-
PDF
- Split View
-
Views
-
Cite
Cite
Pablo Moriña-Vázquez, María Teresa Moraleda-Salas, Ana José Manovel-Sánchez, Juan Manuel Fernández-Gómez, Álvaro Arce-Léon, José Venegas-Gamero, Rafael Barba-Pichardo, Early improvement of left ventricular ejection fraction by cardiac resynchronization through His bundle pacing in patients with heart failure, EP Europace, Volume 22, Issue 1, January 2020, Pages 125–132, https://doi.org/10.1093/europace/euz296
Close - Share Icon Share
Abstract
Permanent His bundle pacing (p-HBP) can correct intraventricular conduction disorders and could be an alternative to traditional cardiac resynchronization therapy (CRT) via the coronary sinus. We describe the short-term impact of HBP on left ventricular ejection fraction (LVEF) and improvement of left intraventricular synchrony.
This prospective descriptive study, performed from January 2018 to February 2019, included patients with left bundle branch block (LBBB) and an CRT indication who were resynchronized by p-HBP. We used the Medtronic C315 His catheter or a combination of the CPS-Direct-Universal introducer, CPS-AIM™-Universal subselector (Abbot), and SelectSecure™ MRI-SureScan™ 3830 lead. Correction of the LBBB by HBP had been previously checked. At 1 month of follow-up, we analysed the quantification of LVEF and measurement of the delay of the septal wall with the posterior wall as a parameter of intraventricular synchrony. We included 48 patients with LBBB and an indication for CRT. With HBP, we corrected the LBBB in 81% of patients (n = 39), and we achieved cardiac resynchronization through permanent HBP in 92% of these patients (n = 36). Left ventricular ejection fraction and intraventricular mechanical resynchronization improved in all patients, which was demonstrated by echocardiography through the improvement of the delay of the septal wall with the posterior wall from 138 ms (range 131–151) to 41 ms (19–63).
There is early improvement after p-HBP in LVEF and left ventricular electromechanical synchronization in patients with LBBB, heart failure, and an indication for CRT.
The main finding of our study is that His bundle pacing (HBP) causes an early improvement in echocardiographic parameters in patients who are candidates for cardiac resynchronization therapy when narrowing of the QRS is achieved due to depolarization of the left ventricle through the native conduction system.
This is the first study to demonstrate early improvement of intraventricular mechanical resynchronization by echocardiography (24 h after implantation and at 1 month of follow-up), as well as the short-term impact of HBP on left ventricular ejection fraction (LVEF) by analysing early improvement in LVEF at 1 month of follow-up.
Introduction
Permanent His bundle pacing (p-HBP) is feasible nowadays. It produces a physiological ventricular contraction, which avoids long-term ventricular dysfunction, and its benefits have been known since the first publications about it in 2000–01.1,2
Furthermore, His bundle pacing (HBP) in patients with left bundle branch block (LBBB) and heart failure (HF) can correct conduction disturbance and mechanical dyssynchrony as well as improve the ejection fraction. These benefits of HBP were reported for the first time by Narula and El-Sherif et al.,3,4 and they are physiopathologically supported by the theory of the longitudinal dissociation in the His bundle. Therefore, in patients with an indication for cardiac resynchronization therapy (CRT), p-HBP could be an alternative to or even replace classic CRT via the coronary sinus,5–9 as it achieves physiological biventricular activation through the native conduction system.
We describe the short-term impact of p-HBP on left ventricular ejection fraction (LVEF) and physiological resynchronization through echocardiographic assessment together with general results of the technique.
Methods
Clinical study design and initial selection of patients
We designed a descriptive, prospective observational study with a 6 months of follow-up to evaluate the early impact of p-HBP on echocardiographic parameters, dyssynchrony at 24 h and LVEF at the first month of follow-up. We included patients with HF and baseline LVEF < 35%, LBBB (QRS >130 ms and QS or rS pattern in lead V1), and CRT indication10 from January 2018 to February 2019. All patients were thoroughly informed of the technique and provided written informed consent. This study was approved by the hospital’s ethics committee.
Definition and technical evaluation of the electrical response to His bundle pacing
The electrical response to HBP was defined based on the current recommendations from the Multicenter HBP Collaborative Working Group.11 Patients were considered candidates for p-HBP when HBP had the ability to recruit the distal conduction system and either normalize the QRS (<120 ms) or narrow it by more than 20%. In non-selective HBP, QRS duration was measured from stimulus to the end of the QRS.
In atrial fibrillation (AF) patients with an indication for a CRT-D, a catheter was introduced to pace the His bundle and verify correction of LBBB. In the remaining patients, we confirmed correction of LBBB with the lead that we used for permanent pacing. We used this method because in AF patients who are candidates for CRT-D, the permanent lead used for HBP would not be used if HBP does not narrow the QRS, while in patients in sinus rhythm, it would be implanted in the right atrium.
In patients in whom HBP did not correct LBBB, a conventional CRT-D was implanted. Patients in whom HBP corrected LBBB underwent p-HBP.
