-
PDF
- Split View
-
Views
-
Cite
Cite
Roman Brenner, Peter Ammann, See-Il Yoon, Stefan Christen, Jens Hellermann, Grégoire Girod, Urs Knaus, Firat Duru, Nazmi Krasniqi, David Ramsay, Christian Sticherling, Kurt Lippuner, Michael Kühne, Reduction of falls and fractures after permanent pacemaker implantation in elderly patients with sinus node dysfunction, EP Europace, Volume 19, Issue 7, July 2017, Pages 1220–1226, https://doi.org/10.1093/europace/euw156
Close - Share Icon Share
Abstract
Elderly patients with sinus node dysfunction (SND) are at increased risk of falls with possible injuries. However, the incidence of these adverse events and its reduction after permanent pacemaker (PPM) implantation are not known.
Eighty-seven patients (mean [SD] age 75.4 [8.3] years, 51% women) with SND and an indication for cardiac pacing were included and were examined by a standardized interview targeting fall history. The incidence and total number of falls, falls with injury, falls requiring treatment, and falls resulting in a fracture were assessed for the time period of 12 months before (retrospectively) and after PPM implantation (prospectively). Furthermore, symptoms such as syncope, dizziness, and dyspnea were evaluated before and after PPM implantation. The implantation of a PPM was associated with a reduced proportion of patients experiencing at least one fall by 71% (from 53 to 15%, P < 0.001) and a reduction of the absolute number of falls by 90% (from 127 to 13, P < 0.001) during the 12 months before vs. after PPM implant. Falls with injury (28 vs. 10%, P = 0.005), falls requiring medical attention (31 vs. 8%, P < 0.001), and falls leading to fracture (8 vs. 0%, P = 0.013) were similarly reduced. Notably, fewer patients had syncope (4 vs. 45%, P < 0.001) and dizziness after PPM implantation (12 vs. 45%, P < 0.001).
Falls, fall-related injuries, and fall-related fractures are frequent in SND patients. Permanent pacemaker implantation is associated with a significantly reduced risk of these adverse events, although no causal relationship could be established due to the study design.
Prevalence of falls and osteoporotic fracture risk in patients with sinus node disease.
Marked reduction of falls, injuries, and fractures after pacemaker implantation (even in patients without previous syncope).
Improvement of symptoms such as syncope and dizziness after pacemaker implantation.
Introduction
Falls represent a serious threat to the lives, health, and independence of older adults and a growing economic burden, especially in industrialized countries where the number of individuals older than 65 years is expected to double between the years 2005 and 2050.1 Falls are the leading cause of injury in people aged 65 years or older, and between 30 and 40% of community-dwelling persons aged 65 years or older fall at least once per year.2 In addition, falls are the underlying cause of half of all injury-related hospitalizations in elderly.1
There is a myriad of causes for falls, some of which have been related to rhythm disorders such as atrioventricular (AV) block,3 carotid sinus syndrome (CSS), and sinus node disease (SND),3 and retrospective data suggest that the incidence of falls may be reduced in patients with SND after permanent pacemaker (PPM) implantation.4 Sinus node dysfunction is a common disorder in elderly patients and encompasses a spectrum that ranges from sinus bradycardia to sinus arrest and the possible development of a bradycardia–tachycardia syndrome with corresponding symptoms and consequences. The incidence of falls in patients with SND is estimated to range between 30 and 60%5 and symptoms related to SND such as syncope and also dizziness with gait disturbance might both contribute to falls with or without injuries. Data from the general population suggest that ∼5% of the patients with falls have a fracture.6
In SND patients, the incidence of falls with clinical consequences is largely unknown. It is not clear whether cardiac pacing in SND patients reduces falls, particularly falls with injuries, and symptoms attributed to SND. Therefore, the aim of this study was to assess the reduction of falls and symptoms in elderly patients referred for PPM implantation due to SND before and after PPM implantation.
Methods
Settings and participants
In this non-randomized, prospective multicentre study, 87 consecutive patients with SND and a class I indication for PPM implantation according to current guidelines were included.7 Inclusion criteria were: 50 years of age or older; symptoms compatible with SND plus ECG characteristics suggestive of SND evidenced by 12-lead ECG or Holter-ECG; availability for follow-up at the study centre for the length of the study; signed informed consent. Exclusion criteria were: impaired cognitive function, permanent second or third degree AV block; permanent atrial fibrillation (AF); remaining life expectancy of <2 years; participation in another clinical trial.
Patient demographics, cardiovascular history and medication, symptoms associated with SND, and left ventricular ejection fraction (LVEF) assessed by transthoracic echocardiography were recorded at study inclusion. Arrhythmias documented by ECG or device interrogation were noted and device interrogation was performed 12 months after PPM implantation. In addition, the FRAX score estimating the bone-related fracture risk was calculated for major osteoporotic fractures (FRAX MOF) and for hip fractures (FRAX HIP) at study entry (www.shef.ac.uk/FRAX).
