-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Zanon, Kenneth A Ellenbogen, Gopi Dandamudi, Parikshit S Sharma, Weijian Huang, Daniel L Lustgarten, Roderick Tung, Hiroshi Tada, Jayanthi N Koneru, Tracy Bergemann, Dedra H Fagan, John Harrison Hudnall, Pugazhendhi Vijayaraman, Permanent His-bundle pacing: a systematic literature review and meta-analysis, EP Europace, Volume 20, Issue 11, November 2018, Pages 1819–1826, https://doi.org/10.1093/europace/euy058
Close - Share Icon Share
Abstract
Permanent cardiac pacing of the His-bundle restores and retains normal electrical activation of the ventricles. Data on His-bundle pacing (HBP) are largely limited to small single-centre reports, and clinical benefits and risks have not been systematically examined. We sought to systematically examine published studies of patients undergoing permanent HBP and quantify the benefits and risks of the therapy.
PubMed, Embase, and Cochrane Library were searched for full-text articles on permanent HBP. Clinical outcomes of interest included implant success rate, procedural and lead complications, pacing thresholds, QRS duration, and ejection fraction at follow-up, and mortality. Data were extracted and summarized. Where possible, meta-analysis of aggregate data was performed. Out of 2876 articles, 26 met the inclusion criteria representing 1438 patients with an implant attempt. Average age of patients was 73 years and 62.1% were implanted due to atrioventricular block. Overall average implant success rate was 84.8% and was higher with use of catheter-delivered systems (92.1%; P < 0.001). Average pacing thresholds were 1.71 V at implant and 1.79 V at >3 months follow-up; although, pulse widths varied at testing. Average left ventricular ejection fractions (LVEFs) were 42.8% at baseline and 49.5% at follow-up. There were 43 complications observed in 907 patients across the 17 studies that reported safety information.
Among 26 articles of permanent HBP, the implant success rate averaged 84.8% and LVEF improved by an average of 5.9% during follow-up. Specific reporting of our clinical outcomes of interest varied widely, highlighting the need for uniform reporting in future HBP trials.
What’s new?
Our study found an overall average implant success rate was 84.8% and was higher with use of catheter-delivered systems (92.1%; P < 0.001).
From baseline to last follow-up, there was an average 5.9% increase in left ventricular ejection fraction (LVEF) with statistical significance (P = 0.001), with a greater increase among patients with a history of heart failure and correspondingly lower baseline LVEF.
Specific reporting of our clinical outcomes of interest varied from 19.2 to 76.9% and definitions of clinical outcomes varied as well, highlighting the need for uniform reporting in future His-bundle pacing trials.
Introduction
For over 50 years the standard approach to permanent cardiac pacing has been to affix a transvenous pacing lead to the endocardium of the right ventricular apex (RVA). For most patients, RVA pacing is safe and highly effective at restoring heart rate and reducing bradycardia-induced symptoms. However, this approach results in a non-physiological and dyssynchronous electrical and mechanical pattern of activation of the ventricles and may predispose some patients to pacing-induced heart failure and cardiomyopathy.1,2 In patients with high-grade atrioventricular (AV) block and >40% requirement for pacing, deterioration in systolic dysfunction and development of new-onset heart failure has been reported in 10–26% of patients.3 The need for more physiological-based cardiac pacing has long been sought after.
Attempts at selective pacing capture of the His-bundle were first described nearly 50 years ago,4 and permanent His-bundle pacing (HBP) was first reported by Deshmukh et al.5 in 2000. His-bundle pacing is aimed to transmit the electrical pulse directly through the normal conduction system, thus restoring physiological activation through the ventricles and avoiding some of the potential hazards associated with RVA pacing. However, localization and placement of a fixation lead at the His-bundle is challenging with existing transvenous systems due to its small anatomic size and the fibrous tissue surrounding the bundle. Further, there is concern about maintaining chronic pacing therapy due to lead dislodgement, exit block, and concern about progressive electrical block distal to the HBP lead. There have been many published case studies and single-centre reports of HBP, but there have been no large randomized clinical trials. Additionally, the published data have not been aggregated to quantify indications, benefits, and risks. Thus, our aim was to systematically review the literature of permanent pacing at or near the His-bundle and to perform a meta-analysis of available data.
Methods
Search strategy
A systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.6 A literature search of Cochrane Library, Embase, and PubMed was performed to locate articles on permanent His-bundle or Para-Hisian pacing published through 5 May 2017. The Boolean search terms utilized for the search were: ‘His bundle’ OR ‘Para hisian’ AND ‘pacing’.
Study selection and data extraction
Titles and abstracts retrieved from the search were reviewed and articles on permanent His-bundle or Para-Hisian pacing were selected. Articles were included if they reported permanent His-bundle or Para-Hisian pacing, were in the English language and included patients ≥18 years. Case reports, review articles, abstracts, editorials/letters, and studies with <5 patients were excluded. In the event of multiple publications from the same study cohort, only the article with the most data was included. Final determination on article inclusion was assessed by three authors (T.B., D.H.F., and J.H.H). Extracted data included: number of patients, follow-up duration, implant success rate, baseline characteristics of patients, indication for implantation, type of delivery tool used for implant (catheter vs. stylet), pacing thresholds at baseline and follow-up, QRS duration at baseline and follow-up, left ventricular ejection fraction (LVEF) at baseline and follow-up, complications, and mortality. Data were extracted by one author (D.H.F.) and verified by additional authors (T.B. and J.H.H.).
Meta-analysis
Random effects models were used to estimate summary statistics for variables of interest where individual studies were treated as a random variable. A test for heterogeneity was performed for each model to determine if the variability in outcomes was larger than that expected by sampling variability. To summarize continuous variables of interest, means, standard deviations, and sample sizes were extracted from papers to estimate the overall average values and confidence intervals (CIs) (e.g. age, baseline LVEF). Numerators and denominators were extracted to estimate overall proportions and CIs for dichotomous variables (e.g. sex, AV block indication). The same approach was used for estimation of outcomes of interest, such as the baseline pacing capture threshold and the implant success rate. To estimate the difference in LVEF over time in multiple studies, only those publications that contained both baseline and follow-up means and standard deviations were used. Follow-up times were not consistent within or between studies. A mixed-effects model tested the difference between baseline LVEF and LVEF at follow-up while treating the study as a random effect. The metafor package (GNU General Public License Version 2) for R was used for all analyses.