Material used for permanent His bundle pacing
For p-HBP, the Medtronic C315 His catheter introducer or a combination of the CPS-Direct-Universal with the CPS-AIM™ Universal Subselector (Abbot®, Chicago, IL, USA) were used to guide the SelectSecure™ MRI-SureScan™ 3830 pacing lead implant (Medtronic®, Minneapolis, MN, USA), depending on the anatomy and His bundle location during the procedure. The CPS tools were used in difficult cases with complex anatomy. We introduced the pacing lead through the combination of those and in this way, the septum could be mapped thoroughly by rotating and moving the subselector forward and backward.
The choice of tools was at the responsible doctor’s discretion, and the decision to implant either a pacemaker or defibrillator was made by the heart team during the clinical session.
When a pacemaker was implanted, a standard single- or dual-chamber device was chosen, and the HBP lead was connected to the ventricular port. When an implantable cardioverter-defibrillator (ICD) was indicated, a CRT-D was used with the HBP lead connected to the left ventricle (LV) port.
Permanent His bundle pacing: viability and implant
The SelectSecure™ MRI-SureScan™ 3830 lead was advanced through the previously described sheaths with the distal electrode/screw located just at the tip of the catheter. We mapped the septum, and then we paced and checked the His bundle capture: either selective (capture of only the His bundle) or non-selective11 (capture of the His bundle and adjacent myocardium) (Figures 1A, B and 2A, B).
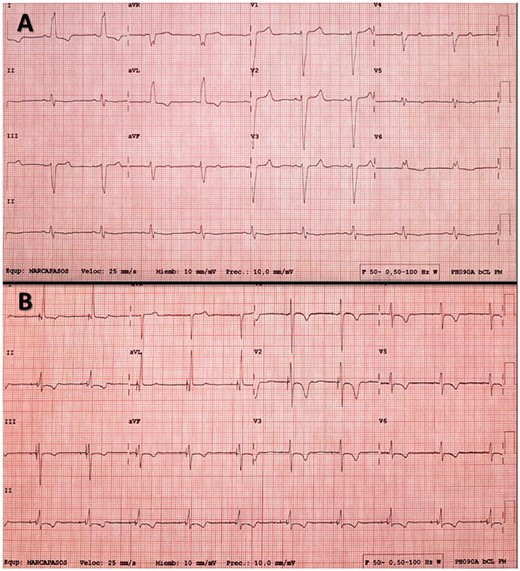
(A) Electrocardiogram of a patient with left bundle branch block (LBBB). (B) Electrocardiogram after permanent His bundle pacing resynchronization; note selective capture of the His bundle (latency between the spike and paced QRS) and disappearance of the LBBB.
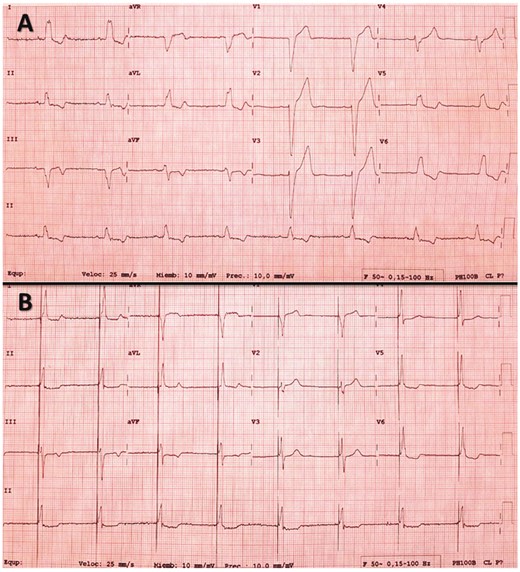
(A) Basal electrocardiogram of a patient with left bundle branch block (LBBB). (B) The same patient after permanent His bundle pacing. The correction of the LBBB was observed; in this case, both the His bundle and adjacent myocardium are captured (fusion), showing a pseudo delta wave at the beginning of the QRS.
In all the cases, after the initial fixation of the electrode with 4–6 clockwise rotations, the electrode was advanced 5 mm and was given an additional 4 rotations in order to attempt to achieve a greater penetration in the tissue.
For safety reasons and better longevity, in cases of non-selective HBP, His bundle thresholds with recruitment of the conduction system at <3.5 V (at 0.4 ms) were considered acceptable, while in cases of selective HBP, we only accepted thresholds <2.5 V (at 0.4 ms). If this was not possible, resynchronization therapy was performed by left ventricular epicardial pacing via the coronary sinus.
All implants were guided by the BV900 equipment (General Electric®, Boston, MA, USA) programmed with low-dose pulsed fluoroscopy. The total dose of radiation and fluoroscopy time were recorded by the equipment.
The implants were placed by the electrophysiologist assigned according to the program of the day.
Permanent programming of the device
The output of the His bundle lead was programmed to 0.5 V above the His bundle threshold if the patient did not require permanent pacing. In patients without intrinsic rhythm, a double output of the minimum capture threshold, either His or myocardial pacing, was programmed. The atrioventricular interval was adjusted to the shortest possible duration to ensure ventricular pacing (VP) and avoid fusion beats between intrinsic and His-paced ventricular depolarization, so as to ensure 100% of ventricular depolarization through Hisian capture and the native conduction system. Similarly, algorithms of the preferential search for intrinsic conduction as well as automatic threshold modulation of the His bundle lead were deactivated.
