-
PDF
- Split View
-
Views
-
Cite
Cite
Susanne Bendesgaard Pedersen, Søren Pihlkjær Hjortshøj, Hans Erik Bøtker, Dóra Körmendiné Farkas, Morten Schmidt, Henrik Toft Sørensen, Jens Cosedis Nielsen, Venous thromboembolism in patients with implantable cardioverter-defibrillators, EP Europace, Volume 19, Issue 6, June 2017, Pages 991–1001, https://doi.org/10.1093/europace/euw124
Close - Share Icon Share
Abstract
To examine the incidence of venous thromboembolism (VTE) and its risk factors among patients with implantable cardioverter-defibrillators (ICDs).
All first-time ICD recipients in Denmark during 2000–12 were identified from medical databases. Incident VTEs were ascertained, overall and according to gender, age, Charlson Comorbidity Index score (no, moderate, or severe comorbidity), prior pacemaker or cardiac resynchronization therapy (CRT-D) implantation, and ICD type (single-chamber, dual-chamber, or CRT-D). We computed the risk of VTE within 3 months and 5 years of implantation, taking death into account as a competing risk. We used Cox proportional hazards regression to compute hazard ratios as estimates of incidence rate ratios (IRRs). Among 8132 ICD recipients, 136 VTEs were diagnosed during up to 13 years of follow-up (median = 3.0 years). The VTE incidence rate was thus 4.5 per 1000 person-years [95% confidence interval (CI): 3.7–5.2]. Venous thromboembolism risk was 0.3% (95% CIs ranging from 0.1 to 0.7%) within 3 months following ICD implantation regardless of comorbidity level. Within 5 years following implantation it was 1.4% (95% CI: 0.8–2.3%), 1.3% (1.0–1.8%), and 3.2% (95% CI: 2.4–4.1%) for patients with no, moderate, and severe comorbidity, respectively. Overall, severe comorbidity conferred a 2.7-fold higher incidence rate ratio than no comorbidity (95% CI: 1.6–4.6). Incidence rate ratios did not differ by gender, age, or ICD type.
Three-month risk of VTE following ICD implantation was 0.3% regardless of comorbidity level. Five-year risk of VTE following ICD implantation was 1.9% and more than twice as high for patients with severe comorbidity as for patients without comorbidity.
This nationwide cohort study with up to 13 years of follow-up showed that the incidence rate of venous thromboembolism among implantable cardioverter-defibrillator recipients was 4.5 per 1000 person-years.
The 3-month risk of VTE following cardioverter-defibrillator implantation was 0.3%.
The 5-year risk of VTE following cardioverter-defibrillator implantation was 1.9%.
While the 3-month risk of VTE did not increase with comorbidity category, the 5-year risk was more than twice as high for patients with severe comorbidity as for patients without comorbidity.
Introduction
Implantable cardioverter-defibrillators (ICDs) are provided increasingly to patients with high comorbidity levels.1 Heart failure, myocardial infarction, and other underlying morbidities frequently found among ICD recipients1 are associated with elevated risk of venous thromboembolism (VTE).2,3 Moreover, lead-related venous thrombosis is a recognized complication of cardiac implantable electronic device (CIED) implantation.4 Mobile thrombi on device leads have been detected during intra-cardiac echocardiography in up to 30% of CIED patients presenting for ablation procedures.4 Identification of pulmonary embolisms (PEs) on autopsy in CIED patients with lead-related venous thrombosis has added further to speculation about a causal relation between CIED treatment and PE.5 First-time ICD implantation carries an 8–16% risk of any complication within 6 months,6 but the specific risk of VTE is unknown. A PE incidence of 3.3 per 1000 person-years recently was observed among CIED patients in a single-centre study with up to 11 years of follow-up, but no predictors of PE were identified.7 As VTE is a common cardiovascular event and PE is the leading cause of preventable hospital death,3 enhanced identification of high-risk patients is of major importance. We therefore examined incidence and risk factors of VTE among ICD recipients in a nationwide setting.
Methods
Design and setting
This population-based cohort study was conducted in Denmark, which had a cumulative population of 6.9 million inhabitants during the period 1 January 2000 through 31 December 2012.8 Healthcare in Denmark is free-of-charge, guaranteeing all inhabitants unfettered access to general practitioners and hospitals.8 Each Danish inhabitant has a unique civil registration number, which is a prerequisite for receiving healthcare. It allows for accurate and unambiguous linkage of nationwide registries.8
The Danish National Patient Registry
The study was based on the Danish National Patient Registry (DNPR), which maintains records of all non-psychiatric hospitalizations in Denmark since 1977, all hospital outpatient clinic and emergency department visits since 1995, and all surgical procedures performed in Denmark since 1996.9 Upon hospital discharge or completion of an outpatient visit, the treating physician records a primary diagnosis, i.e. the main reason for diagnostic work-up and treatment, and one or more secondary diagnoses describing comorbid conditions.9 Diagnoses are coded according to the World Health Organization's International Classification of Diseases, Eighth Revision (ICD-8) before 1993 and Tenth Revision (ICD-10) thereafter. Procedural and diagnosis codes used in the current study are provided in the Supplementary material online, Tables S1–S4. Both primary and secondary hospital inpatient and outpatient discharge diagnoses were included in our analyses.
The Danish National Database of Reimbursed Prescriptions
Danish pharmacies are required by law to register all prescriptions redeemed in Denmark, and electronic records of prescriptions redeemed since 1 January 2004 are kept in the Danish National Database of Reimbursed Prescriptions.10 A barcode identifier on each medication package enables automatic registration of the Anatomical Therapeutic Chemical (ATC) code, strength, pack size, and date of redemption.10Supplementary material online, Table S5 shows ATC codes used in the current study to identify prescription redemptions in the Danish National Database of Reimbursed Prescriptions.
Implantable cardioverter-defibrillator recipients
Patients eligible for the study were registered in the DNPR with a first-time ICD implantation during the study period. Patients receiving single-chamber, dual-chamber, or cardiac resynchronization therapy defibrillators (CRT-Ds) were included. Non-residents and immigrants (n = 483) were excluded because data on previous comorbidities could have been missing for hospitalizations outside Denmark prior to ICD implantation. Patients with a VTE diagnosed prior to ICD implantation were excluded (n = 294) from primary analyses because the validity of recurrent VTE diagnoses has been reported to be lower than for incident VTE diagnoses.11
Comorbidity
The comorbidity burden of each patient was ascertained using Charlson Comorbidity Index (CCI) scores, based on discharge diagnoses registered before and on the day of ICD implantation.12 The CCI has been validated for use with DNPR hospital discharge data12 and has proved to be an adequate tool for measuring the prognostic impact of comorbidity in ICD patients.13 We assigned patients to three categories of comorbidity based on their CCI score: ‘none’ (0 points), ‘moderate’ (1–2 points), and ‘severe’ (≥3 points). Patients also were categorized according to the individual conditions included in the CCI. Codes for these conditions are provided in the Supplementary material online, Table S2.
In addition to the CCI conditions, we obtained diagnoses for other comorbidities known or suspected to be risk factors for VTE (i.e. atrial fibrillation or flutter2 and infection3 within 90 days prior to ICD implantation) as well as information on other recent hospitalizations (defined as any hospitalization within 90 days prior to ICD implantation, lasting for ≥3 consecutive days, with a primary diagnosis other than those listed in Table 1).
