, AV node ablation, or atrial fibrillation ablation
Weight-loss and risk factor management had a dose-dependent effect on freedom from AF. At final follow-up in the overall total arrhythmia-free patients: 45 (39%) patients in Group 1 [5 (13%) without ablation, 15 (34%) with 1 ablation, and 25 (53%) with multiple ablations]; 69 (67%) patients in Group 2 [15 (22%) without ablation, 32 (46%) with 1 ablation, and 22 (32%) with multiple ablations] were free from AF; and in Group 3 116 (86%) patients were free from AF [53 (45.5%) without ablation, 44 (37.5%) with 1 ablation, and 19 (17%) with multiple ablations; Table 2]. There were no differences in the number of patients requiring AV node ablation [6 (5%), 5 (5%), and 5 (4%)] or pacemaker implantation [44 (38%), 35 (34%), and 36 (27%), respectively between the three groups (P = NS)].
Discussion
This study looks at the association of weight loss and the natural progression of AF and would suggests that in over-weight and obese individuals with symptomatic AF, sustained obesity is associated with progression of the AF disease while progressive weight loss has an association with the ‘reversal’ of the natural progression of AF.
Atrial fibrillation is a progressive disease with the majority of patients progressing from paroxysmal to persistent and then permanent AF over time.9,10 There are dynamic adaptive changes in the atria, enhancing the ability of the AF not only to sustain itself, but also to recur (‘AF begets AF’).11 Although postulated that early cardioversion would prevent the remodelling due to AF and allow ‘sinus rhythm to beget sinus rhythm’, restoration of sinus rhythm with early repeated cardioversion reversed electrical remodelling but did not impact the maintenance of sinus rhythm.12 Thus the role of a ‘second factor’, an atrial substrate responsible for propagation of AF has been implicated.9 Indeed, abnormal atrial changes have been observed even in patients with apparently ‘lone AF’.8 A recent study has observed a progressive atrial substrate even after successful catheter ablation of AF.4 These findings argue in favour of an underlying atrial substrate responsible for AF that is promoted by inadequately treated or unrecognized risk factors.13 Cardiac risk factors such as hypertension, diabetes mellitus, obesity, and sleep apnoea have been independently shown to increase incidence of AF.14–16 As shown in the LEGACY Study, weight loss was associated with improvement in risk factors, hypertension, sleep apnoea, improved glycaemic control, and physical activity resulting increased long-term freedom from AF, and a reduction in the need for ablation and the need for multiple procedures.5 Importantly, these have been associated with structural and electrical remodelling of the atria that forms the substrate leading to the development and progression of AF.17–19 This study confirms these observations. We found that AF has a more progressive course when associated with cardiac risk factors.
It has been shown that patients on rhythm control medications are much less likely to progress to more persistent forms of AF as opposed to those on rate control.20 However, successful ablation alone does not halt the progression of the AF substrate.4 Whether earlier intervention may alter disease progression is a subject of ongoing evaluation. In the current study, aggressive weight and risk factor management was associated with reversal in AF progression. We found ≥10% weight loss with management of associated risk factors was associated with significant reversal of the disease state with 88% of AF patients having significant reduction in burden and reversing to PAF or experiencing no further AF. In addition, weight reduction was associated with significant reduction in need for AF ablation; ≥10% weight loss was associated with 45% patients not requiring any ablation and further 37% requiring only single ablation.
The recognition of AF as a progressive disease, determined by ongoing remodelling consequent to the various underlying risk factors, calls for early and aggressive weight and risk factor intervention. This study adds to a growing body of evidence that risk factor management to treat the primary cause of the disease halts this vicious cycle and improves the long-term freedom from AF. Given the rising epidemic of obesity and AF, primary and secondary prevention strategies need to be urgently implemented.
Limitations
Findings from this study are subject to biases that are inherent in observational studies. However, measurement bias has been minimized through standardized processes in our clinic, and the evaluation by operators blinded to the patient’s weight management strategy. Atrial fibrillation burden assessment using 7-day Holter may incompletely detect AF episodes, especially in patients with low AF burden. Continuous monitoring was not available in all patients so this may lead to asymptomatic AF not able to be captured. However, this method was utilized in both groups and thus unlikely to introduce detection bias. Ascertainment bias was reduced through the collection of outcome via routine data sources. Given the observational nature of the data being registry based the outcomes were not pre-specified a priori, this is a limitation which will be addressed in a randomized control study. This study looks at the association of weight loss on progression of AF. Considering the study design, it was statistically impossible to run a multivariate analysis or logistic regression with so many co-variates and therefore was avoided. Weight loss results in improvement in various associated risk factors such as sleep apnoea and hypertension, no adjustments for applied rhythm control therapies were made, which may have also influenced progression of AF. This study does not provide insight into the cause–effect relationship of individual risk factors to the change in AF type.
Conclusion
Atrial fibrillation is a progressive disease. Sustained obesity is associated with progression from paroxysmal to PrsAF; however, this study suggests that weight loss and management of risk factors may reverse the natural progression of AF disease, resulting in those with PrsAF more frequently transiting to either PAF or no AF. These findings provide further insight to the role of upstream intervention to potentially alter the AF disease process.
Funding
Centre for Heart Rhythm Disorders at the University of Adelaide, Adelaide, Australia; Postgraduate Scholarship from the National Health and Medical Research Council of Australia and the Robert J. Craig Scholarship from the University of Adelaide to M.M.; Postdoctoral Fellowship from the National Health and Medical Research Council of Australia to R.P.; Early Career Fellowship from the National Heart Foundation of Australia to A.E.; Health Professional Fellowship co-funded by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia, and by the Leo J. Mahar Lectureship from the University of Adelaide to R.M.; Leo J. Mahar Electrophysiology Scholarships from the University of Adelaide to D.T.; Leo J. Mahar Cardiovascular Nursing Scholarship from the University of Adelaide to C.G.; Derek Frewin Lectureship from the University of Adelaide to J.H.; Beacon Research Fellowship from the University of Adelaide to D.L.; Practitioner Fellowships from the National Health and Medical Research Council of Australia to R.D.M., J.K., and P.S.; National Heart Foundation of Australia to W.A. and P.S.; Robert J. Craig Lectureship from the University of Adelaide to D.L.
Conflict of interest: R.M. reports that the University of Adelaide has received lecture fees and research funding on his behalf from Medtronic and St Jude Medical. D.L. reports having received lecture fees and research funding from Medtronic, ResMed, Sorin, Sanofi-Aventis and Pfizer. R.D.M. reports receiving research funding from Philips Respironics, ResMed, Fisher&Paykel Healthcare, and Air Liquide. J.K. reports having received research funding from St Jude Medical, Biosense-Webster, Medtronic, and Boston Scientific. P.S. reports having served on the advisory board of Biosense-Webster, Medtronic, and St Jude Medical. P.S. reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Biosense-Webster, Medtronic, and St Jude Medical. P.S. reports that the University of Adelaide has received on his behalf research funding from Medtronic, St Jude Medical, Boston Scientific, Biotronik, and Sorin. All other authors have no disclosures.
References
Author notes
Melissa E. Middeldorp and Rajeev K. Pathak authors contributed equally to the study.