Objective assessment of cardiac resynchronization
Immediately after implantation, electrical cardiac resynchronization was verified by comparing the native QRS with the QRS during His pacing. Echocardiography was performed just before implantation, 24 h after implantation, and at 1 month of follow-up to verify mechanical resynchronization and LVEF changes, by using as a parameter of intraventricular synchrony of the delay (in ms) of the septum-posterior wall (SPW) of the LV in M-mode colour tissue Doppler imaging (TDI), and calculating the LVEF by the Simpson biplane method. The first measurement was measured using M-mode colour TDI in the parasternal short-axis at the level of the papillary muscles, and the delay (in ms) was annotated between the peak of the septal wall in systole and peak of the posterior wall in systole (Figure 3A, B). All measurements were performed with the same ultrasound machine (iE33, Philips, Andover, MA, USA) and by the same operator to reduce the inter-observer variability.
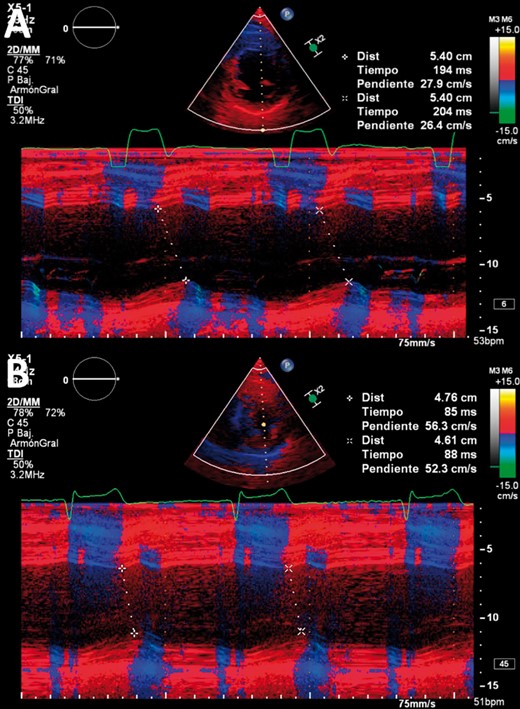
(A) Echocardiogram of a patient with left bundle branch block (M-mode colour tissue Doppler imaging) showing a septum-posterior wall (SPW) delay of 194–204 ms; note the wide QRS in the derivation of the echocardiogram. (B) Echocardiogram after permanent His bundle pacing resynchronization with an SPW delay of 85–88 ms; note the narrow QRS compared to that in (A).
Threshold and type of capture
Thresholds and type of capture were also evaluated at 3 and 6 months after implantation. The thresholds reported are the His capture thresholds with LBBB correction (Figure 4).
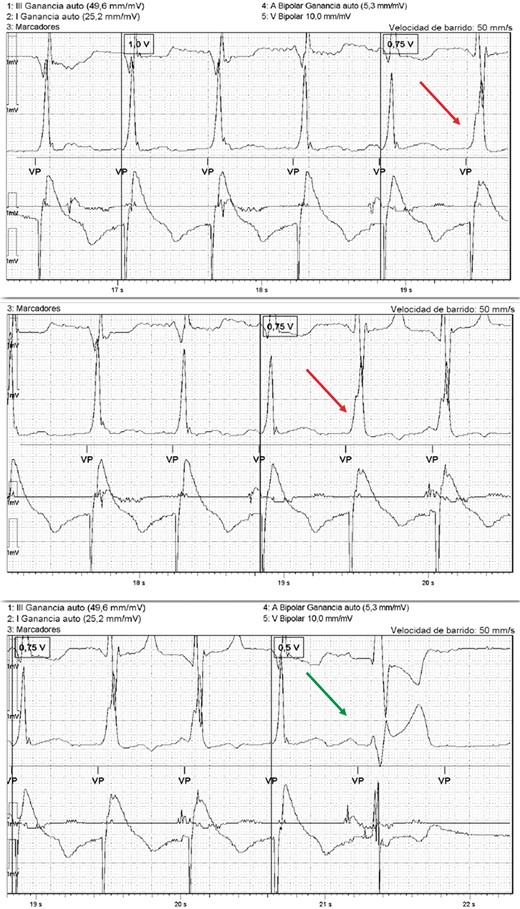
Register of the programmer with the intracardiac recording and the ECG monitor showing that there is a loss of His capture at 0.75 V with a change in the morphology of the QRS (red arrow), which increases in duration. At 0.5 V there is a loss of total capture (green arrow). In this case, the His threshold with LBBB correction is 1 V (0.4 ms) and the myocardium threshold is 0.75 V. ECG, electrocardiogram; LBBB, left bundle branch block.