Results
Studies and patients
A total of 2334 articles were retrieved after excluding duplicates. Articles were screened and 2262 were excluded for not meeting inclusion criteria, leaving 72 articles to assess for eligibility. After assessment of the full-text articles, 46 were excluded for reasons such as: editorial/review article, not permanent HBP, case report, not a human study, overlapping cohort, and an article not in the English language. This left 26 articles to be included in the analysis (Figure 1 and Table 1).
| Publication . | Year . | Study type . | Total number of pointsa . | Implant success (%) . | Follow-up (months) . | Type pacing . | Indication . | 3830 used . |
|---|---|---|---|---|---|---|---|---|
| Ajijola et al.7 | 2017 | Single-arm | 21 | 76.2 | 12 | S, NS HBP | CRT | Yes |
| Huang et al.8 | 2017 | Single-arm | 52 | 80.8 | 21.2 ± 9.3 | DHBP, PHP | AF with AVN ablation | Yes |
| Sharma et al.9 | 2017 | Single-arm | 30 | 93 | NR | S, NS HBP | AV block | Yes |
| Vijayaraman et al.10 | 2016 | Single-arm | 28 | NRb | 21 ± 19 | S, NS HBP | AV conduction disease, SND | Yes |
| Teng et al.11 | 2016 | Single-arm | 29 | NRb | NR | S, NS HBP | LBBB w/pacing or CRT indication | Yes |
| Su et al.12 | 2016 | Single-arm | 38 | NRb | NR | NR | CRT-D/ICD indication | Yes |
| Pastore et al.13 | 2016 | Comparative (HBP vs. RVA vs. RVS) | 148 | NRb | 67.2 ± 28.7 | DHBP, PHP | AVB | NR |
| Lustgarten et al.14 | 2015 | Comparative (HBP vs. BiV) | 29 | 96.6 | NR | S, NS HBP | CRT indication | Yes |
| Vijayaraman et al.15 | 2015 | Single-arm | 67 | 90 | 2–12 | DHBP, PHP | SND, AVB | Yes |
| Vijayaraman et al.16 | 2015 | Single-arm | 100 | 84 | 19 ± 12 | S, NS HBP | AVB | Yes |
| Sharma et al.17 | 2015 | Comparative (HBP vs. RVP) | 94 | 80 | NR | DHBP, PHP | AV conduction disease, SND | Yes |
| Kronborg et al.18 | 2014 | Comparative (HBP vs. RVSP) | 38 | NRb | 12 | DHBP, PHP | AVB | Yes |
| Pastore et al.19 | 2014 | Comparative (HBP vs RVAP) | 37 | NRb | NR | DHBP, PHP | AVB | Yes |
| Catanzariti et al.20 | 2013 | Comparative (HBP vs RVAP) | 26 | NRb | 34.6 ± 11 | DHBP, PHP | AVB, SND, AF | Yes |
| Barba-Pichardo et al.21 | 2013 | Single-arm | 13 | 69 | 31.11 ± 21.45 | DHBP; S, NS | CRT-D indication LBBB | No |
| Zanon et al.22 | 2011 | Comparative (HBP vs. RVA vs. RVS) | 307 | 100 | 20 ± 10 | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.23 | 2010 | Single-arm | 91 | 64.8 | 3 | Pure, fused | AVB | No |
| Pastore et al.24 | 2010 | Comparative (RVA vs. RVS vs. HA) | 44 | 93.6 | None | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.25 | 2008 | Single-arm | 31 | 35.4 | None | Pure, fused | AVB | No |
| Zanon et al.26 | 2008 | Comparative (DHBP vs. RVAP) | 12 | 100 | NR | DHBP | AVB, AF | Yes |
| Occhetta et al.27 | 2007 | Single-arm | 68 | 95.8 | 21 | HBP, PHP | AVB, AF | Yes |
| Catanzariti et al.28 | 2006 | Single-arm | 24 | 95.8 | 7.5 ± 2.9 | DHBP, PHP | AF, AVB, SND | Yes |
| Cantu et al.29 | 2006 | Single-arm | 17 | 100 | None | SHBP, PHP | AF, AVB, SND | Yes |
| Zanon et al.30 | 2006 | Single-arm | 26 | 92 | NR | DHBP | AF, AVB, SND | Yes |
| Deshmukh and Romanyshyn31 | 2004 | Single-arm | 54 | 72.2 | 42 | DHBP, PHP | AF with HF | No |
| Deshmukh et al.5 | 2000 | Single-arm | 14 | 85.7 | 23.4 ± 8.3 | DHBP | AF with HF | No |
| Publication . | Year . | Study type . | Total number of pointsa . | Implant success (%) . | Follow-up (months) . | Type pacing . | Indication . | 3830 used . |
|---|---|---|---|---|---|---|---|---|
| Ajijola et al.7 | 2017 | Single-arm | 21 | 76.2 | 12 | S, NS HBP | CRT | Yes |
| Huang et al.8 | 2017 | Single-arm | 52 | 80.8 | 21.2 ± 9.3 | DHBP, PHP | AF with AVN ablation | Yes |
| Sharma et al.9 | 2017 | Single-arm | 30 | 93 | NR | S, NS HBP | AV block | Yes |
| Vijayaraman et al.10 | 2016 | Single-arm | 28 | NRb | 21 ± 19 | S, NS HBP | AV conduction disease, SND | Yes |
| Teng et al.11 | 2016 | Single-arm | 29 | NRb | NR | S, NS HBP | LBBB w/pacing or CRT indication | Yes |
| Su et al.12 | 2016 | Single-arm | 38 | NRb | NR | NR | CRT-D/ICD indication | Yes |
| Pastore et al.13 | 2016 | Comparative (HBP vs. RVA vs. RVS) | 148 | NRb | 67.2 ± 28.7 | DHBP, PHP | AVB | NR |
| Lustgarten et al.14 | 2015 | Comparative (HBP vs. BiV) | 29 | 96.6 | NR | S, NS HBP | CRT indication | Yes |
| Vijayaraman et al.15 | 2015 | Single-arm | 67 | 90 | 2–12 | DHBP, PHP | SND, AVB | Yes |
| Vijayaraman et al.16 | 2015 | Single-arm | 100 | 84 | 19 ± 12 | S, NS HBP | AVB | Yes |
| Sharma et al.17 | 2015 | Comparative (HBP vs. RVP) | 94 | 80 | NR | DHBP, PHP | AV conduction disease, SND | Yes |
| Kronborg et al.18 | 2014 | Comparative (HBP vs. RVSP) | 38 | NRb | 12 | DHBP, PHP | AVB | Yes |
| Pastore et al.19 | 2014 | Comparative (HBP vs RVAP) | 37 | NRb | NR | DHBP, PHP | AVB | Yes |
| Catanzariti et al.20 | 2013 | Comparative (HBP vs RVAP) | 26 | NRb | 34.6 ± 11 | DHBP, PHP | AVB, SND, AF | Yes |
| Barba-Pichardo et al.21 | 2013 | Single-arm | 13 | 69 | 31.11 ± 21.45 | DHBP; S, NS | CRT-D indication LBBB | No |
| Zanon et al.22 | 2011 | Comparative (HBP vs. RVA vs. RVS) | 307 | 100 | 20 ± 10 | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.23 | 2010 | Single-arm | 91 | 64.8 | 3 | Pure, fused | AVB | No |
| Pastore et al.24 | 2010 | Comparative (RVA vs. RVS vs. HA) | 44 | 93.6 | None | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.25 | 2008 | Single-arm | 31 | 35.4 | None | Pure, fused | AVB | No |
| Zanon et al.26 | 2008 | Comparative (DHBP vs. RVAP) | 12 | 100 | NR | DHBP | AVB, AF | Yes |
| Occhetta et al.27 | 2007 | Single-arm | 68 | 95.8 | 21 | HBP, PHP | AVB, AF | Yes |
| Catanzariti et al.28 | 2006 | Single-arm | 24 | 95.8 | 7.5 ± 2.9 | DHBP, PHP | AF, AVB, SND | Yes |
| Cantu et al.29 | 2006 | Single-arm | 17 | 100 | None | SHBP, PHP | AF, AVB, SND | Yes |
| Zanon et al.30 | 2006 | Single-arm | 26 | 92 | NR | DHBP | AF, AVB, SND | Yes |
| Deshmukh and Romanyshyn31 | 2004 | Single-arm | 54 | 72.2 | 42 | DHBP, PHP | AF with HF | No |
| Deshmukh et al.5 | 2000 | Single-arm | 14 | 85.7 | 23.4 ± 8.3 | DHBP | AF with HF | No |
AF, atrial fibrillation; AV, atrioventricular; AVB, atrioventricular block; AVN, atrioventricular node; BiV, biventricular pacemaker; CRT-D, cardiac resynchronization therapy defibrillator; DHBP, direct His-bundle pacing; HBP, His-bundle pacing; HF, heart failure; ICD, implantable cardioverter defibrillator; LBBB, left bundle-branch block; NR, not reported; NS HBP, non-selective His-bundle pacing; PHP, Para-Hisian pacing; RVAP, right ventricular apical pacing; RVSP, right ventricular septal pacing; SHBP, selective His-bundle pacing; SND, sinus node dysfunction.