The study was approved by the local institutional review boards of the participating centres and was registered at www.clinicaltrials.gov (NCT01037426).
Pacemaker implantation
All patients underwent implantation of a dual-chamber PPM manufactured by Medtronic (Minneapolis, MN, USA). The algorithm to minimize right ventricular pacing (managed ventricular pacing was activated at the time of device implantation. The generators used were: Medtronic Advisa® (n = 29), Medtronic Adapta® (n = 57), and Medtronic EnRhythm® (n = 1).
Outcomes
The primary outcome was the change in the number of patients experiencing any fall (≥1 fall, with or without injury, requiring medical attention or not, with or without fracture) during the year following compared with the year preceding PPM implantation.8 Secondary objectives were: the change in the total number of falls (as events with or without injury, requiring medical attention or not, with or without fracture) during the year following compared with the year preceding PPM implantation; the change in predefined symptoms of SND (syncope, dizziness, fatigue, and dyspnoea) 12 months after compared with before PPM implantation; the occurrence of AF 12 months after compared with before PPM implantation; and the safety profile of PPM implantation and cardiac pacing during the 12 months following implantation.
Fall history, including falls without injury, falls with injury, falls with medical treatment and falls with fracture, and symptoms were assessed by patient recall and history over a 12-month period before (retrospectively) and after PPM implantation (prospectively). In all patients, the family physician of the corresponding patient was also interviewed and asked for fall history of the patient. A fall was defined as an ‘unexpected event where a person falls to the ground from an upper level or the same level’, according to the International Classification of Disease (ICD 9 and 10) definition recommended by the WHO.1 The period of 12 months was chosen because it has been shown to correlate relatively well with the true incidence of falls.8 For falls resulting in a fracture, radiological confirmation of the fracture was required.
Statistical analysis
Baseline variables were analyzed in all patients included (n = 87). All other analyses were performed in the patients with available prospective fall data 1 year after PPM implantation (per protocol analysis, n = 78). No 12-month follow-up data were available for nine patients (six patients discontinued the study, three patients died, none lost to follow-up).
χ2 tests were used for significance testing between categorical variables. Differences involving continuous variables of independent groups were tested by t-test or Mann–Whitney U-test as appropriate. For paired comparisons, paired t-test or Wilcoxon rank sum test was used as appropriate. McNemar test was used to compare the paired proportions before and after PPM insertion considering each individual as his own control. To explore potential predictors of falls, univariable logistic regressions were used. Multiple logistic regression analysis with forced entry was performed to assess the independent importance of clinically relevant predictors of fall reduction. Values are presented as mean and corresponding SDs or as median values and interquartile ranges (IQRs) as appropriate. Two-sided P-value of <0.05 was considered statistically significant. The statistical calculations were performed using StatsDirect version 2.7.7 (StatsDirect Ltd, Atrincham, Cheshire, UK).
Results
Baseline characteristics
The baseline characteristics of all 87 patients are shown in Table 1, characterization of SND by ECG findings and other ECG abnormalities are given in Table 2. FRAX MOF and FRAX HIP to determine osteoporotic fracture risk were significantly higher in women than in men (30 [20, 40] vs. 10 [7.7, 14], P < 0.001 and 13 [4.9, 17] vs. 3.3 [1.5, 5.2], P < 0.001).
Baseline characteristics of the overall study population assessed before pacemaker implantation
| . | Overall study population (n = 87) . |
|---|---|
| Age (years), mean (SD) | 75.4 (8.3) |
| Women, n (%) | 44 (50.6) |
| Weight (kg) | 80.4 (18.9) |