Study objectives
The main objective was to demonstrate the feasibility of p-HBP to acutely correct conduction disturbances and achieve physiological depolarization of the LV in patients with ventricular dysfunction and LBBB. Feasibility was analysed 24 h after the implant through echocardiographic evaluation of the electromechanical resynchronization while at a month follow-up, both resynchronization parameter and LVEF were evaluated.
The secondary objective was to describe the overall success of implantation, acute thresholds at follow-up, the time of fluoroscopy, and rate of displacement of the electrodes.
Statistical analysis
Quantitative variables are expressed as a median and 25th and 75th percentiles, and qualitative variables as a proportion. Paired comparisons were made using the Student’s t-test if the data were normally distributed, two related data were compared using the Wilcoxon non-parametric test, and two independent data were compared using the Mann–Whitney U non-parametric test. In all analyses, a safety level of 95% was considered to establish statistical significance. The statistical package SPSS (version 23.0; IBM Corp., Armonk, NY, USA) was used with the support of calculation complements (Excel; Microsoft Corp., Redmond, WA, USA).
Results
We included 48 patients with LBBB and CRT indication according to current recommendations.10 Although all of them had an indication for CRT-D according to the current guidelines, only 38 patients were implanted. The rest of the patients underwent a p-HBP because of either an important associated comorbidity or the patient refused implantation of an ICD. All patients had at least 1 month of follow-up, while 24 patients completed 6 months of follow-up by the end of the recruitment period. The basal characteristics and indications of the implant are presented in Table 1. In total, 94% (n = 45) presented with non-ischaemic dilated cardiomyopathy (NIDCM), and 6% (n = 3) presented with ischaemic dilated cardiomyopathy. In 83% (N = 40) of patients we used the Medtronic C315 His catheter introducer (Medtronic®, Minneapolis, MN, USA), while in 17% (N = 8) we used a combination of the CPS-Direct-Universal with the CPS-AIM™ Universal Subselector (Abbot®, Chicago, IL, USA).
Baseline characteristics, electrical response to temporal His bundle pacing, and success of the implant
| Basal characteristics | (N = 48) |
| Age (years) | 66 (58–72) |
| Structural heart disease | |
| Non-ischaemic cardiomyopathy | 94% (n = 45) |
| Ischaemic cardiopathy with ventricular dysfunction | 6% (n = 3) |
| Indication of the implant | |
| HF + LBBB (QRS >130 ms) + LVEF <35% | 100% (n = 48) |
| Electrical response to temporal His bundle pacing | (N = 48) |
| QRS narrowing (QRS <120 ms or narrowing by >20% of the baseline) | 81% (n = 39) |
| Non-ischaemic LBBB | 79% (n = 38) |
| Ischaemic LBBB | 2% (n = 1) |
| Non-QRS narrowing | 19% (n = 9) |
| Non-ischaemic LBBB | 15% (n = 7) |
| Ischaemic LBBB | 4% (n = 2) |
| Cardiac resynchronization through p-HBP | (N = 39) |
| Successful implant | 92% (n = 36) |
| Unsuccessful implant | 8% (n = 3) |
| Global success of CRT by p-HBP in our series | (N = 48) |
| CRT through permanent His bundle pacing | 75% (n = 36) |
| CRT through the coronary sinus | 25% (n = 12) |
| Basal characteristics | (N = 48) |
| Age (years) | 66 (58–72) |
| Structural heart disease | |
| Non-ischaemic cardiomyopathy | 94% (n = 45) |
| Ischaemic cardiopathy with ventricular dysfunction | 6% (n = 3) |
| Indication of the implant | |
| HF + LBBB (QRS >130 ms) + LVEF <35% | 100% (n = 48) |
| Electrical response to temporal His bundle pacing | (N = 48) |
| QRS narrowing (QRS <120 ms or narrowing by >20% of the baseline) | 81% (n = 39) |
| Non-ischaemic LBBB | 79% (n = 38) |
| Ischaemic LBBB | 2% (n = 1) |
| Non-QRS narrowing | 19% (n = 9) |
| Non-ischaemic LBBB | 15% (n = 7) |
| Ischaemic LBBB | 4% (n = 2) |
| Cardiac resynchronization through p-HBP | (N = 39) |
| Successful implant | 92% (n = 36) |
| Unsuccessful implant | 8% (n = 3) |
| Global success of CRT by p-HBP in our series | (N = 48) |
| CRT through permanent His bundle pacing | 75% (n = 36) |
| CRT through the coronary sinus | 25% (n = 12) |
CRT, cardiac resynchronization therapy; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; p-HBP, permanent His bundle pacing.