Total number of patients represents number of patients with permanent His-bundle pacing implant attempt.
Only included successful implants.
| Publication . | Year . | Study type . | Total number of pointsa . | Implant success (%) . | Follow-up (months) . | Type pacing . | Indication . | 3830 used . |
|---|---|---|---|---|---|---|---|---|
| Ajijola et al.7 | 2017 | Single-arm | 21 | 76.2 | 12 | S, NS HBP | CRT | Yes |
| Huang et al.8 | 2017 | Single-arm | 52 | 80.8 | 21.2 ± 9.3 | DHBP, PHP | AF with AVN ablation | Yes |
| Sharma et al.9 | 2017 | Single-arm | 30 | 93 | NR | S, NS HBP | AV block | Yes |
| Vijayaraman et al.10 | 2016 | Single-arm | 28 | NRb | 21 ± 19 | S, NS HBP | AV conduction disease, SND | Yes |
| Teng et al.11 | 2016 | Single-arm | 29 | NRb | NR | S, NS HBP | LBBB w/pacing or CRT indication | Yes |
| Su et al.12 | 2016 | Single-arm | 38 | NRb | NR | NR | CRT-D/ICD indication | Yes |
| Pastore et al.13 | 2016 | Comparative (HBP vs. RVA vs. RVS) | 148 | NRb | 67.2 ± 28.7 | DHBP, PHP | AVB | NR |
| Lustgarten et al.14 | 2015 | Comparative (HBP vs. BiV) | 29 | 96.6 | NR | S, NS HBP | CRT indication | Yes |
| Vijayaraman et al.15 | 2015 | Single-arm | 67 | 90 | 2–12 | DHBP, PHP | SND, AVB | Yes |
| Vijayaraman et al.16 | 2015 | Single-arm | 100 | 84 | 19 ± 12 | S, NS HBP | AVB | Yes |
| Sharma et al.17 | 2015 | Comparative (HBP vs. RVP) | 94 | 80 | NR | DHBP, PHP | AV conduction disease, SND | Yes |
| Kronborg et al.18 | 2014 | Comparative (HBP vs. RVSP) | 38 | NRb | 12 | DHBP, PHP | AVB | Yes |
| Pastore et al.19 | 2014 | Comparative (HBP vs RVAP) | 37 | NRb | NR | DHBP, PHP | AVB | Yes |
| Catanzariti et al.20 | 2013 | Comparative (HBP vs RVAP) | 26 | NRb | 34.6 ± 11 | DHBP, PHP | AVB, SND, AF | Yes |
| Barba-Pichardo et al.21 | 2013 | Single-arm | 13 | 69 | 31.11 ± 21.45 | DHBP; S, NS | CRT-D indication LBBB | No |
| Zanon et al.22 | 2011 | Comparative (HBP vs. RVA vs. RVS) | 307 | 100 | 20 ± 10 | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.23 | 2010 | Single-arm | 91 | 64.8 | 3 | Pure, fused | AVB | No |
| Pastore et al.24 | 2010 | Comparative (RVA vs. RVS vs. HA) | 44 | 93.6 | None | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.25 | 2008 | Single-arm | 31 | 35.4 | None | Pure, fused | AVB | No |
| Zanon et al.26 | 2008 | Comparative (DHBP vs. RVAP) | 12 | 100 | NR | DHBP | AVB, AF | Yes |
| Occhetta et al.27 | 2007 | Single-arm | 68 | 95.8 | 21 | HBP, PHP | AVB, AF | Yes |
| Catanzariti et al.28 | 2006 | Single-arm | 24 | 95.8 | 7.5 ± 2.9 | DHBP, PHP | AF, AVB, SND | Yes |
| Cantu et al.29 | 2006 | Single-arm | 17 | 100 | None | SHBP, PHP | AF, AVB, SND | Yes |
| Zanon et al.30 | 2006 | Single-arm | 26 | 92 | NR | DHBP | AF, AVB, SND | Yes |
| Deshmukh and Romanyshyn31 | 2004 | Single-arm | 54 | 72.2 | 42 | DHBP, PHP | AF with HF | No |
| Deshmukh et al.5 | 2000 | Single-arm | 14 | 85.7 | 23.4 ± 8.3 | DHBP | AF with HF | No |
| Publication . | Year . | Study type . | Total number of pointsa . | Implant success (%) . | Follow-up (months) . | Type pacing . | Indication . | 3830 used . |
|---|---|---|---|---|---|---|---|---|
| Ajijola et al.7 | 2017 | Single-arm | 21 | 76.2 | 12 | S, NS HBP | CRT | Yes |
| Huang et al.8 | 2017 | Single-arm | 52 | 80.8 | 21.2 ± 9.3 | DHBP, PHP | AF with AVN ablation | Yes |
| Sharma et al.9 | 2017 | Single-arm | 30 | 93 | NR | S, NS HBP | AV block | Yes |
| Vijayaraman et al.10 | 2016 | Single-arm | 28 | NRb | 21 ± 19 | S, NS HBP | AV conduction disease, SND | Yes |
| Teng et al.11 | 2016 | Single-arm | 29 | NRb | NR | S, NS HBP | LBBB w/pacing or CRT indication | Yes |
| Su et al.12 | 2016 | Single-arm | 38 | NRb | NR | NR | CRT-D/ICD indication | Yes |
| Pastore et al.13 | 2016 | Comparative (HBP vs. RVA vs. RVS) | 148 | NRb | 67.2 ± 28.7 | DHBP, PHP | AVB | NR |
| Lustgarten et al.14 | 2015 | Comparative (HBP vs. BiV) | 29 | 96.6 | NR | S, NS HBP | CRT indication | Yes |
| Vijayaraman et al.15 | 2015 | Single-arm | 67 | 90 | 2–12 | DHBP, PHP | SND, AVB | Yes |
| Vijayaraman et al.16 | 2015 | Single-arm | 100 | 84 | 19 ± 12 | S, NS HBP | AVB | Yes |