| Height (cm) | 168.3 (9.6) |
| Body mass index (kg/m2), mean (SD) | 27.9 (4.5) |
| Heart rate (beats per minute), mean (SD) | 62.6 (17.3) |
| FRAX MOF (%), median (IQR) | 16.0 (9.6, 32.0) |
| FRAX MOF ≥ 20%, n (%) | 37 (42.5) |
| FRAX HIP (%), median (IQR) | 5.1 (2.4, 13.0) |
| FRAX HIP ≥ 3%, n (%) | 63 (72.4) |
| Cardiovascular medical history | |
| Non-ischaemic cardiomyopathy, n (%) | 8 (9.2) |
| Coronary artery disease, n (%) | 31 (35.6) |
| Valvular heart disease, n (%) | 20 (23.0) |
| Stroke/TIA, n (%) | 10 (11.8) |
| Hypertension, n (%) | 59 (67.8) |
| Diabetes mellitus, n (%) | 20 (23.3) |
| LVEF (%), median (IQR) | 60 (50, 62) |
| Medication | |
| RAS inhibitor, n (%) | 56 (64.4) |
| Betablocker, n (%) | 35 (40.2) |
| CCB, n (%) | 24 (27.6) |
| Diuretics, n (%) | 33 (37.9) |
| . | Overall study population (n = 87) . |
|---|---|
| Age (years), mean (SD) | 75.4 (8.3) |
| Women, n (%) | 44 (50.6) |
| Weight (kg) | 80.4 (18.9) |
| Height (cm) | 168.3 (9.6) |
| Body mass index (kg/m2), mean (SD) | 27.9 (4.5) |
| Heart rate (beats per minute), mean (SD) | 62.6 (17.3) |
| FRAX MOF (%), median (IQR) | 16.0 (9.6, 32.0) |
| FRAX MOF ≥ 20%, n (%) | 37 (42.5) |
| FRAX HIP (%), median (IQR) | 5.1 (2.4, 13.0) |
| FRAX HIP ≥ 3%, n (%) | 63 (72.4) |
| Cardiovascular medical history | |
| Non-ischaemic cardiomyopathy, n (%) | 8 (9.2) |
| Coronary artery disease, n (%) | 31 (35.6) |
| Valvular heart disease, n (%) | 20 (23.0) |
| Stroke/TIA, n (%) | 10 (11.8) |
| Hypertension, n (%) | 59 (67.8) |
| Diabetes mellitus, n (%) | 20 (23.3) |
| LVEF (%), median (IQR) | 60 (50, 62) |
| Medication | |
| RAS inhibitor, n (%) | 56 (64.4) |
| Betablocker, n (%) | 35 (40.2) |
| CCB, n (%) | 24 (27.6) |
| Diuretics, n (%) | 33 (37.9) |
CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; RAS, renin–angiotensin system; TIA, transient ischaemic attack; FRAX HIP, 10-year probability of osteoporotic hip fracture; FRAX MOF, 10-year probability of major osteoporotic fracture.
Baseline characteristics of the overall study population assessed before pacemaker implantation
| . | Overall study population (n = 87) . |
|---|---|
| Age (years), mean (SD) | 75.4 (8.3) |
| Women, n (%) | 44 (50.6) |
| Weight (kg) | 80.4 (18.9) |
| Height (cm) | 168.3 (9.6) |
| Body mass index (kg/m2), mean (SD) | 27.9 (4.5) |
| Heart rate (beats per minute), mean (SD) | 62.6 (17.3) |
| FRAX MOF (%), median (IQR) | 16.0 (9.6, 32.0) |
| FRAX MOF ≥ 20%, n (%) | 37 (42.5) |
| FRAX HIP (%), median (IQR) | 5.1 (2.4, 13.0) |
| FRAX HIP ≥ 3%, n (%) | 63 (72.4) |
| Cardiovascular medical history | |
| Non-ischaemic cardiomyopathy, n (%) | 8 (9.2) |
| Coronary artery disease, n (%) | 31 (35.6) |
| Valvular heart disease, n (%) | 20 (23.0) |
| Stroke/TIA, n (%) | 10 (11.8) |
| Hypertension, n (%) | 59 (67.8) |
| Diabetes mellitus, n (%) | 20 (23.3) |
| LVEF (%), median (IQR) | 60 (50, 62) |
| Medication | |
| RAS inhibitor, n (%) | 56 (64.4) |
| Betablocker, n (%) | 35 (40.2) |
| CCB, n (%) | 24 (27.6) |
| Diuretics, n (%) | 33 (37.9) |
| . | Overall study population (n = 87) . |
|---|---|
| Age (years), mean (SD) | 75.4 (8.3) |
| Women, n (%) | 44 (50.6) |
| Weight (kg) | 80.4 (18.9) |
| Height (cm) | 168.3 (9.6) |
| Body mass index (kg/m2), mean (SD) | 27.9 (4.5) |
| Heart rate (beats per minute), mean (SD) | 62.6 (17.3) |
| FRAX MOF (%), median (IQR) | 16.0 (9.6, 32.0) |
| FRAX MOF ≥ 20%, n (%) | 37 (42.5) |
| FRAX HIP (%), median (IQR) | 5.1 (2.4, 13.0) |
| FRAX HIP ≥ 3%, n (%) | 63 (72.4) |
| Cardiovascular medical history | |
| Non-ischaemic cardiomyopathy, n (%) | 8 (9.2) |
| Coronary artery disease, n (%) | 31 (35.6) |
| Valvular heart disease, n (%) | 20 (23.0) |
| Stroke/TIA, n (%) | 10 (11.8) |
| Hypertension, n (%) | 59 (67.8) |
| Diabetes mellitus, n (%) | 20 (23.3) |
| LVEF (%), median (IQR) | 60 (50, 62) |
| Medication | |
| RAS inhibitor, n (%) | 56 (64.4) |
| Betablocker, n (%) | 35 (40.2) |
| CCB, n (%) | 24 (27.6) |
| Diuretics, n (%) | 33 (37.9) |
CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; RAS, renin–angiotensin system; TIA, transient ischaemic attack; FRAX HIP, 10-year probability of osteoporotic hip fracture; FRAX MOF, 10-year probability of major osteoporotic fracture.