Baseline characteristics, electrical response to temporal His bundle pacing, and success of the implant
| Basal characteristics | (N = 48) |
| Age (years) | 66 (58–72) |
| Structural heart disease | |
| Non-ischaemic cardiomyopathy | 94% (n = 45) |
| Ischaemic cardiopathy with ventricular dysfunction | 6% (n = 3) |
| Indication of the implant | |
| HF + LBBB (QRS >130 ms) + LVEF <35% | 100% (n = 48) |
| Electrical response to temporal His bundle pacing | (N = 48) |
| QRS narrowing (QRS <120 ms or narrowing by >20% of the baseline) | 81% (n = 39) |
| Non-ischaemic LBBB | 79% (n = 38) |
| Ischaemic LBBB | 2% (n = 1) |
| Non-QRS narrowing | 19% (n = 9) |
| Non-ischaemic LBBB | 15% (n = 7) |
| Ischaemic LBBB | 4% (n = 2) |
| Cardiac resynchronization through p-HBP | (N = 39) |
| Successful implant | 92% (n = 36) |
| Unsuccessful implant | 8% (n = 3) |
| Global success of CRT by p-HBP in our series | (N = 48) |
| CRT through permanent His bundle pacing | 75% (n = 36) |
| CRT through the coronary sinus | 25% (n = 12) |
| Basal characteristics | (N = 48) |
| Age (years) | 66 (58–72) |
| Structural heart disease | |
| Non-ischaemic cardiomyopathy | 94% (n = 45) |
| Ischaemic cardiopathy with ventricular dysfunction | 6% (n = 3) |
| Indication of the implant | |
| HF + LBBB (QRS >130 ms) + LVEF <35% | 100% (n = 48) |
| Electrical response to temporal His bundle pacing | (N = 48) |
| QRS narrowing (QRS <120 ms or narrowing by >20% of the baseline) | 81% (n = 39) |
| Non-ischaemic LBBB | 79% (n = 38) |
| Ischaemic LBBB | 2% (n = 1) |
| Non-QRS narrowing | 19% (n = 9) |
| Non-ischaemic LBBB | 15% (n = 7) |
| Ischaemic LBBB | 4% (n = 2) |
| Cardiac resynchronization through p-HBP | (N = 39) |
| Successful implant | 92% (n = 36) |
| Unsuccessful implant | 8% (n = 3) |
| Global success of CRT by p-HBP in our series | (N = 48) |
| CRT through permanent His bundle pacing | 75% (n = 36) |
| CRT through the coronary sinus | 25% (n = 12) |
CRT, cardiac resynchronization therapy; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; p-HBP, permanent His bundle pacing.
With temporary HBP, LBBB was corrected in 81% of patients (N = 39); these patients proceeded to p-HPB. It was not possible to correct LBBB in 9 patients (7 with NIDCM and 2 with ischaemic cardiopathy). Details are shown in Table 1.
Resynchronization therapy was attempted by p-HBP in the 39 patients in whom LBBB was corrected. In 92% of cases (N = 36), electrical cardiac resynchronization by p-HBP was possible (14% with selective HBP), while in 3 patients, it failed because it was impossible to fix the pacing lead. In these 3 cases, as in the other 9 in which the correction of LBBB was not previously obtained, CRT was performed via the coronary sinus.
Median fluoroscopy times of device implantation including the time taken for temporary HBP and failed HBP implant were 8.1 min (range 6.1–9.9) in patients resynchronized by p-HBP and 14.9 min (13.7–18.1) in patients in which CRT was performed through the coronary sinus (P = 0.004).
The characteristics of the basal and paced QRS, as well as His thresholds, are shown in Table 2. The median acute His threshold was 1.6 V (0.9–1.9) at 0.4 ms in cases where CRT was performed by p-HBP, compared to a left ventricular threshold of 1.3 V (0.8–1.7) in cases where CRT was performed through the coronary sinus, without significant differences between the two groups (P = 0.186). The His threshold remained stable at 1, 3, and 6 months of follow-up in patients with CRT by p-HBP, excluding 1 patient whose His threshold progressively increased to 5.5 V. There was no dislocation of leads during the follow-up.
| QRS duration in patients resynchronized by HBP | (N = 36) |
| Basal QRS (ms) | 160 (150–161) |
| Paced QRS (ms) | 132 (126–148) |
| His capture thresholds with LBBB correction | |
| Acute threshold (V at 0.4 ms) | 1.6 (0.9–1.9) |
| Threshold at 1 month of follow-up (V at 0.4 ms) (N = 36) | 0.75 (0.7–2.1) |
| Threshold at 3 months of follow-up (V at 0.4 ms) (N = 34) | 0.8 (0.6–1.6) |
| Threshold at 6 months of follow-up (V at 0.4 ms) (N = 24) | 0.9 (0.7–2) |
| QRS duration in patients resynchronized by HBP | (N = 36) |
| Basal QRS (ms) | 160 (150–161) |
| Paced QRS (ms) | 132 (126–148) |
| His capture thresholds with LBBB correction | |
| Acute threshold (V at 0.4 ms) | 1.6 (0.9–1.9) |
| Threshold at 1 month of follow-up (V at 0.4 ms) (N = 36) | 0.75 (0.7–2.1) |
| Threshold at 3 months of follow-up (V at 0.4 ms) (N = 34) | 0.8 (0.6–1.6) |
| Threshold at 6 months of follow-up (V at 0.4 ms) (N = 24) | 0.9 (0.7–2) |
HBP, His bundle pacing; LBBB, left bundle branch block.