Characteristics of ICD recipients and distribution of incident venous thromboembolisms, Denmark, 2000–12
| Characteristics . | Patients, n (%) . | VTE, n (%) . | Pulmonary embolism, n (%) . | Deep venous thrombosis, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Total . | Provoked . | Total . | Provoked . | Total . | Provoked . | ||
| Overall | 8132 (100.0) | 136 (100.0) | 68 (100.0) | 71 (100.0) | 34 (100.0) | 65 (100.0) | 34 (100.0) |
| Gender | |||||||
| Male | 6554 (80.6) | 112 (82.4) | 52 (76.5) | 58 (81.7) | 25 (73.5) | 54 (83.1) | 27 (79.4) |
| Female | 1578 (19.4) | 24 (17.6) | 16 (23.5) | 13 (18.3) | 9 (26.5) | 11 (16.9) | 7 (20.6) |
| Age, years | |||||||
| <60 | 2731 (33.6) | 46 (33.8) | 20 (29.4) | 19 (26.8) | 7 (20.6) | 27 (41.5) | 13 (38.2) |
| 60–69 | 2735 (33.6) | 50 (36.8) | 28 (41.2) | 26 (36.6) | 13 (38.2) | 24 (36.9) | 15 (44.1) |
| ≥70 | 2666 (32.8) | 40 (29.4) | 20 (29.4) | 26 (36.6) | 14 (41.2) | 14 (21.5) | 6 (17.6) |
| ICD type | |||||||
| Single-chamber | 4356 (53.6) | 80 (58.8) | 39 (57.4) | 45 (63.4) | 23 (67.6) | 35 (53.8) | 16 (47.1) |
| Dual-chamber | 1948 (24.0) | 32 (23.5) | 17 (25.0) | 13 (18.3) | 5 (14.7) | 19 (29.2) | 12 (35.3) |
| CRT-D | 1828 (22.5) | 24 (17.6) | 12 (17.6) | 13 (18.3) | 6 (17.6) | 11 (16.9) | 6 (17.6) |
| Prior device implantation | 679 (8.3) | 13 (9.6) | 5 (7.4) | 6 (8.5) | 1 (2.9) | 7 (10.8) | 4 (11.8) |
| Comorbidity (CCI score) | |||||||
| None (0) | 1427 (17.5) | 19 (14.0) | 11 (16.2) | 10 (14.1) | 6 (17.6) | 9 (13.8) | 5 (14.7) |
| Moderate (1–2) | 3905 (48.0) | 50 (36.8) | 23 (33.8) | 27 (38.0) | 12 (35.3) | 23 (35.4) | 11 (32.4) |
| Severe (≥3) | 2800 (34.4) | 67 (49.3) | 34 (50.0) | 34 (47.9) | 16 (47.1) | 33 (50.8) | 18 (52.9) |
| CCI conditions | |||||||
| Myocardial infarction | 4297 (52.8) | 79 (58.1) | 33 (48.5) | 39 (54.9) | 14 (41.2) | 40 (61.5) | 19 (55.9) |
| Heart failure | 4383 (53.9) | 70 (51.5) | 41 (60.3) | 38 (53.5) | 20 (58.8) | 32 (49.2) | 21 (61.8) |
| Peripheral vascular disease | 1011 (12.4) | 24 (17.6) | 12 (17.6) | 14 (19.7) | 5 (14.7) | 10 (15.4) | 7 (20.6) |
| Cerebrovascular disease | 1191 (14.6) | 25 (18.4) | 10 (14.7) | 15 (21.1) | 6 (17.6) | 10 (15.4) | 4 (11.8) |
| Dementia | 36 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chronic pulmonary disease | 1009 (12.4) | 27 (19.9) | 15 (22.1) | 13 (18.3) | 8 (23.5) | 14 (21.5) | 7 (20.6) |
| Connective tissue disease | 259 (3.2) | 9 (6.6) | 4 (5.9) | 5 (7.0) | 2 (5.9) | 4 (6.2) | 2 (5.9) |
| Ulcer disease | 480 (5.9) | 13 (9.6) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 8 (12.3) | 2 (5.9) |
| Mild liver disease | 97 (1.2) | 5 (3.7) | 3 (4.4) | 3 (4.2) | 2 (5.9) | 2 (3.1) | 1 (2.9) |
| Diabetes without end-organ damage | 544 (6.7) | 4 (2.9) | 2 (2.9) | 2 (2.8) | 1 (2.9) | 2 (3.1) | 1 (2.9) |
| Hemiplegia | 16 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe renal disease | 458 (5.6) | 15 (11.0) | 9 (13.2) | 8 (11.3) | 4 (11.8) | 7 (10.8) | 5 (14.7) |
| Diabetes with end-organ damage | 716 (8.8) | 12 (8.8) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 7 (10.8) | 2 (5.9) |
| Non-metastatic solid tumour | 560 (6.9) | 9 (6.6) | 9 (13.2) | 5 (7.0) | 5 (14.7) | 4 (6.2) | 4 (11.8) |
| Leukaemia | 26 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 63 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe liver disease | 27 (0.3) | 1 (0.7) | 1 (1.5) | 1 (1.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Metastatic cancer | 39 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AIDS | 5 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Other comorbidities | |||||||
| Atrial fibrillation or flutter | 2125 (26.1) | 37 (27.2) | 24 (35.3) | 22 (31.0) | 14 (41.2) | 15 (23.1) | 10 (29.4) |
| Infectiona | 536 (6.6) | 15 (11.0) | 6 (8.8) | 8 (11.3) | 3 (8.8) | 7 (10.8) | 3 (8.8) |
| Other recent hospitalizationb | 2410 (29.6) | 60 (44.1) | 30 (44.1) | 26 (36.6) | 11 (32.4) | 34 (52.3) | 19 (55.9) |
| Use of antithrombotic therapya,c | |||||||
| Antiplatelet agents | 3007 (43.1) | 38 (38.4) | 15 (32.6) | 22 (41.5) | 9 (34.6) | 16 (34.8) | 6 (30.0) |
| Anticoagulant agents | 1231 (17.6) | 13 (13.1) | 8 (17.4) | 6 (11.3) | 3 (11.5) | 7 (15.2) | 5 (25.0) |
| Characteristics . | Patients, n (%) . | VTE, n (%) . | Pulmonary embolism, n (%) . | Deep venous thrombosis, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Total . | Provoked . | Total . | Provoked . | Total . | Provoked . | ||
| Overall | 8132 (100.0) | 136 (100.0) | 68 (100.0) | 71 (100.0) | 34 (100.0) | 65 (100.0) | 34 (100.0) |
| Gender | |||||||
| Male | 6554 (80.6) | 112 (82.4) | 52 (76.5) | 58 (81.7) | 25 (73.5) | 54 (83.1) | 27 (79.4) |
| Female | 1578 (19.4) | 24 (17.6) | 16 (23.5) | 13 (18.3) | 9 (26.5) | 11 (16.9) | 7 (20.6) |
| Age, years | |||||||
| <60 | 2731 (33.6) | 46 (33.8) | 20 (29.4) | 19 (26.8) | 7 (20.6) | 27 (41.5) | 13 (38.2) |
| 60–69 | 2735 (33.6) | 50 (36.8) | 28 (41.2) | 26 (36.6) | 13 (38.2) | 24 (36.9) | 15 (44.1) |
| ≥70 | 2666 (32.8) | 40 (29.4) | 20 (29.4) | 26 (36.6) | 14 (41.2) | 14 (21.5) | 6 (17.6) |
| ICD type | |||||||
| Single-chamber | 4356 (53.6) | 80 (58.8) | 39 (57.4) | 45 (63.4) | 23 (67.6) | 35 (53.8) | 16 (47.1) |
| Dual-chamber | 1948 (24.0) | 32 (23.5) | 17 (25.0) | 13 (18.3) | 5 (14.7) | 19 (29.2) | 12 (35.3) |
| CRT-D | 1828 (22.5) | 24 (17.6) | 12 (17.6) | 13 (18.3) | 6 (17.6) | 11 (16.9) | 6 (17.6) |
| Prior device implantation | 679 (8.3) | 13 (9.6) | 5 (7.4) | 6 (8.5) | 1 (2.9) | 7 (10.8) | 4 (11.8) |
| Comorbidity (CCI score) | |||||||
| None (0) | 1427 (17.5) | 19 (14.0) | 11 (16.2) | 10 (14.1) | 6 (17.6) | 9 (13.8) | 5 (14.7) |
| Moderate (1–2) | 3905 (48.0) | 50 (36.8) | 23 (33.8) | 27 (38.0) | 12 (35.3) | 23 (35.4) | 11 (32.4) |
| Severe (≥3) | 2800 (34.4) | 67 (49.3) | 34 (50.0) | 34 (47.9) | 16 (47.1) | 33 (50.8) | 18 (52.9) |
| CCI conditions | |||||||
| Myocardial infarction | 4297 (52.8) | 79 (58.1) | 33 (48.5) | 39 (54.9) | 14 (41.2) | 40 (61.5) | 19 (55.9) |
| Heart failure | 4383 (53.9) | 70 (51.5) | 41 (60.3) | 38 (53.5) | 20 (58.8) | 32 (49.2) | 21 (61.8) |
| Peripheral vascular disease | 1011 (12.4) | 24 (17.6) | 12 (17.6) | 14 (19.7) | 5 (14.7) | 10 (15.4) | 7 (20.6) |
| Cerebrovascular disease | 1191 (14.6) | 25 (18.4) | 10 (14.7) | 15 (21.1) | 6 (17.6) | 10 (15.4) | 4 (11.8) |
| Dementia | 36 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chronic pulmonary disease | 1009 (12.4) | 27 (19.9) | 15 (22.1) | 13 (18.3) | 8 (23.5) | 14 (21.5) | 7 (20.6) |
| Connective tissue disease | 259 (3.2) | 9 (6.6) | 4 (5.9) | 5 (7.0) | 2 (5.9) | 4 (6.2) | 2 (5.9) |
| Ulcer disease | 480 (5.9) | 13 (9.6) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 8 (12.3) | 2 (5.9) |
| Mild liver disease | 97 (1.2) | 5 (3.7) | 3 (4.4) | 3 (4.2) | 2 (5.9) | 2 (3.1) | 1 (2.9) |
| Diabetes without end-organ damage | 544 (6.7) | 4 (2.9) | 2 (2.9) | 2 (2.8) | 1 (2.9) | 2 (3.1) | 1 (2.9) |
| Hemiplegia | 16 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe renal disease | 458 (5.6) | 15 (11.0) | 9 (13.2) | 8 (11.3) | 4 (11.8) | 7 (10.8) | 5 (14.7) |
| Diabetes with end-organ damage | 716 (8.8) | 12 (8.8) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 7 (10.8) | 2 (5.9) |
| Non-metastatic solid tumour | 560 (6.9) | 9 (6.6) | 9 (13.2) | 5 (7.0) | 5 (14.7) | 4 (6.2) | 4 (11.8) |
| Leukaemia | 26 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 63 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe liver disease | 27 (0.3) | 1 (0.7) | 1 (1.5) | 1 (1.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Metastatic cancer | 39 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AIDS | 5 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Other comorbidities | |||||||
| Atrial fibrillation or flutter | 2125 (26.1) | 37 (27.2) | 24 (35.3) | 22 (31.0) | 14 (41.2) | 15 (23.1) | 10 (29.4) |
| Infectiona | 536 (6.6) | 15 (11.0) | 6 (8.8) | 8 (11.3) | 3 (8.8) | 7 (10.8) | 3 (8.8) |
| Other recent hospitalizationb | 2410 (29.6) | 60 (44.1) | 30 (44.1) | 26 (36.6) | 11 (32.4) | 34 (52.3) | 19 (55.9) |
| Use of antithrombotic therapya,c | |||||||
| Antiplatelet agents | 3007 (43.1) | 38 (38.4) | 15 (32.6) | 22 (41.5) | 9 (34.6) | 16 (34.8) | 6 (30.0) |
| Anticoagulant agents | 1231 (17.6) | 13 (13.1) | 8 (17.4) | 6 (11.3) | 3 (11.5) | 7 (15.2) | 5 (25.0) |
AIDS, acquired immune-deficiency syndrome; CCI, Charlson Comorbidity Index; CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter-defibrillator; VTE, venous thromboembolism.