Electrocardiographic characterization of SND and history of arrhythmias at baseline in the overall study population
| . | Overall study population (n = 87) . |
|---|---|
| Sinus arrest, n (%) | 46 (52.9) |
| Brady/tachycardia syndrome, n (%) | 27 (31.0) |
| Chronotropic incompetence, n (%) | 5 (5.7) |
| History of paroxysmal AF, n (%) | 35 (40.2) |
| History of persistent AF, n (%) | 3 (3.4) |
| AV block I°, n (%) | 15 (17.2) |
| Intermittent AV block II°, n (%) | 8 (9.2) |
| Intermittent AV block III°, n (%) | 6 (6.9) |
| Right bundle branch block, n (%) | 7 (8.0) |
| Left bundle branch block, n (%) | 7 (8.0) |
| . | Overall study population (n = 87) . |
|---|---|
| Sinus arrest, n (%) | 46 (52.9) |
| Brady/tachycardia syndrome, n (%) | 27 (31.0) |
| Chronotropic incompetence, n (%) | 5 (5.7) |
| History of paroxysmal AF, n (%) | 35 (40.2) |
| History of persistent AF, n (%) | 3 (3.4) |
| AV block I°, n (%) | 15 (17.2) |
| Intermittent AV block II°, n (%) | 8 (9.2) |
| Intermittent AV block III°, n (%) | 6 (6.9) |
| Right bundle branch block, n (%) | 7 (8.0) |
| Left bundle branch block, n (%) | 7 (8.0) |
AF, atrial fibrillation; AV, atrioventricular.
Electrocardiographic characterization of SND and history of arrhythmias at baseline in the overall study population
| . | Overall study population (n = 87) . |
|---|---|
| Sinus arrest, n (%) | 46 (52.9) |
| Brady/tachycardia syndrome, n (%) | 27 (31.0) |
| Chronotropic incompetence, n (%) | 5 (5.7) |
| History of paroxysmal AF, n (%) | 35 (40.2) |
| History of persistent AF, n (%) | 3 (3.4) |
| AV block I°, n (%) | 15 (17.2) |
| Intermittent AV block II°, n (%) | 8 (9.2) |
| Intermittent AV block III°, n (%) | 6 (6.9) |
| Right bundle branch block, n (%) | 7 (8.0) |
| Left bundle branch block, n (%) | 7 (8.0) |
| . | Overall study population (n = 87) . |
|---|---|
| Sinus arrest, n (%) | 46 (52.9) |
| Brady/tachycardia syndrome, n (%) | 27 (31.0) |
| Chronotropic incompetence, n (%) | 5 (5.7) |
| History of paroxysmal AF, n (%) | 35 (40.2) |
| History of persistent AF, n (%) | 3 (3.4) |
| AV block I°, n (%) | 15 (17.2) |
| Intermittent AV block II°, n (%) | 8 (9.2) |
| Intermittent AV block III°, n (%) | 6 (6.9) |
| Right bundle branch block, n (%) | 7 (8.0) |
| Left bundle branch block, n (%) | 7 (8.0) |
AF, atrial fibrillation; AV, atrioventricular.
Occurrence of falls and sinus node dysfunction-related symptoms after pacemaker implantation
Overall, 47/87 (54%) of the patients reported at least one fall within 12 months before PPM implantation, which lead to an injury in 25/47 (53%), need for medical attention in 28/47 (59%) and fracture in 7/47 (13%) of them. The reported fall-related fractures affected the ribs and the jaw (two patients each), the vertebrae, the distal radius, and the proximal humerus in one patient each.
Compared with the 12 months before PPM implant, the proportion of patients with one or more falls during the 12 months after PPM implantation was significantly reduced (Figure 1). Falls with injury, falls requiring medical attention and falls leading to fracture were reduced to a similar extent (Figure 1). Of the 41 patients who reported one or more falls and of the 20 patients who experienced at least two falls during the 12 months preceding PPM implantation, 32/41 (78%) and 14/20 (70%) patients were free from any fall after PPM implantation. Predictors of falls, assessed in univariable regression models, are presented in Table 3. Whereas syncope was a significant predictor of falls, intermittent AV block and any of the antihypertensive therapies were not associated with falls before PPM implantation. In a multivariable logistic regression model including a history of syncope (OR 11.1 [95% CI 3.7–33.8]), any intermittent AV block (OR 0.9 [95% CI 0.2–4.8]), diabetes mellitus (OR 0.6 [95% CI 0.1–2.5]), and hypertension status (OR 1.0 [0.3–3.5]), only a history of syncope was a significant independent predictor for reduction of falls after PPM implantation. Accordingly, 34/47 (72%) of patients with falls before PPM implantation and 0/12 of the patients with falls after the procedure reported at least one syncope (P < 0.001). However, even in patients with no syncope but a fall history before PPM implantation (11/78, 14%), the number of falls was significantly reduced by 91% from 47 to 4 falls after the intervention (P = 0.002).