| QRS duration in patients resynchronized by HBP | (N = 36) |
| Basal QRS (ms) | 160 (150–161) |
| Paced QRS (ms) | 132 (126–148) |
| His capture thresholds with LBBB correction | |
| Acute threshold (V at 0.4 ms) | 1.6 (0.9–1.9) |
| Threshold at 1 month of follow-up (V at 0.4 ms) (N = 36) | 0.75 (0.7–2.1) |
| Threshold at 3 months of follow-up (V at 0.4 ms) (N = 34) | 0.8 (0.6–1.6) |
| Threshold at 6 months of follow-up (V at 0.4 ms) (N = 24) | 0.9 (0.7–2) |
| QRS duration in patients resynchronized by HBP | (N = 36) |
| Basal QRS (ms) | 160 (150–161) |
| Paced QRS (ms) | 132 (126–148) |
| His capture thresholds with LBBB correction | |
| Acute threshold (V at 0.4 ms) | 1.6 (0.9–1.9) |
| Threshold at 1 month of follow-up (V at 0.4 ms) (N = 36) | 0.75 (0.7–2.1) |
| Threshold at 3 months of follow-up (V at 0.4 ms) (N = 34) | 0.8 (0.6–1.6) |
| Threshold at 6 months of follow-up (V at 0.4 ms) (N = 24) | 0.9 (0.7–2) |
HBP, His bundle pacing; LBBB, left bundle branch block.
In patients who could be resynchronized by p-HBP (n = 36), the differences in the delay of the SPW of the LV in M-mode colour TDI at baseline and immediately after CRT using p-HBP were analysed as a measure of interventricular asynchrony and objective assessment of mechanical resynchronization. Of these, 35 patients presented with NIDCM, and one of them had severe ischaemic dysfunction with LBBB. The median baseline delays of the SPW were 138 ms (131–151) and then 41 ms (19–63) after CRT by p-HBP. There were no relevant changes in the delay of the SPW at 1 month of follow-up compared to the echocardiography performed 24 h after the implant. The median basal LVEFs were 30% (27–34) and then 51% (48–58) at the 1 month of follow-up, showing recovery in all patients after Hisian resynchronization. At 1 month of follow-up, the 50% of patients experienced an absolute increase of LVEF of more than 20% (Table 3).
| Patients witd LBBB and CRT indication | (N = 36) |
| Non-ischaemic cardiomyopathy (%) | 97 (n = 35) |
| Ischaemic cardiopathy (%) | 3 (n = 1) |
| Delay of the septum-posterior wall | (N = 36) |
| Basal (ms) | 138 (131–151) |
| 24 h after CRT (ms) | 41 (19–63) |
| One month of follow-up (ms) | 41 (19–63) |
| LVEF | (N = 36) |
| Basal (%) | 30 (27–34) |
| One month of follow-up (%) | 51 (48–58) |
| Echocardiography response (1 month of follow-up) | (N = 36) |
| Absolute improvement of LVEF between 5% and 10% (%) | 8 (n = 3) |
| Absolute improvement of LVEF between 10% and 20% (%) | 42 (n = 15) |
| Absolute improvement of LVEF >20% (%) | 50 (n = 18) |
| Patients witd LBBB and CRT indication | (N = 36) |
| Non-ischaemic cardiomyopathy (%) | 97 (n = 35) |
| Ischaemic cardiopathy (%) | 3 (n = 1) |
| Delay of the septum-posterior wall | (N = 36) |
| Basal (ms) | 138 (131–151) |
| 24 h after CRT (ms) | 41 (19–63) |
| One month of follow-up (ms) | 41 (19–63) |
| LVEF | (N = 36) |
| Basal (%) | 30 (27–34) |
| One month of follow-up (%) | 51 (48–58) |
| Echocardiography response (1 month of follow-up) | (N = 36) |
| Absolute improvement of LVEF between 5% and 10% (%) | 8 (n = 3) |
| Absolute improvement of LVEF between 10% and 20% (%) | 42 (n = 15) |
| Absolute improvement of LVEF >20% (%) | 50 (n = 18) |
CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction.
| Patients witd LBBB and CRT indication | (N = 36) |
| Non-ischaemic cardiomyopathy (%) | 97 (n = 35) |
| Ischaemic cardiopathy (%) | 3 (n = 1) |
| Delay of the septum-posterior wall | (N = 36) |
| Basal (ms) | 138 (131–151) |
| 24 h after CRT (ms) | 41 (19–63) |
| One month of follow-up (ms) | 41 (19–63) |
| LVEF | (N = 36) |
| Basal (%) | 30 (27–34) |
| One month of follow-up (%) | 51 (48–58) |
| Echocardiography response (1 month of follow-up) | (N = 36) |
| Absolute improvement of LVEF between 5% and 10% (%) | 8 (n = 3) |
| Absolute improvement of LVEF between 10% and 20% (%) | 42 (n = 15) |
| Absolute improvement of LVEF >20% (%) | 50 (n = 18) |
| Patients witd LBBB and CRT indication | (N = 36) |
| Non-ischaemic cardiomyopathy (%) | 97 (n = 35) |
| Ischaemic cardiopathy (%) | 3 (n = 1) |
| Delay of the septum-posterior wall | (N = 36) |
| Basal (ms) | 138 (131–151) |
| 24 h after CRT (ms) | 41 (19–63) |
| One month of follow-up (ms) | 41 (19–63) |
| LVEF | (N = 36) |
| Basal (%) | 30 (27–34) |
| One month of follow-up (%) | 51 (48–58) |
| Echocardiography response (1 month of follow-up) | (N = 36) |
| Absolute improvement of LVEF between 5% and 10% (%) | 8 (n = 3) |
| Absolute improvement of LVEF between 10% and 20% (%) | 42 (n = 15) |
| Absolute improvement of LVEF >20% (%) | 50 (n = 18) |
CRT, cardiac resynchronization therapy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction.