aWithin 90 days prior to ICD implantation.
bFor a primary diagnosis not listed in this table within 90 days prior to ICD implantation and lasting for ≥3 consecutive days.
cPatients included from 1 April 2004 through 31 December 2012 only (N = 6984).
Characteristics of ICD recipients and distribution of incident venous thromboembolisms, Denmark, 2000–12
| Characteristics . | Patients, n (%) . | VTE, n (%) . | Pulmonary embolism, n (%) . | Deep venous thrombosis, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Total . | Provoked . | Total . | Provoked . | Total . | Provoked . | ||
| Overall | 8132 (100.0) | 136 (100.0) | 68 (100.0) | 71 (100.0) | 34 (100.0) | 65 (100.0) | 34 (100.0) |
| Gender | |||||||
| Male | 6554 (80.6) | 112 (82.4) | 52 (76.5) | 58 (81.7) | 25 (73.5) | 54 (83.1) | 27 (79.4) |
| Female | 1578 (19.4) | 24 (17.6) | 16 (23.5) | 13 (18.3) | 9 (26.5) | 11 (16.9) | 7 (20.6) |
| Age, years | |||||||
| <60 | 2731 (33.6) | 46 (33.8) | 20 (29.4) | 19 (26.8) | 7 (20.6) | 27 (41.5) | 13 (38.2) |
| 60–69 | 2735 (33.6) | 50 (36.8) | 28 (41.2) | 26 (36.6) | 13 (38.2) | 24 (36.9) | 15 (44.1) |
| ≥70 | 2666 (32.8) | 40 (29.4) | 20 (29.4) | 26 (36.6) | 14 (41.2) | 14 (21.5) | 6 (17.6) |
| ICD type | |||||||
| Single-chamber | 4356 (53.6) | 80 (58.8) | 39 (57.4) | 45 (63.4) | 23 (67.6) | 35 (53.8) | 16 (47.1) |
| Dual-chamber | 1948 (24.0) | 32 (23.5) | 17 (25.0) | 13 (18.3) | 5 (14.7) | 19 (29.2) | 12 (35.3) |
| CRT-D | 1828 (22.5) | 24 (17.6) | 12 (17.6) | 13 (18.3) | 6 (17.6) | 11 (16.9) | 6 (17.6) |
| Prior device implantation | 679 (8.3) | 13 (9.6) | 5 (7.4) | 6 (8.5) | 1 (2.9) | 7 (10.8) | 4 (11.8) |
| Comorbidity (CCI score) | |||||||
| None (0) | 1427 (17.5) | 19 (14.0) | 11 (16.2) | 10 (14.1) | 6 (17.6) | 9 (13.8) | 5 (14.7) |
| Moderate (1–2) | 3905 (48.0) | 50 (36.8) | 23 (33.8) | 27 (38.0) | 12 (35.3) | 23 (35.4) | 11 (32.4) |
| Severe (≥3) | 2800 (34.4) | 67 (49.3) | 34 (50.0) | 34 (47.9) | 16 (47.1) | 33 (50.8) | 18 (52.9) |
| CCI conditions | |||||||
| Myocardial infarction | 4297 (52.8) | 79 (58.1) | 33 (48.5) | 39 (54.9) | 14 (41.2) | 40 (61.5) | 19 (55.9) |
| Heart failure | 4383 (53.9) | 70 (51.5) | 41 (60.3) | 38 (53.5) | 20 (58.8) | 32 (49.2) | 21 (61.8) |
| Peripheral vascular disease | 1011 (12.4) | 24 (17.6) | 12 (17.6) | 14 (19.7) | 5 (14.7) | 10 (15.4) | 7 (20.6) |
| Cerebrovascular disease | 1191 (14.6) | 25 (18.4) | 10 (14.7) | 15 (21.1) | 6 (17.6) | 10 (15.4) | 4 (11.8) |
| Dementia | 36 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chronic pulmonary disease | 1009 (12.4) | 27 (19.9) | 15 (22.1) | 13 (18.3) | 8 (23.5) | 14 (21.5) | 7 (20.6) |
| Connective tissue disease | 259 (3.2) | 9 (6.6) | 4 (5.9) | 5 (7.0) | 2 (5.9) | 4 (6.2) | 2 (5.9) |
| Ulcer disease | 480 (5.9) | 13 (9.6) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 8 (12.3) | 2 (5.9) |
| Mild liver disease | 97 (1.2) | 5 (3.7) | 3 (4.4) | 3 (4.2) | 2 (5.9) | 2 (3.1) | 1 (2.9) |
| Diabetes without end-organ damage | 544 (6.7) | 4 (2.9) | 2 (2.9) | 2 (2.8) | 1 (2.9) | 2 (3.1) | 1 (2.9) |
| Hemiplegia | 16 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe renal disease | 458 (5.6) | 15 (11.0) | 9 (13.2) | 8 (11.3) | 4 (11.8) | 7 (10.8) | 5 (14.7) |
| Diabetes with end-organ damage | 716 (8.8) | 12 (8.8) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 7 (10.8) | 2 (5.9) |
| Non-metastatic solid tumour | 560 (6.9) | 9 (6.6) | 9 (13.2) | 5 (7.0) | 5 (14.7) | 4 (6.2) | 4 (11.8) |
| Leukaemia | 26 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 63 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe liver disease | 27 (0.3) | 1 (0.7) | 1 (1.5) | 1 (1.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Metastatic cancer | 39 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AIDS | 5 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Other comorbidities | |||||||
| Atrial fibrillation or flutter | 2125 (26.1) | 37 (27.2) | 24 (35.3) | 22 (31.0) | 14 (41.2) | 15 (23.1) | 10 (29.4) |
| Infectiona | 536 (6.6) | 15 (11.0) | 6 (8.8) | 8 (11.3) | 3 (8.8) | 7 (10.8) | 3 (8.8) |
| Other recent hospitalizationb | 2410 (29.6) | 60 (44.1) | 30 (44.1) | 26 (36.6) | 11 (32.4) | 34 (52.3) | 19 (55.9) |
| Use of antithrombotic therapya,c | |||||||
| Antiplatelet agents | 3007 (43.1) | 38 (38.4) | 15 (32.6) | 22 (41.5) | 9 (34.6) | 16 (34.8) | 6 (30.0) |
| Anticoagulant agents | 1231 (17.6) | 13 (13.1) | 8 (17.4) | 6 (11.3) | 3 (11.5) | 7 (15.2) | 5 (25.0) |
| Characteristics . | Patients, n (%) . | VTE, n (%) . | Pulmonary embolism, n (%) . | Deep venous thrombosis, n (%) . | |||
|---|---|---|---|---|---|---|---|
| Total . | Provoked . | Total . | Provoked . | Total . | Provoked . | ||
| Overall | 8132 (100.0) | 136 (100.0) | 68 (100.0) | 71 (100.0) | 34 (100.0) | 65 (100.0) | 34 (100.0) |
| Gender | |||||||
| Male | 6554 (80.6) | 112 (82.4) | 52 (76.5) | 58 (81.7) | 25 (73.5) | 54 (83.1) | 27 (79.4) |
| Female | 1578 (19.4) | 24 (17.6) | 16 (23.5) | 13 (18.3) | 9 (26.5) | 11 (16.9) | 7 (20.6) |
| Age, years | |||||||
| <60 | 2731 (33.6) | 46 (33.8) | 20 (29.4) | 19 (26.8) | 7 (20.6) | 27 (41.5) | 13 (38.2) |
| 60–69 | 2735 (33.6) | 50 (36.8) | 28 (41.2) | 26 (36.6) | 13 (38.2) | 24 (36.9) | 15 (44.1) |
| ≥70 | 2666 (32.8) | 40 (29.4) | 20 (29.4) | 26 (36.6) | 14 (41.2) | 14 (21.5) | 6 (17.6) |
| ICD type | |||||||
| Single-chamber | 4356 (53.6) | 80 (58.8) | 39 (57.4) | 45 (63.4) | 23 (67.6) | 35 (53.8) | 16 (47.1) |
| Dual-chamber | 1948 (24.0) | 32 (23.5) | 17 (25.0) | 13 (18.3) | 5 (14.7) | 19 (29.2) | 12 (35.3) |
| CRT-D | 1828 (22.5) | 24 (17.6) | 12 (17.6) | 13 (18.3) | 6 (17.6) | 11 (16.9) | 6 (17.6) |
| Prior device implantation | 679 (8.3) | 13 (9.6) | 5 (7.4) | 6 (8.5) | 1 (2.9) | 7 (10.8) | 4 (11.8) |
| Comorbidity (CCI score) | |||||||
| None (0) | 1427 (17.5) | 19 (14.0) | 11 (16.2) | 10 (14.1) | 6 (17.6) | 9 (13.8) | 5 (14.7) |
| Moderate (1–2) | 3905 (48.0) | 50 (36.8) | 23 (33.8) | 27 (38.0) | 12 (35.3) | 23 (35.4) | 11 (32.4) |
| Severe (≥3) | 2800 (34.4) | 67 (49.3) | 34 (50.0) | 34 (47.9) | 16 (47.1) | 33 (50.8) | 18 (52.9) |
| CCI conditions | |||||||
| Myocardial infarction | 4297 (52.8) | 79 (58.1) | 33 (48.5) | 39 (54.9) | 14 (41.2) | 40 (61.5) | 19 (55.9) |
| Heart failure | 4383 (53.9) | 70 (51.5) | 41 (60.3) | 38 (53.5) | 20 (58.8) | 32 (49.2) | 21 (61.8) |
| Peripheral vascular disease | 1011 (12.4) | 24 (17.6) | 12 (17.6) | 14 (19.7) | 5 (14.7) | 10 (15.4) | 7 (20.6) |
| Cerebrovascular disease | 1191 (14.6) | 25 (18.4) | 10 (14.7) | 15 (21.1) | 6 (17.6) | 10 (15.4) | 4 (11.8) |
| Dementia | 36 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chronic pulmonary disease | 1009 (12.4) | 27 (19.9) | 15 (22.1) | 13 (18.3) | 8 (23.5) | 14 (21.5) | 7 (20.6) |