| Sharma et al.17 | 2015 | Comparative (HBP vs. RVP) | 94 | 80 | NR | DHBP, PHP | AV conduction disease, SND | Yes |
| Kronborg et al.18 | 2014 | Comparative (HBP vs. RVSP) | 38 | NRb | 12 | DHBP, PHP | AVB | Yes |
| Pastore et al.19 | 2014 | Comparative (HBP vs RVAP) | 37 | NRb | NR | DHBP, PHP | AVB | Yes |
| Catanzariti et al.20 | 2013 | Comparative (HBP vs RVAP) | 26 | NRb | 34.6 ± 11 | DHBP, PHP | AVB, SND, AF | Yes |
| Barba-Pichardo et al.21 | 2013 | Single-arm | 13 | 69 | 31.11 ± 21.45 | DHBP; S, NS | CRT-D indication LBBB | No |
| Zanon et al.22 | 2011 | Comparative (HBP vs. RVA vs. RVS) | 307 | 100 | 20 ± 10 | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.23 | 2010 | Single-arm | 91 | 64.8 | 3 | Pure, fused | AVB | No |
| Pastore et al.24 | 2010 | Comparative (RVA vs. RVS vs. HA) | 44 | 93.6 | None | DHBP, PHP | AVB, SND | Yes |
| Barba-Pichardo et al.25 | 2008 | Single-arm | 31 | 35.4 | None | Pure, fused | AVB | No |
| Zanon et al.26 | 2008 | Comparative (DHBP vs. RVAP) | 12 | 100 | NR | DHBP | AVB, AF | Yes |
| Occhetta et al.27 | 2007 | Single-arm | 68 | 95.8 | 21 | HBP, PHP | AVB, AF | Yes |
| Catanzariti et al.28 | 2006 | Single-arm | 24 | 95.8 | 7.5 ± 2.9 | DHBP, PHP | AF, AVB, SND | Yes |
| Cantu et al.29 | 2006 | Single-arm | 17 | 100 | None | SHBP, PHP | AF, AVB, SND | Yes |
| Zanon et al.30 | 2006 | Single-arm | 26 | 92 | NR | DHBP | AF, AVB, SND | Yes |
| Deshmukh and Romanyshyn31 | 2004 | Single-arm | 54 | 72.2 | 42 | DHBP, PHP | AF with HF | No |
| Deshmukh et al.5 | 2000 | Single-arm | 14 | 85.7 | 23.4 ± 8.3 | DHBP | AF with HF | No |
AF, atrial fibrillation; AV, atrioventricular; AVB, atrioventricular block; AVN, atrioventricular node; BiV, biventricular pacemaker; CRT-D, cardiac resynchronization therapy defibrillator; DHBP, direct His-bundle pacing; HBP, His-bundle pacing; HF, heart failure; ICD, implantable cardioverter defibrillator; LBBB, left bundle-branch block; NR, not reported; NS HBP, non-selective His-bundle pacing; PHP, Para-Hisian pacing; RVAP, right ventricular apical pacing; RVSP, right ventricular septal pacing; SHBP, selective His-bundle pacing; SND, sinus node dysfunction.
Total number of patients represents number of patients with permanent His-bundle pacing implant attempt.
Only included successful implants.
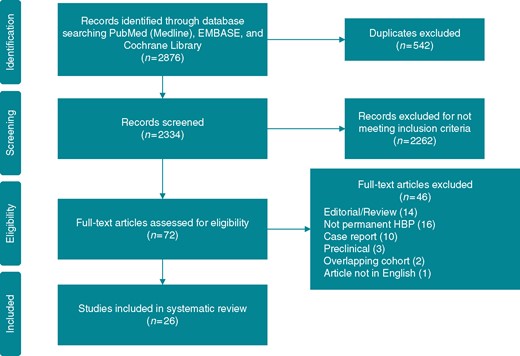
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. Depiction of selection of studies. HBP, His-bundle pacing.
The total population included 1438 patients with a permanent HBP implant attempt across 16 centres. Initial enrolment ranged from 1995 to 2014, with the median being 2006. There were 17 single-arm studies and nine comparative studies included in the analysis. Types of HBP reported included: direct HBP, Para-Hisian pacing, selective HBP, and non-selective HBP. His-bundle pacing was used for a cardiac resynchronization therapy indication in five studies. The reporting rates of the outcomes of interest across the 26 included studies ranged from 19.2% (QRS duration at baseline and follow-up) to 76.9% (implant success rate, Supplementary material online, Table S1). When performing meta-analyses, tests of heterogeneity were highly statistically significant for all variables analysed, and therefore, random effects models were fit to estimate the amount of heterogeneity attributable at the study level.
Patient baseline characteristics were reported in 25 of 26 articles, among 1453 patients (Table 2). The number and types of baseline characteristics reported varied greatly across studies. The average age was 73.0 years (n = 1148; 95% CI 71.5–74.4) and 65.1% (n = 1206; 95% CI 62.5–67.8%) of patients were male. History of atrial fibrillation was present in 41.5% (n = 983; 95% CI 27.4–55.6%), and the most commonly reported indication for implant was AV block (62.1%; n = 1177). Average native QRS duration was 118 ms (n = 960; 95% CI 108–128). Patients did not have heart failure or heart failure status was not reported in the majority of studies (16 of 25).