Predictors of falls before PPM implantation in the overall study population calculated in univariable, logistic regression models with independent variables assessed before PPM implantation
| Independent variable . | Odds ratio (95% CI) for fall . | P-value . |
|---|---|---|
| Age (years)a | 0.97 (0.92, 1.0) | 0.201 |
| Sex (male vs. female) | 0.96 (0.41, 2.22) | 0.921 |
| Diabetes mellitus | 1.08 (0.40, 3.0) | 0.877 |
| Hypertension | 1.57 (0.64, 3.88) | 0.329 |
| Sinus arrest | 1.39 (0.60, 3.24) | 0.447 |
| Brady/tachycardia syndrome | 1.09 (0.44, 2.73) | 0.847 |
| Chronotropic incompetence | 0.55 (0.09, 3.46) | 0.522 |
| AV block I° | 0.36 (0.11, 1.15) | 0.076 |
| Intermittent AV block II° | 0.48 (0.11, 2.13) | 0.333 |
| Intermittent AV block III° | 1.77 (0.31, 10.20) | 0.524 |
| Bundle branch block | 0.71 (0.24, 2.04) | 0.522 |
| Syncope | 14.82 (5.04, 43.54) | <0.001 |
| Fatigue | 1.80 (0.42, 7.73) | 0.427 |
| Dizziness | 0.51 (0.22, 1.21) | 0.125 |
| Dyspnoea | 0.65 (0.16, 2.61) | 0.545 |
| LVEF (%)a | 1.06 (0.99, 1.1) | 0.097 |
| Heart rate (beats per minute)a | 1.01 (0.99, 1.04) | 0.295 |
| RAS inhibitor | 0.63 (0.26, 1.54) | 0.313 |
| Calcium channel blocker | 1.27 (0.49, 3.29) | 0.620 |
| β-blocker | 1.23 (0.52, 2.93) | 0.632 |
| Diuretic | 0.85 (0.36, 2.03) | 0.714 |
| Independent variable . | Odds ratio (95% CI) for fall . | P-value . |
|---|---|---|
| Age (years)a | 0.97 (0.92, 1.0) | 0.201 |
| Sex (male vs. female) | 0.96 (0.41, 2.22) | 0.921 |
| Diabetes mellitus | 1.08 (0.40, 3.0) | 0.877 |
| Hypertension | 1.57 (0.64, 3.88) | 0.329 |
| Sinus arrest | 1.39 (0.60, 3.24) | 0.447 |
| Brady/tachycardia syndrome | 1.09 (0.44, 2.73) | 0.847 |
| Chronotropic incompetence | 0.55 (0.09, 3.46) | 0.522 |
| AV block I° | 0.36 (0.11, 1.15) | 0.076 |
| Intermittent AV block II° | 0.48 (0.11, 2.13) | 0.333 |
| Intermittent AV block III° | 1.77 (0.31, 10.20) | 0.524 |
| Bundle branch block | 0.71 (0.24, 2.04) | 0.522 |
| Syncope | 14.82 (5.04, 43.54) | <0.001 |
| Fatigue | 1.80 (0.42, 7.73) | 0.427 |
| Dizziness | 0.51 (0.22, 1.21) | 0.125 |
| Dyspnoea | 0.65 (0.16, 2.61) | 0.545 |
| LVEF (%)a | 1.06 (0.99, 1.1) | 0.097 |
| Heart rate (beats per minute)a | 1.01 (0.99, 1.04) | 0.295 |
| RAS inhibitor | 0.63 (0.26, 1.54) | 0.313 |
| Calcium channel blocker | 1.27 (0.49, 3.29) | 0.620 |
| β-blocker | 1.23 (0.52, 2.93) | 0.632 |
| Diuretic | 0.85 (0.36, 2.03) | 0.714 |
CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; RAS, renin–angiotensin system.
aOdds ratio is calculated per unit increase of the respective independent variable.