| Connective tissue disease | 259 (3.2) | 9 (6.6) | 4 (5.9) | 5 (7.0) | 2 (5.9) | 4 (6.2) | 2 (5.9) |
| Ulcer disease | 480 (5.9) | 13 (9.6) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 8 (12.3) | 2 (5.9) |
| Mild liver disease | 97 (1.2) | 5 (3.7) | 3 (4.4) | 3 (4.2) | 2 (5.9) | 2 (3.1) | 1 (2.9) |
| Diabetes without end-organ damage | 544 (6.7) | 4 (2.9) | 2 (2.9) | 2 (2.8) | 1 (2.9) | 2 (3.1) | 1 (2.9) |
| Hemiplegia | 16 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe renal disease | 458 (5.6) | 15 (11.0) | 9 (13.2) | 8 (11.3) | 4 (11.8) | 7 (10.8) | 5 (14.7) |
| Diabetes with end-organ damage | 716 (8.8) | 12 (8.8) | 5 (7.4) | 5 (7.0) | 3 (8.8) | 7 (10.8) | 2 (5.9) |
| Non-metastatic solid tumour | 560 (6.9) | 9 (6.6) | 9 (13.2) | 5 (7.0) | 5 (14.7) | 4 (6.2) | 4 (11.8) |
| Leukaemia | 26 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphoma | 63 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate/severe liver disease | 27 (0.3) | 1 (0.7) | 1 (1.5) | 1 (1.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Metastatic cancer | 39 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AIDS | 5 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Other comorbidities | |||||||
| Atrial fibrillation or flutter | 2125 (26.1) | 37 (27.2) | 24 (35.3) | 22 (31.0) | 14 (41.2) | 15 (23.1) | 10 (29.4) |
| Infectiona | 536 (6.6) | 15 (11.0) | 6 (8.8) | 8 (11.3) | 3 (8.8) | 7 (10.8) | 3 (8.8) |
| Other recent hospitalizationb | 2410 (29.6) | 60 (44.1) | 30 (44.1) | 26 (36.6) | 11 (32.4) | 34 (52.3) | 19 (55.9) |
| Use of antithrombotic therapya,c | |||||||
| Antiplatelet agents | 3007 (43.1) | 38 (38.4) | 15 (32.6) | 22 (41.5) | 9 (34.6) | 16 (34.8) | 6 (30.0) |
| Anticoagulant agents | 1231 (17.6) | 13 (13.1) | 8 (17.4) | 6 (11.3) | 3 (11.5) | 7 (15.2) | 5 (25.0) |
AIDS, acquired immune-deficiency syndrome; CCI, Charlson Comorbidity Index; CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter-defibrillator; VTE, venous thromboembolism.
aWithin 90 days prior to ICD implantation.
bFor a primary diagnosis not listed in this table within 90 days prior to ICD implantation and lasting for ≥3 consecutive days.
cPatients included from 1 April 2004 through 31 December 2012 only (N = 6984).
Venous thromboembolism
All incident VTEs diagnosed after ICD implantation were identified and categorized as PEs or deep venous thromboses (DVTs). We used the same discharge diagnoses as in earlier work,2 excluding emergency room diagnoses due to their low validity.14 Patients diagnosed with both PE and DVT during the same hospital contact were categorized as having PE only. Venous thromboembolisms in conjunction with the occurrence within the previous 90 days of known VTE risk factors, i.e. surgery, fracture, trauma, pregnancy, cancer, or prescription redemption for hormone replacement therapy, were classified as ‘provoked’.3 Thus, all VTEs diagnosed within 3 months of ICD implantation were classified as provoked. Venous thromboembolism diagnosis with a subsequent diagnosis of cancer within 90 days also was classified as ‘provoked’ because occult cancer is a strong risk factor for VTE.3 Remaining VTEs were classified as ‘unprovoked’.3
Antithrombotic therapy
We obtained all available information about redeemed prescriptions during the study period for antiplatelet agents (i.e. aspirin and clopidogrel) and anticoagulant medication (i.e. warfarin and phenprocoumon) from the Danish National Database of Reimbursed Prescriptions.10 Use of antithrombotic therapy was defined as prescription redemption within 90 days prior to ICD implantation.
Statistical analysis
Follow-up began on the day of ICD implantation and continued until a VTE diagnosis, death, emigration, or 31 December 2012, whichever came first. As of the date of implantation, we characterized patients according to gender, age (<60, 60–69, or ≥70 years), ICD type (single-chamber, dual-chamber, or CRT-D), prior device implantation (pacemaker or CRT implantation prior to ICD implantation), comorbidity level (none, moderate, or severe), individual comorbidities (all individual conditions included in the CCI and the other comorbidities listed in Table 1), and use of antithrombotic therapy (use of antiplatelet agents and/or use of anticoagulant agents).
Incident VTEs were ascertained overall and within each patient subgroup. Comorbidity subgroups with less than five VTEs diagnosed during follow-up were excluded from further statistical analysis because of imprecise estimates. Incidence rates (IRs) were computed as the number of VTEs per 1000 person-years at risk. In a sensitivity analysis of the PE IR, patients with a VTE diagnosed prior to ICD implantation were included.
Treating death as a competing risk, we computed the absolute risk of VTE within 3 months and 5 years following ICD implantation, using the cumulative incidence function.
To assess associations between known or possible risk factors and VTE occurrence, Cox proportional hazards regression was used to compute hazard ratios as estimates of incidence rate ratios (IRRs) according to gender (using men as reference), age (using age <60 years as reference), ICD type (using single-chamber device as reference), prior device implantation (using no prior device implantation as reference), comorbidity level (using no comorbidity as reference), and individual comorbidities (using absence of the condition in question as reference).
In estimating the effect of individual risk factors, IRRs were mutually adjusted for (i) gender, age, and comorbidity level, or (ii) gender, age, ICD type, prior device implantation, and individual comorbidities. We did not test for interactions, as testing was not planned a priori. For ICD recipients with available data in the Danish National Database of Reimbursed Prescriptions, i.e. patients included from 1 April 2004 through 31 December 2012, IRRs were additionally computed according to and adjusted for use of antithrombotics (using no use of the antithrombotic in question as reference). Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study was approved by the Danish Data Protection Agency (record nos. 1-16-02-1-08 and 2012-41-0793). Ethics approval was not needed for this registry-based study.
Results
Patient characteristics and venous thromboembolism distribution
A total of 8132 ICD recipients were followed for up to 13 years, with a median follow-up time of 3.0 years [interquartile range (IQR): 1.3–5.4 years]. Median age at time of implantation was 65 years (IQR: 57–72 years). Patient characteristics are presented in Table 1.