| Baseline characteristics . | Papers reported number . | Sample size of data reported (n) . | Value (95% CI) . |
|---|---|---|---|
| Total | 25 | 1453 | |
| Age (years) | 20 | 1148 | 73.0 (71.5–74.4) |
| Male sex (%) | 22 | 1206 | 65.1% (62.5–67.8%) |
| AF | 17 | 983 | 41.5% (27.4–55.6%) |
| Indication | |||
| AV block | 16 | 1177 | 62.1% (49.2–75.1%) |
| SND | 8 | 645 | 34.2% (21.4–47.1%) |
| AV nodal ablation | 8 | 329 | 30.9% (4.9–56.9%) |
| Infranodal block | 3 | 158 | 33.6% (1.2–66.0%) |
| CRT | 14 | 665 | 29.1% (7.4–50.9%) |
| ICD | 11 | 597 | 20.3% (0–43.1%) |
| QRS | |||
| Native duration | 17 | 960 | 118 (108–128) |
| LBBB | 7 | 254 | 53.4% (24.1–82.7%) |
| RBBB | 6 | 229 | 18.8% (6.7–30.9%) |
| IVCD | 1 | 30 | 3.30% |
| Heart failure | |||
| NYHA class | |||
| I | 7 | 231 | 10.9% (0–27.3%) |
| II | 7 | 231 | 11.0% (0–24.1%) |
| III | 7 | 231 | 44.4% (13.2–75.6%) |
| IV | 7 | 231 | 1% (0–2.5%) |
| Any (unspecified class) | 10 | 634 | 69.4% (42.7–96.2%) |
| None/not reported | 15 | 637 | NA |
| Cardiac function | |||
| LVEF | 17 | 1204 | 47.3 (42.1–52.5) |
| Hypertension | 13 | 695 | 67.8% (55.6–80.0%) |
| Coronary artery disease | 11 | 614 | 28.3% (19.0–37.6%) |
| Valvular disease | 6 | 175 | 23.9% (0–53.8%) |
| Baseline characteristics . | Papers reported number . | Sample size of data reported (n) . | Value (95% CI) . |
|---|---|---|---|
| Total | 25 | 1453 | |
| Age (years) | 20 | 1148 | 73.0 (71.5–74.4) |
| Male sex (%) | 22 | 1206 | 65.1% (62.5–67.8%) |
| AF | 17 | 983 | 41.5% (27.4–55.6%) |
| Indication | |||
| AV block | 16 | 1177 | 62.1% (49.2–75.1%) |
| SND | 8 | 645 | 34.2% (21.4–47.1%) |
| AV nodal ablation | 8 | 329 | 30.9% (4.9–56.9%) |
| Infranodal block | 3 | 158 | 33.6% (1.2–66.0%) |
| CRT | 14 | 665 | 29.1% (7.4–50.9%) |
| ICD | 11 | 597 | 20.3% (0–43.1%) |
| QRS | |||
| Native duration | 17 | 960 | 118 (108–128) |
| LBBB | 7 | 254 | 53.4% (24.1–82.7%) |
| RBBB | 6 | 229 | 18.8% (6.7–30.9%) |
| IVCD | 1 | 30 | 3.30% |
| Heart failure | |||
| NYHA class | |||
| I | 7 | 231 | 10.9% (0–27.3%) |
| II | 7 | 231 | 11.0% (0–24.1%) |
| III | 7 | 231 | 44.4% (13.2–75.6%) |
| IV | 7 | 231 | 1% (0–2.5%) |
| Any (unspecified class) | 10 | 634 | 69.4% (42.7–96.2%) |
| None/not reported | 15 | 637 | NA |
| Cardiac function | |||
| LVEF | 17 | 1204 | 47.3 (42.1–52.5) |
| Hypertension | 13 | 695 | 67.8% (55.6–80.0%) |
| Coronary artery disease | 11 | 614 | 28.3% (19.0–37.6%) |
| Valvular disease | 6 | 175 | 23.9% (0–53.8%) |
AF, atrial fibrillation; AV, atrioventricular; CI, confidence interval; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IVCD, intraventricular conduction delay; LBBB, left bundle-branch block; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; RBBB, right bundle-branch block; SND, sinus node dysfunction.
| Baseline characteristics . | Papers reported number . | Sample size of data reported (n) . | Value (95% CI) . |
|---|---|---|---|
| Total | 25 | 1453 | |
| Age (years) | 20 | 1148 | 73.0 (71.5–74.4) |
| Male sex (%) | 22 | 1206 | 65.1% (62.5–67.8%) |
| AF | 17 | 983 | 41.5% (27.4–55.6%) |
| Indication | |||
| AV block | 16 | 1177 | 62.1% (49.2–75.1%) |
| SND | 8 | 645 | 34.2% (21.4–47.1%) |
| AV nodal ablation | 8 | 329 | 30.9% (4.9–56.9%) |
| Infranodal block | 3 | 158 | 33.6% (1.2–66.0%) |
| CRT | 14 | 665 | 29.1% (7.4–50.9%) |
| ICD | 11 | 597 | 20.3% (0–43.1%) |
| QRS | |||
| Native duration | 17 | 960 | 118 (108–128) |
| LBBB | 7 | 254 | 53.4% (24.1–82.7%) |
| RBBB | 6 | 229 | 18.8% (6.7–30.9%) |
| IVCD | 1 | 30 | 3.30% |
| Heart failure | |||
| NYHA class | |||
| I | 7 | 231 | 10.9% (0–27.3%) |
| II | 7 | 231 | 11.0% (0–24.1%) |
| III | 7 | 231 | 44.4% (13.2–75.6%) |
| IV | 7 | 231 | 1% (0–2.5%) |
| Any (unspecified class) | 10 | 634 | 69.4% (42.7–96.2%) |
| None/not reported | 15 | 637 | NA |
| Cardiac function | |||
| LVEF | 17 | 1204 | 47.3 (42.1–52.5) |
| Hypertension | 13 | 695 | 67.8% (55.6–80.0%) |
| Coronary artery disease | 11 | 614 | 28.3% (19.0–37.6%) |
| Valvular disease | 6 | 175 | 23.9% (0–53.8%) |
| Baseline characteristics . | Papers reported number . | Sample size of data reported (n) . | Value (95% CI) . |
|---|---|---|---|
| Total | 25 | 1453 | |
| Age (years) | 20 | 1148 | 73.0 (71.5–74.4) |
| Male sex (%) | 22 | 1206 | 65.1% (62.5–67.8%) |
| AF | 17 | 983 | 41.5% (27.4–55.6%) |
| Indication | |||
| AV block | 16 | 1177 | 62.1% (49.2–75.1%) |
| SND | 8 | 645 | 34.2% (21.4–47.1%) |
| AV nodal ablation | 8 | 329 | 30.9% (4.9–56.9%) |
| Infranodal block | 3 | 158 | 33.6% (1.2–66.0%) |
| CRT | 14 | 665 | 29.1% (7.4–50.9%) |
| ICD | 11 | 597 | 20.3% (0–43.1%) |
| QRS | |||
| Native duration | 17 | 960 | 118 (108–128) |
| LBBB | 7 | 254 | 53.4% (24.1–82.7%) |
| RBBB | 6 | 229 | 18.8% (6.7–30.9%) |
| IVCD | 1 | 30 | 3.30% |
| Heart failure | |||
| NYHA class | |||
| I | 7 | 231 | 10.9% (0–27.3%) |
| II | 7 | 231 | 11.0% (0–24.1%) |
| III | 7 | 231 | 44.4% (13.2–75.6%) |
| IV | 7 | 231 | 1% (0–2.5%) |
| Any (unspecified class) | 10 | 634 | 69.4% (42.7–96.2%) |
| None/not reported | 15 | 637 | NA |
| Cardiac function | |||
| LVEF | 17 | 1204 | 47.3 (42.1–52.5) |
| Hypertension | 13 | 695 | 67.8% (55.6–80.0%) |
| Coronary artery disease | 11 | 614 | 28.3% (19.0–37.6%) |
| Valvular disease | 6 | 175 | 23.9% (0–53.8%) |
AF, atrial fibrillation; AV, atrioventricular; CI, confidence interval; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; IVCD, intraventricular conduction delay; LBBB, left bundle-branch block; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; RBBB, right bundle-branch block; SND, sinus node dysfunction.