Predictors of falls before PPM implantation in the overall study population calculated in univariable, logistic regression models with independent variables assessed before PPM implantation
| Independent variable . | Odds ratio (95% CI) for fall . | P-value . |
|---|---|---|
| Age (years)a | 0.97 (0.92, 1.0) | 0.201 |
| Sex (male vs. female) | 0.96 (0.41, 2.22) | 0.921 |
| Diabetes mellitus | 1.08 (0.40, 3.0) | 0.877 |
| Hypertension | 1.57 (0.64, 3.88) | 0.329 |
| Sinus arrest | 1.39 (0.60, 3.24) | 0.447 |
| Brady/tachycardia syndrome | 1.09 (0.44, 2.73) | 0.847 |
| Chronotropic incompetence | 0.55 (0.09, 3.46) | 0.522 |
| AV block I° | 0.36 (0.11, 1.15) | 0.076 |
| Intermittent AV block II° | 0.48 (0.11, 2.13) | 0.333 |
| Intermittent AV block III° | 1.77 (0.31, 10.20) | 0.524 |
| Bundle branch block | 0.71 (0.24, 2.04) | 0.522 |
| Syncope | 14.82 (5.04, 43.54) | <0.001 |
| Fatigue | 1.80 (0.42, 7.73) | 0.427 |
| Dizziness | 0.51 (0.22, 1.21) | 0.125 |
| Dyspnoea | 0.65 (0.16, 2.61) | 0.545 |
| LVEF (%)a | 1.06 (0.99, 1.1) | 0.097 |
| Heart rate (beats per minute)a | 1.01 (0.99, 1.04) | 0.295 |
| RAS inhibitor | 0.63 (0.26, 1.54) | 0.313 |
| Calcium channel blocker | 1.27 (0.49, 3.29) | 0.620 |
| β-blocker | 1.23 (0.52, 2.93) | 0.632 |
| Diuretic | 0.85 (0.36, 2.03) | 0.714 |
| Independent variable . | Odds ratio (95% CI) for fall . | P-value . |
|---|---|---|
| Age (years)a | 0.97 (0.92, 1.0) | 0.201 |
| Sex (male vs. female) | 0.96 (0.41, 2.22) | 0.921 |
| Diabetes mellitus | 1.08 (0.40, 3.0) | 0.877 |
| Hypertension | 1.57 (0.64, 3.88) | 0.329 |
| Sinus arrest | 1.39 (0.60, 3.24) | 0.447 |
| Brady/tachycardia syndrome | 1.09 (0.44, 2.73) | 0.847 |
| Chronotropic incompetence | 0.55 (0.09, 3.46) | 0.522 |
| AV block I° | 0.36 (0.11, 1.15) | 0.076 |
| Intermittent AV block II° | 0.48 (0.11, 2.13) | 0.333 |
| Intermittent AV block III° | 1.77 (0.31, 10.20) | 0.524 |
| Bundle branch block | 0.71 (0.24, 2.04) | 0.522 |
| Syncope | 14.82 (5.04, 43.54) | <0.001 |
| Fatigue | 1.80 (0.42, 7.73) | 0.427 |
| Dizziness | 0.51 (0.22, 1.21) | 0.125 |
| Dyspnoea | 0.65 (0.16, 2.61) | 0.545 |
| LVEF (%)a | 1.06 (0.99, 1.1) | 0.097 |
| Heart rate (beats per minute)a | 1.01 (0.99, 1.04) | 0.295 |
| RAS inhibitor | 0.63 (0.26, 1.54) | 0.313 |
| Calcium channel blocker | 1.27 (0.49, 3.29) | 0.620 |
| β-blocker | 1.23 (0.52, 2.93) | 0.632 |
| Diuretic | 0.85 (0.36, 2.03) | 0.714 |
CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; RAS, renin–angiotensin system.
aOdds ratio is calculated per unit increase of the respective independent variable.
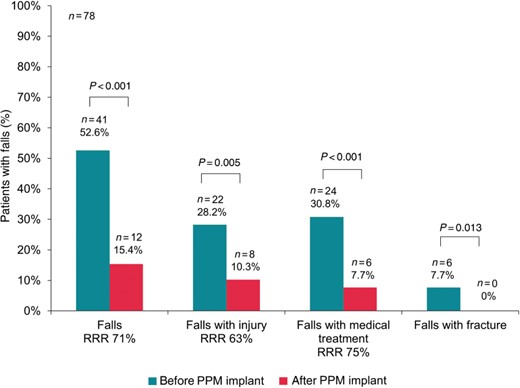
Proportion of patients with different categories of falls before and after pacemaker implantation. PPM, permanent pacemaker; RRR, relative risk reduction.
Similar results were obtained with regard to the total number of fall events by category (Figure 2). The overall number of falls was reduced by 90% (127 falls before vs. 13 after PPM implantation, P < 0.001), the number of falls with injury was reduced by 77% (35 vs. 8, P = 0.002), the number of falls requiring medical attention was reduced by 85% (41 vs. 6, P < 0.001), and the number of falls resulting in a fracture by 100% (6 vs. 0, P = 0.013).
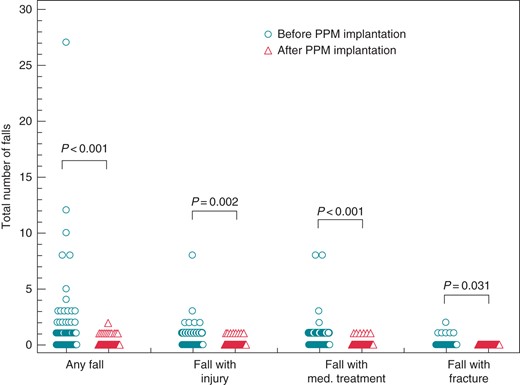
Total number of falls grouped according to its consequences. Circles symbolize patients before, triangle patients after permanent pacemaker implantation.