During follow-up, 1721 (21.2%) patients died, corresponding to a mortality rate of 56.7 (95% CI: 54.0–59.3) deaths per 1000 person-years, and 136 patients had a VTE diagnosed (71 PEs and 65 DVTs). Six patients with PE had a concurrent DVT diagnosis. The distribution of VTEs is shown in Table 1. Absolute risks are shown in Figure 1. As shown in Table 2, the VTE IR per 1000 person-years was 4.5 (95% CI: 3.7–5.2), slightly higher for PE than for DVT. Including patients with a VTE diagnosed prior to ICD implantation, we found a PE incidence of 2.4 (95% CI: 1.9–3.0) per 1000 person-years.
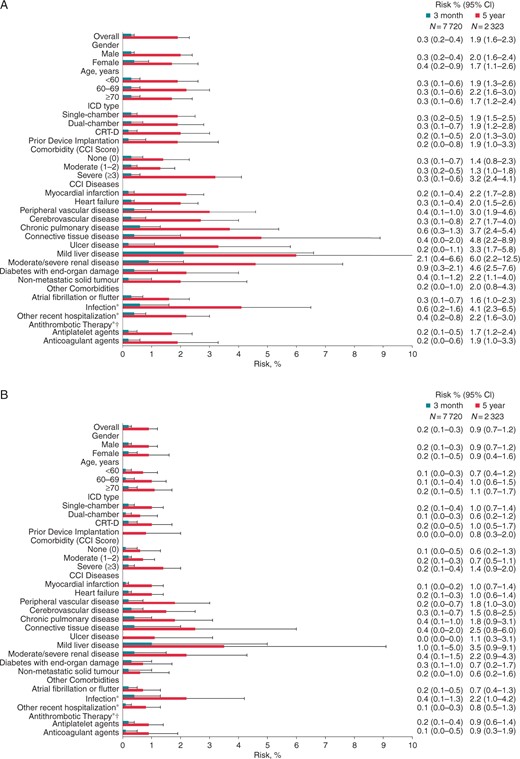
Absolute risks of (A) venous thromboembolism, (B) pulmonary embolism, and (C) deep venous thrombosis within 3 months (blue) and 5 years (red) following ICD implantation, Denmark, 2000–12. Whiskers illustrate upper 95% CIs. CCI, Charlson Comorbidity Index; CI, confidence interval; CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter-defibrillator; N, number of patients at risk; asterisk indicates within 90 days prior to ICD implantation; dagger indicates patients included from 1 April 2004 through 31 December 2012 only (N = 6984).
Incidence rates of venous thromboembolism following ICD implantation, Denmark, 2000–12
| . | VTE . | PE . | DVT . |
|---|---|---|---|
| IR (95% CI) . | IR (95% CI) . | IR (95% CI) . | |
| Overall | 4.5 (3.7–5.2) | 2.3 (1.8–2.9) | 2.1 (1.6–2.7) |
| Gender | |||
| Male | 4.6 (3.7–5.4) | 2.4 (1.8–3.0) | 2.2 (1.6–2.8) |
| Female | 4.0 (2.4–5.6) | 2.2 (1.0–3.3) | 1.8 (0.7–2.9) |
| Age, years | |||
| <60 | 3.8 (2.7–4.9) | 1.6 (0.9–2.3) | 2.2 (1.4–3.1) |
| 60–69 | 4.9 (3.6–6.3) | 2.6 (1.6–3.5) | 2.4 (1.4–3.3) |
| ≥70 | 5.0 (3.4–6.5) | 3.2 (2.0–4.5) | 1.7 (0.8–2.7) |
| ICD type | |||
| Single-chamber | 4.5 (3.5–5.5) | 2.5 (1.8–3.3) | 2.0 (1.3–2.6) |
| Dual-chamber | 4.3 (2.8–5.8) | 1.7 (0.8–2.7) | 2.5 (1.4–3.7) |
| CRT-D | 4.8 (2.9–6.7) | 2.6 (1.2–4.0) | 2.2 (0.9–3.5) |
| Prior device implantation | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.9) | 2.1 (1.5–2.6) |
| Yes | 6.0 (2.8–9.3) | 2.8 (0.6–5.0) | 3.2 (0.8–5.7) |
| Comorbidity (CCI score) | |||
| None (0) | 2.9 (1.6–4.2) | 1.5 (0.6–2.5) | 1.4 (0.5–2.3) |
| Moderate (1–2) | 3.2 (2.3–4.1) | 1.7 (1.1–2.4) | 1.5 (0.9–2.1) |
| Severe (≥ 3) | 8.1 (6.2–10.0) | 4.1 (2.7–5.5) | 4.0 (2.6–5.3) |
| CCI conditions | |||
| Myocardial infarction | |||
| No | 3.9 (2.9–4.9) | 2.2 (1.4–3.0) | 1.7 (1.0–2.4) |
| Yes | 5.0 (3.9–6.1) | 2.5 (1.7–3.2) | 2.5 (1.7–3.3) |
| Heart failure | |||
| No | 4.0 (3.0–4.9) | 2.0 (1.3–2.7) | 2.0 (1.3–2.7) |
| Yes | 5.1 (3.9–6.3) | 2.8 (1.9–3.6) | 2.3 (1.5–3.1) |
| Peripheral vascular disease | |||
| No | 4.1 (3.3–4.9) | 2.1 (1.5–2.6) | 2.0 (1.5–2.5) |
| Yes | 7.8 (4.7–10.9) | 4.5 (2.2–6.9) | 3.2 (1.2–5.3) |
| Cerebrovascular disease | |||
| No | 4.2 (3.4–5.0) | 2.1 (1.6–2.7) | 2.1 (1.5–2.6) |
| Yes | 6.5 (4.0–9.1) | 3.9 (1.9–5.9) | 2.6 (1.0–4.2) |
| Chronic pulmonary disease | |||
| No | 4.0 (3.2–4.7) | 2.1 (1.6–2.7) | 1.9 (1.4–2.4) |
| Yes | 8.8 (5.5–12.1) | 4.2 (1.9–6.5) | 4.6 (2.2–7.0) |
| Connective tissue disease | |||
| No | 4.3 (3.6–5.1) | 2.2 (1.7–2.8) | 2.1 (1.5–2.6) |
| Yes | 10.1 (3.5–16.8) | 5.6 (0.7–10.6) | 4.5 (0.1–8.9) |
| Ulcer disease | |||
| No | 4.3 (3.5–5.0) | 2.3 (1.7–2.8) | 2.0 (1.5–2.5) |
| Yes | 8.7 (4.0–13.5) | 3.4 (0.4–6.3) | 5.4 (1.7–9.1) |
| Mild liver disease | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.8) | 2.1 (1.6–2.6) |
| Yes | 17.3 (2.1–32.4) | 10.4 (0.0–22.1) | 6.9 (0.0–16.5) |
| Moderate/severe renal disease | |||
| No | 4.1 (3.4–4.9) | 2.2 (1.6–2.7) | 2.0 (1.5–2.5) |
| Yes | 12.4 (6.1–18.7) | 6.6 (2.0–11.2) | 5.8 (1.5–10.1) |
| Diabetes with end-organ damage | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.8–2.9) | 2.0 (1.5–2.6) |
| Yes | 6.2 (2.7–9.7) | 2.6 (0.3–4.8) | 3.6 (0.9–6.3) |
| Non-metastatic solid tumour | |||
| No | 4.4 (3.7–5.2) | 2.3 (1.7–2.9) | 2.1 (1.6–2.7) |
| Yes | 5.4 (1.9–8.8) | 3.0 (0.4–5.6) | 2.4 (0.0–4.7) |
| Other comorbidities | |||
| Atrial fibrillation or flutter | |||
| No | 4.3 (3.4–5.1) | 2.1 (1.5–2.7) | 2.2 (1.6–2.8) |
| Yes | 5.1 (3.4–6.7) | 3.0 (1.8–4.3) | 2.1 (1.0–3.1) |
| Infectiona | |||
| No | 4.2 (3.5–5.0) | 2.2 (1.7–2.7) | 2.0 (1.5–2.6) |
| Yes | 8.4 (4.2–12.7) | 4.5 (1.4–7.6) | 3.9 (1.0–6.8) |
| Other recent hospitalizationb | |||
| No | 3.7 (2.9–4.6) | 2.2 (1.6–2.9) | 1.5 (1.0–2.1) |