Procedure assessment
The average implant success rate was 84.8% (range 35.425–100%22,26,29). Among studies with stylet delivery, the implant success rate was 54.6%, whereas the implant success rate among studies with catheter delivery was 92.1% (P < 0.001). There was no discernible trend in implant success rate by first year of enrolment (Figure 2).
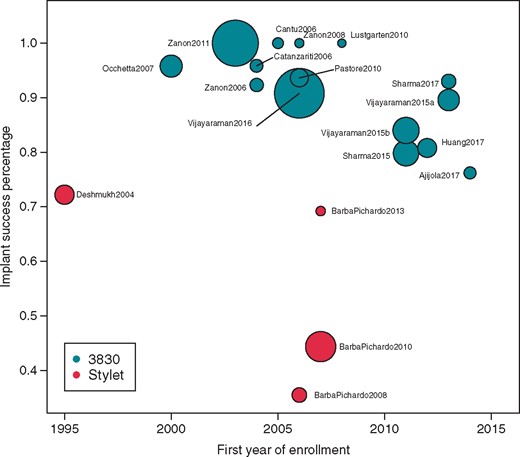
Implant success by year. Scatterplot indicating the implant success percentage by the year of first enrolment in a study for each publication. The size of the bubble per publication reflects the overall study sample size.
Nine studies provided some measure of procedure duration; however, the definition of procedure duration varied (i.e. procedure duration, fluoroscopy duration). Six studies reported procedure time, which ranged from 6415 to 188 min.7 Additionally, six studies reported fluoroscopy time, which ranged from 10 to 17 min.20,29
Efficacy assessment
Paced QRS duration at implant was reported in 15 studies, with an average value of 114 ± 3 ms (95% CI 108–120). Baseline pacing thresholds were reported in 19 studies (Figure 3), with an average value of 1.71 V (95% CI 1.42–2.01 V), most often reported at a pulse width of 0.5 ms, although this was not the case in all studies (some did not specify, while others were reported at 1.0 ms or a range was provided). Acute (≤3 months post-implant) thresholds were reported in six studies, with an average value of 1.76 V (95% CI 1.47–2.05 V). Chronic pacing thresholds >3 months post-implant were reported in eight studies, with an average value of 1.79 V (95% CI 1.27–2.32 V). Although the definitions of selective vs. non-selective and direct vs. Para-Hisian pacing varied by study, of the four manuscripts that separated thresholds based upon type of HBP, thresholds tended to be lower with Para-Hisian pacing/non-selective HBP.9,22,27,29
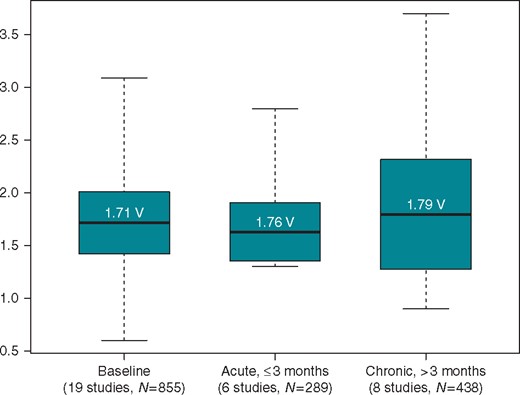
Acute and chronic pacing capture thresholds. Box plot shows the distribution of pacing capture thresholds. The average value is the centre of the box; the black line represents median; the edges of the boxes denote the 95% confidence interval and the whiskers show the minimum and maximum values.
Eight studies reported both baseline and follow-up LVEF values with means and standard deviations (Table 3). Among the eight studies reporting LVEF values, the average follow-up was 16.9 months (range 3–42 months). Average LVEF at baseline was 42.8% ± 4.5% (eight studies, n = 263) and at follow-up of at least 3 months was 49.5% ± 3.1% (eight studies, n = 252). From baseline to last follow-up, there was an average 5.9% increase in LVEF with statistical significance (P = 0.001). The increase in LVEF was greater among those studies of patients with a history of heart failure who correspondingly also had a lower average baseline LVEF (average of 31% ± 4%), with an estimated increase of 10.8% in the four studies with the lowest baseline LVEF (Figure 4). In contrast, the change in the four studies with higher baseline LVEF (average of 54% ± 2%) was estimated to be 2.0%.