A sub-analysis excluding patients with intermittent AV block showed similar results compared with the overall population: the proportion of patients with falls, falls with injuries, and falls with fracture was reduced after PPM implantation by 72% (from 36/67 [53.7%] to 10/67 [14.9%], P < 0.001), 67% (from 18/67 [26.9%] to 6/67 [9%], P = 0.008), and 100% (from 4/67 [6%] to 0/67 [0%], P < 0.001), respectively. The number of falls, falls with injury, and falls with fracture was reduced by 91% (from 116 to 11 falls, P < 0.001), 80% (from 30 to 6 falls, P = 0.002), and 100% (from 4 to 0 falls, P = 0.13), respectively, after PPM implantation.
The presence of a PPM was also associated with improvement of typical symptoms of SND such as syncope and dizziness (Figure 3). In contrast, the proportion of patients reporting dyspnoea increased, although no shift in NYHA (New York Heart Association) class I/II and III/IV patients was observed after PPM implantation compared with before PPM implantation (31 vs. 32% in NYHA I/II, P = 1.0 and 12 vs. 8% in NYHA III/IV, P = 0.58, respectively). New onset of dyspnoea was reported by 15 (19%) patients after PPM implantation, whereas five (6%) patients reported improvement of dyspnoea. The median percentages of atrial and ventricular pacing 12 months after PPM implantation were 63% (12, 88%) and 0% (0, 2%), respectively. New onset of dyspnoea was significantly associated with the percentage of right ventricular pacing (OR 1.03 [95% CI 1.00–1.54] per increase of 1% of ventricular stimulation, P = 0.041), but the median percentage of ventricular pacing was still low (0%, IQR 0–30%) in these 15 patients. Of the 32 patients with a history of paroxysmal AF at baseline, 7 (22%) developed persistent or permanent AF as evidenced by device diagnostics. New onset AF was found in two patients (3%). Persistent or permanent AF was significantly more frequent 12 months after PPM implantation compared with baseline (11 vs. 4%, P = 0.046) and was more prevalent in patients with new onset dyspnoea than in those without (33 vs. 6%, P = 0.010).
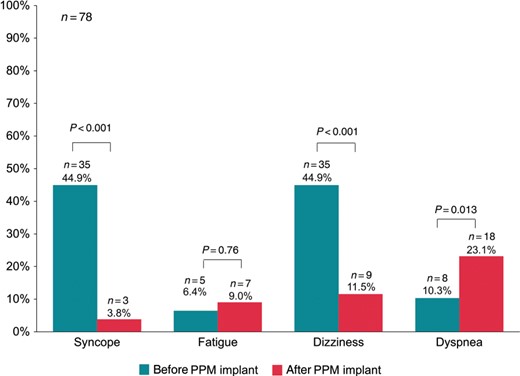
Incidence of reported symptoms 12 months before and 12 months after implantation of a permanent pacemaker. PPM, permanent pacemaker.
At the visit 12 months after PPM implantation, 46 (59%) patients took an inhibitor of the renin–angiotensin–aldosterone system, 21 (27%) were on a calcium channel blocker, 37 (47%) on a β-blocker, and 32 (41%) on a diuretic. This was not significantly different compared with the respective proportion at the initial visit before PPM insertion.
Safety
During the follow-up period of 12 months, three patients (3%) died. Causes of death were stroke, septic shock unrelated to the PPM, and unknown. No implantation-related death occurred. Thirteen patients (17%) were hospitalized due to heart failure (n = 3), lead dislodgement (n = 3), syncope (n = 2), and AF, stroke, renal failure, pneumonia, and back pain (n = 1 each).
Discussion
During the 12 months preceding PPM implantation, more than half of the patients with falls in our study suffered from fall-related injuries and the need for medical treatment, and 13% had a clinically relevant fracture. The implantation of a PPM was associated with a significant reduction of falls and a significant reduction of injuries following a fall by up to 70%. Furthermore, symptoms such as syncope and dizziness were less frequently reported after PPM implantation compared with before the procedure. The overall safety profile of PPM implantation was comparable with that reported in previous studies.9,10
The incidence of falls before PPM implantation was high in our study population compared with studies examining a general elderly population over a similar time period of 1 year.11 Arrhythmias are not rare in patients with unexplained falls, depending on the method of monitoring, and the American Geriatric Society/British Geriatric Society and the National Institute of Health and Care Excellence recommend standardized cardiovascular investigations in patients with recurrent falls.12,13 A recently published single centre, prospective, observational cohort study of patients with recurrent, unexplained falls implanted with an internal loop recorder found arrhythmias correlating with falls in 20% of these patients and 14% were subsequently implanted with a PPM.14 Unexplained fall recurrence within the 9 months follow-up period was 51% despite guideline-based falls assessment and intervention, and the presence of arrhythmia (mainly bradyarrhythmia) was the single most important risk factor for fall recurrence. Other studies suggest that patients with previous falls have a nearly 70% risk of experiencing another fall during the subsequent year.15 In contrast, we observed a fall recurrence rate of only 22% in patients with a previous fall after PPM implantation, and therefore, despite the fact that no control group without PPM implantation is available, it may be hypothesized that PPM implantation had a major effect on fall reduction. While a significant contribution of a placebo effect of PPM implantation and also regression to the mean cannot be excluded, several other factors might have influenced the association of PPM implantation and fall reduction. Routinely taught measures to avoid falls and syncope in patients prone to these adverse events might have had an effect on the observed reduction of falls during the year after PPM implantation. In contrast, the awareness for falls after PPM implantation is expected to increase rather than decrease due to entry into the prospective part of the study (informed consent). Therefore, underreporting of falls is probably more likely in the period before PPM implantation, potentially even leading to an underestimation of the effect of PPM implantation.