There were no relevant complications during implantation or the follow-up, and all patients showed subjective clinical improvement.
Discussion
The main finding of our study is that HBP causes an early improvement in echocardiographic parameters in patients who are candidates for CRT when a narrowing of the QRS is achieved because of depolarization of the LV through the native conduction system.
Heart failure is a cardiovascular disease of increasing prevalence in our society with high morbidity and mortality. Ventricular asynchrony derived primarily from LBBB, but also from right bundle branch block (RBBB), is a well-known cause of left ventricular dysfunction and HF. Cardiac resynchronization therapy is currently available via the coronary sinus as a therapeutic measure for patients with HF who present with LBBB or RBBB and LV dysfunction when optimal pharmacological treatment is insufficient.12,13 In this way, it is possible to improve biventricular synchrony by pacing simultaneously from the right VP lead and LV pacing lead implanted in the coronary sinus. However, about 30% of patients do not respond for different reasons. A basic limitation of current CRT is that it synchronizes the LV in a non-physiological way; that is, it is not performed through the native cardiac electrical conduction system, but through the lateral wall of the muscle of the LV via epicardial activation. This issue has been related to some cases of non-response and the potential development of ventricular arrhythmias.
Left ventricular endocardial pacing through the transseptal approach emerged as an alternative to solve this aforementioned limitation;14 however, this is a challenging technique, and it requires permanent anticoagulation.
Permanent His bundle pacing can correct BBB by recruiting the native conduction system and achieving ‘ideal’ resynchronization, with a biventricular contraction identical to the physiological one; this is why it might be an alternative to resynchronization via the coronary sinus.
Previously, Narula3 described the theory of the longitudinal dissociation in the His bundle, which maintains that the fibres destined to the right and left bundle branches of the conduction system are arranged longitudinally and already differentiated and separated in the trunk of His. Accordingly, an injury that only affects a part of the Hisian trunk can cause electrocardiographic disorders typical of BBB. El-Sherif et al.4 proved that pacing distal to the blockage can correct BBB. However, it was not until 2005 that the first case of CRT with His pacing was described in humans,5 and a few years later, the first series of patients with CRT were reported.15–18 Upadhyay et al.19 recently described three intracardiac activation patterns of the left septum in LBBB: Intact Purkinje activation (IPA), Complete conduction block (CCB) with proximal block, and CCB with distal block. The QRS correction was achieved in the 94% of the cases with CCB and proximal block, and in the 62% of the cases with CCB and distal block. It was not possible to correct the QRS in the case of IPA. Nevertheless, a problem that can be attributed to resynchronization via HBP is that, a priori, we cannot determine at which level the alteration of the conduction is located unless we perform an electrophysiological test. In our study, HBP achieved narrowing of the QRS in 81% of patients. It is noteworthy that in our series, 94% of patients had NIDCM, but we cannot predict whether this high rate of narrowing of the QRS will occur in ischaemic patients.
Sharma et al.18 recently reported the largest multicentre cohort of patients with CRT using HBP. In this study, only 34% of patients (N = 36) had LBBB; HBP resulted in narrowing of the QRS in 33 patients (92%). In our series, all the patients (N = 48) had intrinsic LBBB; we achieved narrowing of the QRS in 81% of those patients. Although the narrowing rate is somewhat lower, we believe that it may be comparable and the differences may be attributed to the fact that there is still no uniformity in the criteria to determine when the electrocardiographic response to HBP may be acceptable or even in how to measure the width of the QRS during HBP.
Ajijola et al. previously analysed the impact of CRT using HBP on LVEF.9 They described 16 patients with a mean baseline LVEF of 25 ± 8%. The mean follow-up was 12 months (range 2–19), but only 6 patients completed exactly 1 year of follow-up; in these patients, LVEF improved from 27 ± 10% to 41 ± 13%. The short-term improvement of LVEF was not described. Sharma et al.18 reported an improvement of the LVEF (>5%) in 73% of the global patients (from 30% to 43%); among patients with LBBB, there was a significant improvement (26% to 41%). Huang et al.17 have reported recently another observational study of patients with HBP, HF, and LBBB; they described an improvement in the mean of LVEF from 32.4 ± 8.9% to 48.4 ± 12.2% at 3 months of follow-up. In our series, at the 1 month of follow-up, the median LVEF was 51% (48–58) (basal 30% [27–34%]), and it improved by >10% in 92% and >20% in 50% of cases. Our level of improvement is slightly better, probably because of the low rate of ischaemic patients in our study; only 3% of patients in contrast to 37% and 38% in the series by Sharma et al.18 and Ajiloa et al.,9 respectively.