| References . | Year . | Baseline . | Follow-up . | Difference (mean ± SD) . | |||
|---|---|---|---|---|---|---|---|
| Sample size . | Mean ± SD . | Sample size . | Follow-up (months) . | Mean ± SD . | |||
| Deshmukh and Romanyshyn31 | 2004 | 29 | 23.0 ± 11.0 | 29 | Mean of 42 | 33.0 ± 15.0 | 10.0 ± 3.5 |
| Ajijola et al.7 | 2017 | 11 | 26.9 ± 9.0 | 11 | Median of 12 | 40.8 ± 13.1 | 13.9 ± 4.8 |
| Barba-Pichardo et al.21 | 2013 | 16 | 29.0 ± 5.0 | 16 | At 6 | 36.0 ± 5.0 | 7.0 ± 1.8 |
| Huang et al.8 | 2017 | 42 | 44.9 ± 14.6 | 42 | At 12 | 59.7 ± 9.8 | 14.8 ± 2.7 |
| Barba-Pichardo et al.23 | 2010 | 59 | 50.0 ± 6.0 | 59 | At 3 | 54.0 ± 3.0 | 4.0 ± 0.9 |
| Occhetta et al.27 | 2007 | 68 | 51.3 ± 11.2 | 57 | Mean of 21 | 51.1 ± 9.9 | −0.2 ± 1.9 |
| Catanzariti et al.28 | 2013 | 26 | 57.2 ± 7.4 | 26 | 34.6 ± 11 | 57.3 ± 8.5 | 0.1 ± 2.2 |
| Zanon et al.26 | 2008 | 12 | 59.8 ± 7.0 | 12 | At 3 | 63.0 ± 12.0 | 3.2 ± 4.0 |
| Total | 263 | 42.8 ± 4.5 | 252 | NA | 49.5 ± 3.1 | 5.9 ± 1.7a | |
| 95% CI (34.1–51.6) | 95% CI (43.4–55.6) | 95% CI (2.6–9.3) | |||||
| References . | Year . | Baseline . | Follow-up . | Difference (mean ± SD) . | |||
|---|---|---|---|---|---|---|---|
| Sample size . | Mean ± SD . | Sample size . | Follow-up (months) . | Mean ± SD . | |||
| Deshmukh and Romanyshyn31 | 2004 | 29 | 23.0 ± 11.0 | 29 | Mean of 42 | 33.0 ± 15.0 | 10.0 ± 3.5 |
| Ajijola et al.7 | 2017 | 11 | 26.9 ± 9.0 | 11 | Median of 12 | 40.8 ± 13.1 | 13.9 ± 4.8 |
| Barba-Pichardo et al.21 | 2013 | 16 | 29.0 ± 5.0 | 16 | At 6 | 36.0 ± 5.0 | 7.0 ± 1.8 |
| Huang et al.8 | 2017 | 42 | 44.9 ± 14.6 | 42 | At 12 | 59.7 ± 9.8 | 14.8 ± 2.7 |
| Barba-Pichardo et al.23 | 2010 | 59 | 50.0 ± 6.0 | 59 | At 3 | 54.0 ± 3.0 | 4.0 ± 0.9 |
| Occhetta et al.27 | 2007 | 68 | 51.3 ± 11.2 | 57 | Mean of 21 | 51.1 ± 9.9 | −0.2 ± 1.9 |
| Catanzariti et al.28 | 2013 | 26 | 57.2 ± 7.4 | 26 | 34.6 ± 11 | 57.3 ± 8.5 | 0.1 ± 2.2 |
| Zanon et al.26 | 2008 | 12 | 59.8 ± 7.0 | 12 | At 3 | 63.0 ± 12.0 | 3.2 ± 4.0 |
| Total | 263 | 42.8 ± 4.5 | 252 | NA | 49.5 ± 3.1 | 5.9 ± 1.7a | |
| 95% CI (34.1–51.6) | 95% CI (43.4–55.6) | 95% CI (2.6–9.3) | |||||
CI, confidence interval; NA, not applicable; SD, standard deviation.
Estimate reflects a weighted average of the differences over time from a mixed-effects model.
| References . | Year . | Baseline . | Follow-up . | Difference (mean ± SD) . | |||
|---|---|---|---|---|---|---|---|
| Sample size . | Mean ± SD . | Sample size . | Follow-up (months) . | Mean ± SD . | |||
| Deshmukh and Romanyshyn31 | 2004 | 29 | 23.0 ± 11.0 | 29 | Mean of 42 | 33.0 ± 15.0 | 10.0 ± 3.5 |
| Ajijola et al.7 | 2017 | 11 | 26.9 ± 9.0 | 11 | Median of 12 | 40.8 ± 13.1 | 13.9 ± 4.8 |
| Barba-Pichardo et al.21 | 2013 | 16 | 29.0 ± 5.0 | 16 | At 6 | 36.0 ± 5.0 | 7.0 ± 1.8 |
| Huang et al.8 | 2017 | 42 | 44.9 ± 14.6 | 42 | At 12 | 59.7 ± 9.8 | 14.8 ± 2.7 |
| Barba-Pichardo et al.23 | 2010 | 59 | 50.0 ± 6.0 | 59 | At 3 | 54.0 ± 3.0 | 4.0 ± 0.9 |
| Occhetta et al.27 | 2007 | 68 | 51.3 ± 11.2 | 57 | Mean of 21 | 51.1 ± 9.9 | −0.2 ± 1.9 |
| Catanzariti et al.28 | 2013 | 26 | 57.2 ± 7.4 | 26 | 34.6 ± 11 | 57.3 ± 8.5 | 0.1 ± 2.2 |
| Zanon et al.26 | 2008 | 12 | 59.8 ± 7.0 | 12 | At 3 | 63.0 ± 12.0 | 3.2 ± 4.0 |
| Total | 263 | 42.8 ± 4.5 | 252 | NA | 49.5 ± 3.1 | 5.9 ± 1.7a | |
| 95% CI (34.1–51.6) | 95% CI (43.4–55.6) | 95% CI (2.6–9.3) | |||||
| References . | Year . | Baseline . | Follow-up . | Difference (mean ± SD) . | |||
|---|---|---|---|---|---|---|---|
| Sample size . | Mean ± SD . | Sample size . | Follow-up (months) . | Mean ± SD . | |||
| Deshmukh and Romanyshyn31 | 2004 | 29 | 23.0 ± 11.0 | 29 | Mean of 42 | 33.0 ± 15.0 | 10.0 ± 3.5 |
| Ajijola et al.7 | 2017 | 11 | 26.9 ± 9.0 | 11 | Median of 12 | 40.8 ± 13.1 | 13.9 ± 4.8 |
| Barba-Pichardo et al.21 | 2013 | 16 | 29.0 ± 5.0 | 16 | At 6 | 36.0 ± 5.0 | 7.0 ± 1.8 |
| Huang et al.8 | 2017 | 42 | 44.9 ± 14.6 | 42 | At 12 | 59.7 ± 9.8 | 14.8 ± 2.7 |
| Barba-Pichardo et al.23 | 2010 | 59 | 50.0 ± 6.0 | 59 | At 3 | 54.0 ± 3.0 | 4.0 ± 0.9 |
| Occhetta et al.27 | 2007 | 68 | 51.3 ± 11.2 | 57 | Mean of 21 | 51.1 ± 9.9 | −0.2 ± 1.9 |
| Catanzariti et al.28 | 2013 | 26 | 57.2 ± 7.4 | 26 | 34.6 ± 11 | 57.3 ± 8.5 | 0.1 ± 2.2 |
| Zanon et al.26 | 2008 | 12 | 59.8 ± 7.0 | 12 | At 3 | 63.0 ± 12.0 | 3.2 ± 4.0 |
| Total | 263 | 42.8 ± 4.5 | 252 | NA | 49.5 ± 3.1 | 5.9 ± 1.7a | |
| 95% CI (34.1–51.6) | 95% CI (43.4–55.6) | 95% CI (2.6–9.3) | |||||
CI, confidence interval; NA, not applicable; SD, standard deviation.
Estimate reflects a weighted average of the differences over time from a mixed-effects model.