Our findings confirm the results of previous studies with regard to the reduction of syncope after PPM implantation.16,17 In the study from Ng Kam et al.,17 syncope within 12 months after PPM implantation in patients with SND was 4.5%, whereas the incidence was 50% before PPM implantation; this result is in line with our data. Multivariable analysis identified syncope as a significant predictor of fall reduction after PPM implantation; however, even in patients with falls but no syncope before PPM implantation, the fall rate was significantly reduced after PPM implantation. This underscores that an arrhythmia that does not lead to syncope may be associated with falls,14 and that a reduction of falls may be achieved by correcting the arrhythmia.
In other series, the proportion of falls leading to injury was similar (40–60%) compared with our study, but the proportion of falls leading to fractures was lower (5%).6,11 This might be due to a high bone-related fracture risk observed in our study population, since 43 and 72% of our patients had an FRAX MOF ≥20% and an FRAX HIP ≥3%, respectively (intervention thresholds in several countries).18
Health-related quality of life was recently shown to be increased in the short term and preserved in the long term in a prospective Dutch cohort in patients undergoing PPM implantation.19 Apart from the reduction of falls and syncope, we observed a significant reduction of dizziness after PPM implantation. Whether and to what extent our findings translated into improvements of quality of life was not studied. Interestingly, however, the proportion of patients reporting dyspnoea was increased in our study 12 months after PPM implantation. Since the percentage of ventricular pacing was very low (median 0%), RV pacing is unlikely to be a relevant cause for this finding. It is conceivable that the higher percentage of persistent AF in patients with new onset dyspnoea might play a role. Whether the development of persistent AF is linked to pacing,5 to the natural course of the disease in a patient population with a high prevalence of hypertension or to the availability of device diagnostics with continuous monitoring capacities cannot be answered by the present study and merits further investigation.
Beyond quality-of-life aspects, falls in elderly patients are an important economic problem. The inflation-adjusted direct medical costs to the US health care system of falls and related injuries have been estimated at about USD 20 billion.20,21 Although the monitoring of health care resource utilization was not analyzed in our study, the treatment of SND with PPM implantation was shown to be associated with a statistically significant reduction of falls and related injuries of a clinically relevant magnitude. Overall, the reduction of falls after PPM implantation may be of relevant health economic value to patients, payers, and society.
Limitations
Our study has several limitations. In patients with a clear indication for PPM implantation, a control group was not feasible for ethical reasons. Due to the non-randomized, observational study design, we were able to show an association of PPM implantation with a reduction of falls during the following 12 months but not a causal relationship. Fall history was based on patient recall, which may be subject to bias. In order to minimize this, patients with cognitive dysfunction were excluded, and the family physician was contacted in order to confirm fall events. Finally, we enrolled patients at the time of PPM implantation according to the study protocol and potentially raised the patients' awareness for falls during the following 12 months. This suggests that the rate of falls in the period before PPM implantation might even have been underestimated. In addition, the protocol allowed the inclusion of patients with intermittent AV conduction disturbances if the symptomatic arrhythmia responsible for PPM implantation was documented SND. However, no differences in outcomes between patients with and without additional AV conduction disturbance were found. Finally, another limitation is the potential overlap between SND and the cardioinhibitory type of CSS. According to our study protocol carotid sinus massage was not performed routinely. The fact that the Safepace 2 trial, although underpowered, did not show a reduction of falls with PPM implantation in patients with CCS (compared with loop recorder implantation alone), suggests that the positive results with regard to fall reduction after PPM implantation in this study were mainly driven by treatment of SND.22
Conclusion
Falls, fall-related injuries and fall-related fractures are frequent in SND patients. Although no causal relationship could be established due to the non-randomized study design, PPM implantation was associated with a marked reduction of the incidence of falls and fall-related injuries and fractures in elderly patients with SND.
Funding
The present study was sponsored by Medtronic.
Conflict of interest: P.A. grant/research support and consultant for Medtronic. C.S. grant/research support from Medtronic, consultant for Medtronic. M.K. grant/research support from Medtronic, proctor for Medtronic (Cryoballoon).
Acknowledgements
We are grateful to Philippe Kress, MD, Glattbrugg, Switzerland for the critical review of our manuscript. Raymond Moser PhD (employee of Medtronic) was responsible for the overall study management and Monika Koller (employee of Medtronic) supported the study as a Clinical Research Associate.
References
Author notes
R.B. and P.A. contributed equally to this manuscript.