| Yes | 6.0 (4.4–7.5) | 2.6 (1.6–3.6) | 3.4 (2.2–4.5) |
| Use of antithrombotic therapya,c | |||
| Antiplatelet agents | |||
| No | 4.7 (3.5–5.8) | 2.4 (1.5–3.2) | 2.3 (1.5–3.1) |
| Yes | 4.5 (3.1–5.9) | 2.6 (1.5–3.7) | 1.9 (1.0–2.8) |
| Anticoagulant agents | |||
| No | 4.8 (3.8–5.8) | 2.6 (1.9–3.4) | 2.2 (1.5–2.9) |
| Yes | 3.6 (1.6–5.5) | 1.6 (0.3–3.0) | 1.9 (0.5–3.3) |
| . | VTE . | PE . | DVT . |
|---|---|---|---|
| IR (95% CI) . | IR (95% CI) . | IR (95% CI) . | |
| Overall | 4.5 (3.7–5.2) | 2.3 (1.8–2.9) | 2.1 (1.6–2.7) |
| Gender | |||
| Male | 4.6 (3.7–5.4) | 2.4 (1.8–3.0) | 2.2 (1.6–2.8) |
| Female | 4.0 (2.4–5.6) | 2.2 (1.0–3.3) | 1.8 (0.7–2.9) |
| Age, years | |||
| <60 | 3.8 (2.7–4.9) | 1.6 (0.9–2.3) | 2.2 (1.4–3.1) |
| 60–69 | 4.9 (3.6–6.3) | 2.6 (1.6–3.5) | 2.4 (1.4–3.3) |
| ≥70 | 5.0 (3.4–6.5) | 3.2 (2.0–4.5) | 1.7 (0.8–2.7) |
| ICD type | |||
| Single-chamber | 4.5 (3.5–5.5) | 2.5 (1.8–3.3) | 2.0 (1.3–2.6) |
| Dual-chamber | 4.3 (2.8–5.8) | 1.7 (0.8–2.7) | 2.5 (1.4–3.7) |
| CRT-D | 4.8 (2.9–6.7) | 2.6 (1.2–4.0) | 2.2 (0.9–3.5) |
| Prior device implantation | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.9) | 2.1 (1.5–2.6) |
| Yes | 6.0 (2.8–9.3) | 2.8 (0.6–5.0) | 3.2 (0.8–5.7) |
| Comorbidity (CCI score) | |||
| None (0) | 2.9 (1.6–4.2) | 1.5 (0.6–2.5) | 1.4 (0.5–2.3) |
| Moderate (1–2) | 3.2 (2.3–4.1) | 1.7 (1.1–2.4) | 1.5 (0.9–2.1) |
| Severe (≥ 3) | 8.1 (6.2–10.0) | 4.1 (2.7–5.5) | 4.0 (2.6–5.3) |
| CCI conditions | |||
| Myocardial infarction | |||
| No | 3.9 (2.9–4.9) | 2.2 (1.4–3.0) | 1.7 (1.0–2.4) |
| Yes | 5.0 (3.9–6.1) | 2.5 (1.7–3.2) | 2.5 (1.7–3.3) |
| Heart failure | |||
| No | 4.0 (3.0–4.9) | 2.0 (1.3–2.7) | 2.0 (1.3–2.7) |
| Yes | 5.1 (3.9–6.3) | 2.8 (1.9–3.6) | 2.3 (1.5–3.1) |
| Peripheral vascular disease | |||
| No | 4.1 (3.3–4.9) | 2.1 (1.5–2.6) | 2.0 (1.5–2.5) |
| Yes | 7.8 (4.7–10.9) | 4.5 (2.2–6.9) | 3.2 (1.2–5.3) |
| Cerebrovascular disease | |||
| No | 4.2 (3.4–5.0) | 2.1 (1.6–2.7) | 2.1 (1.5–2.6) |
| Yes | 6.5 (4.0–9.1) | 3.9 (1.9–5.9) | 2.6 (1.0–4.2) |
| Chronic pulmonary disease | |||
| No | 4.0 (3.2–4.7) | 2.1 (1.6–2.7) | 1.9 (1.4–2.4) |
| Yes | 8.8 (5.5–12.1) | 4.2 (1.9–6.5) | 4.6 (2.2–7.0) |
| Connective tissue disease | |||
| No | 4.3 (3.6–5.1) | 2.2 (1.7–2.8) | 2.1 (1.5–2.6) |
| Yes | 10.1 (3.5–16.8) | 5.6 (0.7–10.6) | 4.5 (0.1–8.9) |
| Ulcer disease | |||
| No | 4.3 (3.5–5.0) | 2.3 (1.7–2.8) | 2.0 (1.5–2.5) |
| Yes | 8.7 (4.0–13.5) | 3.4 (0.4–6.3) | 5.4 (1.7–9.1) |
| Mild liver disease | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.8) | 2.1 (1.6–2.6) |
| Yes | 17.3 (2.1–32.4) | 10.4 (0.0–22.1) | 6.9 (0.0–16.5) |
| Moderate/severe renal disease | |||
| No | 4.1 (3.4–4.9) | 2.2 (1.6–2.7) | 2.0 (1.5–2.5) |
| Yes | 12.4 (6.1–18.7) | 6.6 (2.0–11.2) | 5.8 (1.5–10.1) |
| Diabetes with end-organ damage | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.8–2.9) | 2.0 (1.5–2.6) |
| Yes | 6.2 (2.7–9.7) | 2.6 (0.3–4.8) | 3.6 (0.9–6.3) |
| Non-metastatic solid tumour | |||
| No | 4.4 (3.7–5.2) | 2.3 (1.7–2.9) | 2.1 (1.6–2.7) |
| Yes | 5.4 (1.9–8.8) | 3.0 (0.4–5.6) | 2.4 (0.0–4.7) |
| Other comorbidities | |||
| Atrial fibrillation or flutter | |||
| No | 4.3 (3.4–5.1) | 2.1 (1.5–2.7) | 2.2 (1.6–2.8) |
| Yes | 5.1 (3.4–6.7) | 3.0 (1.8–4.3) | 2.1 (1.0–3.1) |
| Infectiona | |||
| No | 4.2 (3.5–5.0) | 2.2 (1.7–2.7) | 2.0 (1.5–2.6) |
| Yes | 8.4 (4.2–12.7) | 4.5 (1.4–7.6) | 3.9 (1.0–6.8) |
| Other recent hospitalizationb | |||
| No | 3.7 (2.9–4.6) | 2.2 (1.6–2.9) | 1.5 (1.0–2.1) |
| Yes | 6.0 (4.4–7.5) | 2.6 (1.6–3.6) | 3.4 (2.2–4.5) |
| Use of antithrombotic therapya,c | |||
| Antiplatelet agents | |||
| No | 4.7 (3.5–5.8) | 2.4 (1.5–3.2) | 2.3 (1.5–3.1) |
| Yes | 4.5 (3.1–5.9) | 2.6 (1.5–3.7) | 1.9 (1.0–2.8) |
| Anticoagulant agents | |||
| No | 4.8 (3.8–5.8) | 2.6 (1.9–3.4) | 2.2 (1.5–2.9) |
| Yes | 3.6 (1.6–5.5) | 1.6 (0.3–3.0) | 1.9 (0.5–3.3) |
CCI, Charlson Comorbidity Index; CI, confidence interval; CRT-D, cardiac resyncronization therapy defibrillator; DVT, deep venous thrombosis; ICD, implantable cardioverter-defibrillator; IR, incidence rate per 1000 person-years; PE, pulmonary embolism; VTE, venous thromboembolism.
aWithin 90 days prior to ICD implantation.
bFor a primary diagnosis not listed in Table 1 within 90 days prior to ICD implantation and lasting for ≥3 consecutive days.
cPatients included from 1 April 2004 through 31 December 2012 only (N = 6984).