Average LVEF values from implant to follow-up for baseline LVEF ≥ 50% vs. LVEF <50%. Box plot shows the distribution of LVEF. The average value is the centre of the box; the black line represents median; the edges of the boxes denote the 95% confidence interval and the whiskers show the minimum and maximum values. EF, ejection fraction; LVEF, left ventricular ejection fraction.
Safety assessment
Eighteen studies report safety information on at least one of the following: total complications, dislodgements, exit block, or loss of therapy. Among the 18 studies, there were 46 complications observed in 966 patients. The most commonly reported complication was lead revision (26 total complications) due to dislodgement (six complications) or elevated thresholds (20 complications), followed by early device replacement due to battery depletion (six complications). Other complications reported included: pocket infection, device dehiscence, elevated thresholds, exit block, device erosion, loss of capture, and sensing issues. The definitions and scope of safety assessments differed by study. Kaplan–Meier estimates for overall complication rates were not possible due to lack of information about timing and follow-up.
Discussion
We systematically assessed publications on permanent pacing at or near the His-bundle which was comprised of 26 original research articles reporting nearly 20 years of experience from 1438 patients across 16 centres around the world. To our knowledge, this study is the first systematic analysis of a large pool of patients from various centres to demonstrate a high success for HBP, demonstrating its wide applicability with acceptable reliability and feasibility. Due to a lack of randomized trials, the current meta-analysis represents at the moment the single largest analysis of a large cohort of patients with pacing targeted at the His region. Our meta-analysis shows an 85% implant success rate and that pacing capture thresholds were on average 1.7 V at implant and at chronic follow-up. Furthermore, these data show that during longitudinal follow-up, HBP may sustain cardiac function with the potential for significant improvement in LVEF in patients with systolic dysfunction and heart failure. Although there is significant heterogeneity in these data, the cumulative experience reported supports that permanent HBP is feasible for the treatment of symptomatic bradycardia, and should seriously be considered in patients who might require a high percentage of ventricular pacing.
These data shed some light on whom the target patient may be for HBP. The majority of patients undergoing HBP were treated for AV block (62% of patients from 15 papers), and patient age, sex, and comorbidities reflect that of a typical pacemaker population. Conversely, a minority of patients had heart failure symptoms with an established indication for cardiac resynchronization therapy (CRT) (29%); although, the application in this patient population has gained more interest for replacing traditional right ventricular (RV) apical pacing or biventricular pacing. Recent evidence has also suggested a role for HBP in patients with bundle branch block and left ventricular dysfunction who are candidates for CRT because it could lead to QRS narrowing by pacing the distal part of the damaged His-bundle.7,32 The long-term comparative effectiveness will need to be better understood with clinical data from prospective clinical trials.
The tools for delivering the lead to the His-bundle appear to be adequate as measured by implant success and pacing capture thresholds, as notable improvement was observed with the use of catheter-delivered leads. The 92% implant success with a catheter-delivered systems is a clear improvement over stylet-delivered leads but is still shy of the >99% implant success with transvenous systems.33,34 The criteria for implant success varied by study, with some studies having a maximum threshold value, number of attempts at lead placement, and total fluoroscopy time. Furthermore, this implant success may come at a cost of longer and more variable procedure times. Thus, there remains room for improvement in the design of implant procedure tools, which could make the implant success rate and replicability approximate that of traditional transvenous pacing implantation. This could result in HBP gaining broader acceptance.
The benefit of HBP relative to RVA pacing is a more normal QRS complex, which would be expected to improve cardiac function. Kaye et al.35 reported on the effects of RV apical and septal pacing on cardiac function in patients without systolic dysfunction and demonstrated that LVEF was reduced by 2% at 2 years follow-up. St John Sutton et al.36 reported on patients with systolic heart failure from the BLOCK-HF study that RV pacing reduced LVEF by 2% but that biventricular pacing improved LVEF by 2% at 2 years follow-up. Our analysis showed that cardiac function is not diminished in patients without systolic dysfunction (2% improvement in LVEF), and that LVEF may actually improve by 10% in patients with systolic dysfunction.
Limitations
There are important limitations of this analysis that should caution interpretation of the results as well as motivate future research in this therapy. Reported data were limited to physicians from 16 centres and these data relied on the physician reporting of the various applications of HBP, thus we were not able to confirm nor assess each application independently. For example, the definitions of implant success and selective/non-selective HBP appear to vary from one study to another. When fitting a model to the implant success percentage, the test of heterogeneity was highly statistically significant (P < 0.001), and therefore, a random effects model was fit to estimate the amount of heterogeneity attributable at the study level. Reporting rates for outcomes of interest ranged from 19.2 to 76.9% of studies. Patient outcomes and estimates of safety risks could not be evaluated due to gaps in reporting across the studies. For example, an overall estimate of mortality could not be derived due to inadequate reporting. Pulse widths varied for pacing capture thresholds. Pacing capture threshold and LVEF follow-up data were not paired, and follow-up times were inconsistent and variable by study. There were not sufficient data to perform a meta-analysis on cumulative percentage of ventricular pacing or on QRS duration and morphology over follow-up. These limitations highlight the need for uniform definitions and essential data collection points to be established for HBP in order to ensure consistency in reported outcomes. A recent publication by an International HBP Collaborative working group has been published to address these needs.37
Conclusion
This analysis represents the first worldwide cumulative experience collected from many centres in China, the USA, and Europe in a real life non-clinical trial environment indicating that HBP is practical and feasible in most patients with an acceptable pacing threshold and low rate of complications. Ventricular function may be maintained in patients with HBP and may significantly improve in patients with systolic dysfunction. Ongoing prospective, multi-centre studies are necessary to advance the field with uniformity in definitions and clinical follow-up.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: F.Z.: Speaker: Boston Scientific, Medtronic, St. Jude Medical, Sorin; K.A.E.: Speaker: Biotronik, Boston Scientific, Medtronic, St. Jude Medical; Consultant/Honorarium: Boston Scientific, Medtronic, St. Jude Medical; Research support: Boston Scientific, Medtronic; G.D.: Speaker/Consultant: Medtronic; Research support: Medtronic; P.S.S.: Honoraria, Medtronic; Consultant, St. Jude Medical/Abbott; W.H.: Nothing to disclose; D.L.L.: Consulting, Medtronic, Biotronik; Speaking fees, Boston Scientific, Medtronic; Advisory Board, Medtronic; Research support, Medtronic, Boston Scientific, Biotronik; R.T.: Nothing to disclose; H.T.: Nothing to disclose; J.N.K.: Consultant/Honoraria, Medtronic, Boston Scientific, St. Jude Medical; Fellowship support, Boston Scientific and Biosense Webster; T.B.: Employment, Shareholder, Medtronic; D.H.F.: Employment, Medtronic; J.H.H.: Employment, Shareholder, Medtronic; P.V.: Speaker/Consultant, Medtronic; Advisory Board, Boston Scientific.