Another observation is that in our series, the QRS shortened from 160 ms to 132 ms (86% with non-selective capture), whereas in Sharma et al.18 and Ajiloa et al.’s studies,9 the QRS shortened from 157 to 117 ms (57% with non-selective capture) and from 180 to 129 ms, respectively (94% with non-selective capture).
An issue that could be questionable is the best mode to measure QRS during HBP. According to the Recommendations from a Multicenter HBP Collaborative Working Group,11 in the case of non-selective HBP, the QRS duration is the interval (ms) between the stimulus and QRS end, while in the case of selective HBP the QRS duration excludes the isoelectric interval between the stimulus and QRS onset (which is equivalent to local HV). We think that the QRS derived from non-selective capture should be measured by subtracting the local HV from the total QRS duration; in this way, the results of different studies would be more comparable because we would be measuring the real recruitment of the conduction system, excluding the local capture of neighbouring myocardium. In our practice, we try to capture the native conduction system as distal as possible. This could explain the high rate non-selective HBP and the slightly longer QRS duration in comparison with Sharma.
The HBP thresholds with LBBB correction are an aspect to be highlighted in our series. During the implant, the median of acute threshold (V at 0.4 ms) was 1.6 (0.9–1.9). At follow-up, we observed 24 h after the implant that the HBP thresholds improved, perhaps due to injury and oedema at the acute moment, which then disappeared. At 1 and 3 months of follow-up, they tend to remain stable. These thresholds reported in our series are somewhat better than those previously reported; we hypothesize that this fact could be due to our implant technique seeking deeper penetration.
A topic that has not been studied so far regarding CRT by p-HBP is the objective assessment of intraventricular mechanical resynchronization by echocardiography. We have used the delay of SPW of the LV in M-mode colour TDI, but more novel echocardiographic methods based on myocardial deformation exist for assessing intraventricular dyssynchrony. Nonetheless, the mere purpose of using this echocardiographic method in our study was to easily demonstrate that LV electromechanical synchrony can be achieved by HBP. Unfortunately, a standardized parameter to accurately assess LV dyssynchonization is still lacking, and no echocardiographic method described until now is free of limitations. M-mode has been a widely used conventional echocardiographic technique because of its high temporal and spatial resolution. A delay in M-mode colour TDI of the septal movement compared to the posterior wall ≥130 ms has a sensitivity of 100% and specificity of 63% when considering ventricular dyssynchrony,20 which was exceeded in all our cases, except in one patient in whom the value was 130 ms, as the delay was remarkably corrected early after cardiac resynchronization. This early improvement, which remained unchanged at 1 month of follow-up, could predict optimal cardiac resynchronization through the specific conduction system and early recovery of LVEF.
Our study is the first single-centre experience to demonstrate the early improvement of intraventricular mechanical resynchronization by echocardiography (24 h after implantation and at 1 month of follow-up), as well as the short-term impact of p-HBP on LVEF by analysing early improvement in LVEF at 1 month of follow-up, which has not yet been described in literature. Although randomized studies are lacking, all previous data support that p-HBP is now an effective alternative to classic CRT, and that it could replace CRT through the coronary sinus in the near future as first-line therapy. Moreover, considering the success rate of implants, the total procedure time and fluoroscopy doses of p-HBP are comparable to those of CRT via the coronary sinus, and the safety of p-HBP has already been demonstrated in large series. We think that the physiological correction of BBB and LV asynchrony, through the native cardiac conduction system, allows for prediction of a higher rate of responders in patients with biventricular asynchrony, LV dysfunction, and HF.
However, this was a descriptive, single-centre, non-randomized study. It would be advisable to design other clinical randomized trials with a larger number of patients to corroborate these results.
Limitations
This is an observational and non-randomized study, and there is not a comparison to the early improvements in LVEF in the case of the biventricular resynchronization through classic CRT.
The high success rates of cardiac resynchronization through HBP achieved by experienced operators need to be replicated in prospective studies.
Although more novel echocardiography methods based on myocardial dysfunction exist for assessing intraventricular dyssynchrony, we used the delay of SPW of the LV in M-mode colour because this is the most extended and reproducible method, and the mere purpose of using this echocardiographic method in our study was to easily demonstrate that LV electromechanical synchrony can be achieved by HBP.
Conclusions
There is early improvement in LVEF and left ventricular electromechanical synchronization after p-HBP in patients with HF, LBBB, and an indication for CRT.
Cardiac resynchronization therapy by p-HBP is feasible and safe in a high percentage of patients, with reasonable fluoroscopy times and acceptable capture thresholds.
However, studies with larger numbers of patients and long-term follow-up periods are needed in order to generalize these results.
Acknowledgements
We thank Costa Lawrence and Thomas Fernandez for critically reading and editing the manuscript.
Conflict of interest: none declared.
Funding
The Andalusian Society of Cardiology.