Incidence rates of venous thromboembolism following ICD implantation, Denmark, 2000–12
| . | VTE . | PE . | DVT . |
|---|---|---|---|
| IR (95% CI) . | IR (95% CI) . | IR (95% CI) . | |
| Overall | 4.5 (3.7–5.2) | 2.3 (1.8–2.9) | 2.1 (1.6–2.7) |
| Gender | |||
| Male | 4.6 (3.7–5.4) | 2.4 (1.8–3.0) | 2.2 (1.6–2.8) |
| Female | 4.0 (2.4–5.6) | 2.2 (1.0–3.3) | 1.8 (0.7–2.9) |
| Age, years | |||
| <60 | 3.8 (2.7–4.9) | 1.6 (0.9–2.3) | 2.2 (1.4–3.1) |
| 60–69 | 4.9 (3.6–6.3) | 2.6 (1.6–3.5) | 2.4 (1.4–3.3) |
| ≥70 | 5.0 (3.4–6.5) | 3.2 (2.0–4.5) | 1.7 (0.8–2.7) |
| ICD type | |||
| Single-chamber | 4.5 (3.5–5.5) | 2.5 (1.8–3.3) | 2.0 (1.3–2.6) |
| Dual-chamber | 4.3 (2.8–5.8) | 1.7 (0.8–2.7) | 2.5 (1.4–3.7) |
| CRT-D | 4.8 (2.9–6.7) | 2.6 (1.2–4.0) | 2.2 (0.9–3.5) |
| Prior device implantation | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.9) | 2.1 (1.5–2.6) |
| Yes | 6.0 (2.8–9.3) | 2.8 (0.6–5.0) | 3.2 (0.8–5.7) |
| Comorbidity (CCI score) | |||
| None (0) | 2.9 (1.6–4.2) | 1.5 (0.6–2.5) | 1.4 (0.5–2.3) |
| Moderate (1–2) | 3.2 (2.3–4.1) | 1.7 (1.1–2.4) | 1.5 (0.9–2.1) |
| Severe (≥ 3) | 8.1 (6.2–10.0) | 4.1 (2.7–5.5) | 4.0 (2.6–5.3) |
| CCI conditions | |||
| Myocardial infarction | |||
| No | 3.9 (2.9–4.9) | 2.2 (1.4–3.0) | 1.7 (1.0–2.4) |
| Yes | 5.0 (3.9–6.1) | 2.5 (1.7–3.2) | 2.5 (1.7–3.3) |
| Heart failure | |||
| No | 4.0 (3.0–4.9) | 2.0 (1.3–2.7) | 2.0 (1.3–2.7) |
| Yes | 5.1 (3.9–6.3) | 2.8 (1.9–3.6) | 2.3 (1.5–3.1) |
| Peripheral vascular disease | |||
| No | 4.1 (3.3–4.9) | 2.1 (1.5–2.6) | 2.0 (1.5–2.5) |
| Yes | 7.8 (4.7–10.9) | 4.5 (2.2–6.9) | 3.2 (1.2–5.3) |
| Cerebrovascular disease | |||
| No | 4.2 (3.4–5.0) | 2.1 (1.6–2.7) | 2.1 (1.5–2.6) |
| Yes | 6.5 (4.0–9.1) | 3.9 (1.9–5.9) | 2.6 (1.0–4.2) |
| Chronic pulmonary disease | |||
| No | 4.0 (3.2–4.7) | 2.1 (1.6–2.7) | 1.9 (1.4–2.4) |
| Yes | 8.8 (5.5–12.1) | 4.2 (1.9–6.5) | 4.6 (2.2–7.0) |
| Connective tissue disease | |||
| No | 4.3 (3.6–5.1) | 2.2 (1.7–2.8) | 2.1 (1.5–2.6) |
| Yes | 10.1 (3.5–16.8) | 5.6 (0.7–10.6) | 4.5 (0.1–8.9) |
| Ulcer disease | |||
| No | 4.3 (3.5–5.0) | 2.3 (1.7–2.8) | 2.0 (1.5–2.5) |
| Yes | 8.7 (4.0–13.5) | 3.4 (0.4–6.3) | 5.4 (1.7–9.1) |
| Mild liver disease | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.8) | 2.1 (1.6–2.6) |
| Yes | 17.3 (2.1–32.4) | 10.4 (0.0–22.1) | 6.9 (0.0–16.5) |
| Moderate/severe renal disease | |||
| No | 4.1 (3.4–4.9) | 2.2 (1.6–2.7) | 2.0 (1.5–2.5) |
| Yes | 12.4 (6.1–18.7) | 6.6 (2.0–11.2) | 5.8 (1.5–10.1) |
| Diabetes with end-organ damage | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.8–2.9) | 2.0 (1.5–2.6) |
| Yes | 6.2 (2.7–9.7) | 2.6 (0.3–4.8) | 3.6 (0.9–6.3) |
| Non-metastatic solid tumour | |||
| No | 4.4 (3.7–5.2) | 2.3 (1.7–2.9) | 2.1 (1.6–2.7) |
| Yes | 5.4 (1.9–8.8) | 3.0 (0.4–5.6) | 2.4 (0.0–4.7) |
| Other comorbidities | |||
| Atrial fibrillation or flutter | |||
| No | 4.3 (3.4–5.1) | 2.1 (1.5–2.7) | 2.2 (1.6–2.8) |
| Yes | 5.1 (3.4–6.7) | 3.0 (1.8–4.3) | 2.1 (1.0–3.1) |
| Infectiona | |||
| No | 4.2 (3.5–5.0) | 2.2 (1.7–2.7) | 2.0 (1.5–2.6) |
| Yes | 8.4 (4.2–12.7) | 4.5 (1.4–7.6) | 3.9 (1.0–6.8) |
| Other recent hospitalizationb | |||
| No | 3.7 (2.9–4.6) | 2.2 (1.6–2.9) | 1.5 (1.0–2.1) |
| Yes | 6.0 (4.4–7.5) | 2.6 (1.6–3.6) | 3.4 (2.2–4.5) |
| Use of antithrombotic therapya,c | |||
| Antiplatelet agents | |||
| No | 4.7 (3.5–5.8) | 2.4 (1.5–3.2) | 2.3 (1.5–3.1) |
| Yes | 4.5 (3.1–5.9) | 2.6 (1.5–3.7) | 1.9 (1.0–2.8) |
| Anticoagulant agents | |||
| No | 4.8 (3.8–5.8) | 2.6 (1.9–3.4) | 2.2 (1.5–2.9) |
| Yes | 3.6 (1.6–5.5) | 1.6 (0.3–3.0) | 1.9 (0.5–3.3) |
| . | VTE . | PE . | DVT . |
|---|---|---|---|
| IR (95% CI) . | IR (95% CI) . | IR (95% CI) . | |
| Overall | 4.5 (3.7–5.2) | 2.3 (1.8–2.9) | 2.1 (1.6–2.7) |
| Gender | |||
| Male | 4.6 (3.7–5.4) | 2.4 (1.8–3.0) | 2.2 (1.6–2.8) |
| Female | 4.0 (2.4–5.6) | 2.2 (1.0–3.3) | 1.8 (0.7–2.9) |
| Age, years | |||
| <60 | 3.8 (2.7–4.9) | 1.6 (0.9–2.3) | 2.2 (1.4–3.1) |
| 60–69 | 4.9 (3.6–6.3) | 2.6 (1.6–3.5) | 2.4 (1.4–3.3) |
| ≥70 | 5.0 (3.4–6.5) | 3.2 (2.0–4.5) | 1.7 (0.8–2.7) |
| ICD type | |||
| Single-chamber | 4.5 (3.5–5.5) | 2.5 (1.8–3.3) | 2.0 (1.3–2.6) |
| Dual-chamber | 4.3 (2.8–5.8) | 1.7 (0.8–2.7) | 2.5 (1.4–3.7) |
| CRT-D | 4.8 (2.9–6.7) | 2.6 (1.2–4.0) | 2.2 (0.9–3.5) |
| Prior device implantation | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.9) | 2.1 (1.5–2.6) |
| Yes | 6.0 (2.8–9.3) | 2.8 (0.6–5.0) | 3.2 (0.8–5.7) |
| Comorbidity (CCI score) | |||
| None (0) | 2.9 (1.6–4.2) | 1.5 (0.6–2.5) | 1.4 (0.5–2.3) |
| Moderate (1–2) | 3.2 (2.3–4.1) | 1.7 (1.1–2.4) | 1.5 (0.9–2.1) |
| Severe (≥ 3) | 8.1 (6.2–10.0) | 4.1 (2.7–5.5) | 4.0 (2.6–5.3) |
| CCI conditions | |||
| Myocardial infarction | |||
| No | 3.9 (2.9–4.9) | 2.2 (1.4–3.0) | 1.7 (1.0–2.4) |
| Yes | 5.0 (3.9–6.1) | 2.5 (1.7–3.2) | 2.5 (1.7–3.3) |
| Heart failure | |||
| No | 4.0 (3.0–4.9) | 2.0 (1.3–2.7) | 2.0 (1.3–2.7) |
| Yes | 5.1 (3.9–6.3) | 2.8 (1.9–3.6) | 2.3 (1.5–3.1) |
| Peripheral vascular disease | |||
| No | 4.1 (3.3–4.9) | 2.1 (1.5–2.6) | 2.0 (1.5–2.5) |
| Yes | 7.8 (4.7–10.9) | 4.5 (2.2–6.9) | 3.2 (1.2–5.3) |
| Cerebrovascular disease | |||
| No | 4.2 (3.4–5.0) | 2.1 (1.6–2.7) | 2.1 (1.5–2.6) |
| Yes | 6.5 (4.0–9.1) | 3.9 (1.9–5.9) | 2.6 (1.0–4.2) |
| Chronic pulmonary disease | |||
| No | 4.0 (3.2–4.7) | 2.1 (1.6–2.7) | 1.9 (1.4–2.4) |
| Yes | 8.8 (5.5–12.1) | 4.2 (1.9–6.5) | 4.6 (2.2–7.0) |
| Connective tissue disease | |||
| No | 4.3 (3.6–5.1) | 2.2 (1.7–2.8) | 2.1 (1.5–2.6) |
| Yes | 10.1 (3.5–16.8) | 5.6 (0.7–10.6) | 4.5 (0.1–8.9) |
| Ulcer disease | |||
| No | 4.3 (3.5–5.0) | 2.3 (1.7–2.8) | 2.0 (1.5–2.5) |
| Yes | 8.7 (4.0–13.5) | 3.4 (0.4–6.3) | 5.4 (1.7–9.1) |
| Mild liver disease | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.7–2.8) | 2.1 (1.6–2.6) |
| Yes | 17.3 (2.1–32.4) | 10.4 (0.0–22.1) | 6.9 (0.0–16.5) |
| Moderate/severe renal disease | |||
| No | 4.1 (3.4–4.9) | 2.2 (1.6–2.7) | 2.0 (1.5–2.5) |
| Yes | 12.4 (6.1–18.7) | 6.6 (2.0–11.2) | 5.8 (1.5–10.1) |
| Diabetes with end-organ damage | |||
| No | 4.4 (3.6–5.1) | 2.3 (1.8–2.9) | 2.0 (1.5–2.6) |
| Yes | 6.2 (2.7–9.7) | 2.6 (0.3–4.8) | 3.6 (0.9–6.3) |
| Non-metastatic solid tumour | |||
| No | 4.4 (3.7–5.2) | 2.3 (1.7–2.9) | 2.1 (1.6–2.7) |
| Yes | 5.4 (1.9–8.8) | 3.0 (0.4–5.6) | 2.4 (0.0–4.7) |
| Other comorbidities | |||
| Atrial fibrillation or flutter | |||
| No | 4.3 (3.4–5.1) | 2.1 (1.5–2.7) | 2.2 (1.6–2.8) |
| Yes | 5.1 (3.4–6.7) | 3.0 (1.8–4.3) | 2.1 (1.0–3.1) |
| Infectiona | |||
| No | 4.2 (3.5–5.0) | 2.2 (1.7–2.7) | 2.0 (1.5–2.6) |
| Yes | 8.4 (4.2–12.7) | 4.5 (1.4–7.6) | 3.9 (1.0–6.8) |
| Other recent hospitalizationb | |||
| No | 3.7 (2.9–4.6) | 2.2 (1.6–2.9) | 1.5 (1.0–2.1) |
| Yes | 6.0 (4.4–7.5) | 2.6 (1.6–3.6) | 3.4 (2.2–4.5) |
| Use of antithrombotic therapya,c | |||
| Antiplatelet agents | |||
| No | 4.7 (3.5–5.8) | 2.4 (1.5–3.2) | 2.3 (1.5–3.1) |
| Yes | 4.5 (3.1–5.9) | 2.6 (1.5–3.7) | 1.9 (1.0–2.8) |
| Anticoagulant agents | |||
| No | 4.8 (3.8–5.8) | 2.6 (1.9–3.4) | 2.2 (1.5–2.9) |
| Yes | 3.6 (1.6–5.5) | 1.6 (0.3–3.0) | 1.9 (0.5–3.3) |
CCI, Charlson Comorbidity Index; CI, confidence interval; CRT-D, cardiac resyncronization therapy defibrillator; DVT, deep venous thrombosis; ICD, implantable cardioverter-defibrillator; IR, incidence rate per 1000 person-years; PE, pulmonary embolism; VTE, venous thromboembolism.
aWithin 90 days prior to ICD implantation.
bFor a primary diagnosis not listed in Table 1 within 90 days prior to ICD implantation and lasting for ≥3 consecutive days.
cPatients included from 1 April 2004 through 31 December 2012 only (N = 6984).
Association of gender, age, implantable cardioverter-defibrillator type, and prior device implantation with venous thromboembolism
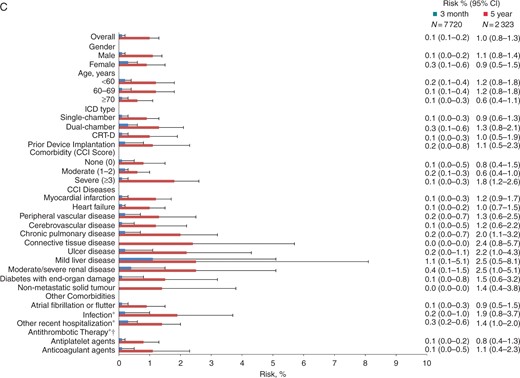
Overall, male ICD recipients had a higher IR for VTE, particularly for DVT (Table 2). However, IRRs were similar for men and women after adjustment for known and possible risk factors (Figure 2 and Supplementary material online, FigureS1), and there was no substantial gender difference in absolute risks of VTE within 3 months or 5 years following ICD implantation (Figure 1).
Crude and adjusted incidence rate ratios for venous thromboembolism (A), pulmonary embolism (B), and deep venous thrombosis (C) following ICD implantation, Denmark, 2000–12. Adjustments were made for gender, age, and comorbidity category (red), or gender, age, individual comorbidities, ICD type, and prior device implantation (blue). Whiskers illustrate 95% CIs. CCI, Charlson Comorbidity Index; CI, confidence interval; ICD, implantable cardioverter-defibrillator; IRR, incidence rate ratio; ref, reference; asterisk indicates using absence of the condition in question as reference.
After adjustment for other risk factors, the IRR for PE was higher among patients ≥70 years than among patients <60 years (Figure 2B). However, this counterbalanced a lower IRR for DVT among older patients (Figure 2C), and adjusted IRRs for VTE were similar across age groups (Figure 2A and Supplementary material online, FigureS1). The risk of VTE did not increase with age (Figure 1A).
Patients receiving CRT-Ds did not have a higher risk of VTE than patients receiving single-chamber ICDs, neither within 3 months following ICD implantation, nor within 5 years (Figure 1A). Risks were similar for PE and DVT (Figure 1B and C). Similarly, IRRs did not increase according to ICD type and number of leads (Figure 2 and Supplementary material online, FigureS1).
Association of comorbidity with venous thromboembolism
The risk of VTE was 0.3% within the first 3 months following ICD implantation regardless of comorbidity level (Figure 1A). However, the risk of VTE within 5 years following implantation was 3.2% for patients with severe comorbidity vs. 1.3–1.4% for patients with no or moderate comorbidity (Figure 1A). Overall, patients with severe comorbidity had a 2.7-fold higher IRR for VTE than patients with no comorbidity (Figure 2A). In the study cohort restricted to patients with available prescription data, the IRR was twice as high for patients with severe comorbidity as for patients with no comorbidity and remained significantly higher after adjustment for risk factors including use of antithrombotic therapy (see Supplementary material online, Figure S1).
Incidence rate ratios were particularly high for patients with mild liver disease compared with patients without this disease (Figure 2 and Supplementary material online, FigureS1). The risk of VTE among these patients was 2.1% within 3 months of implantation, increasing to 6.0% within 5 years (Figure 1A). Moderate-to-severe renal disease also was associated with an elevated IRR for VTE (Figure 2A and Supplementary material online, FigureS1) and a 5-year risk of 4.6% (Figure 1A). While the adjusted IRR comparing patients with connective tissue disease to patients without this disease was not substantially elevated (Figure 2A), the 5-year risk associated with connective tissue disease was 4.8% (Figure 1A). Surprisingly, diabetes with end-organ damage, heart failure, and atrial fibrillation or flutter were not associated with elevated incidence of VTE (Figure 2A), and additional adjustment for use of antithrombotics did not alter this finding notably (see Supplementary material online, Figure S1).
Discussion
Main findings
The VTE risk was 0.3% within the first 3 months following ICD implantation regardless of comorbidity level. We also found that comorbidity was associated with VTE following ICD implantation and that the incidence of VTE within 5 years was more than twice as high for ICD recipients with severe comorbidity as for ICD recipients with no comorbidity.
Comparison with the existing literature
In a US study of 5646 CIED patients during year 2000–10, Noheria et al.7 found a higher incidence of PE than that found in our study. Some of the difference likely arose because Noheria et al.7 included patients with a VTE diagnosed prior to CIED implantation, constituting 15% of the study cohort. However, the number of patients with a history of VTE that were excluded in our study corresponded to only 3.6% of our study cohort and inclusion of these patients in a sensitivity analysis did not increase our estimate considerably. International differences in ethnicity3,15 and prevalence of obesity3 and genetic risk factors3 may also contribute to the differing PE incidences. For instance, the prevalence of the prothrombotic mutations factor V Leiden and the prothrombin 20210A mutation vary widely between ethnic groups15 and north-Americans of African origin have a higher incidence of VTE than Caucasians.3 Consistent with Noheria et al.,7 we demonstrated that gender, age, and number of implanted leads were not associated with PE occurrence, indicating that other factors prevail in development of PE among CIED patients.
Impact of age, implantable cardioverter-defibrillator type, and prior device implantation
The lack of association between age and risk of VTE in our study may reflect a healthy candidate bias, i.e. older ICD recipients constitute a selected patient group with a lower comorbidity burden than younger ICD recipients. Interestingly, age ≥70 years was associated with a higher IRR of PE than age <60 years. In agreement with this finding, other studies reported that PE was more often diagnosed among the elderly compared with DVT.15 An age-related predilection for PE may explain why more than 50% of VTEs in our study were PEs, a considerably larger proportion than reported in the general population.15
We expected VTE risk to increase with newer ICD types, reflecting the larger lead surface available for thrombus formation with an increasing number of leads. This was not the case, nor was prior device implantation associated with risk of PE among ICD recipients. Thus, detachment of lead-associated thrombus material seems to play a minor role in development of VTE among ICD recipients.
Comorbidity as risk factor for venous thromboembolism
As reported in the general population,3 comorbidity was associated with VTE among ICD recipients. Contrary to expectations based on results from other studies,2,3 heart failure and atrial fibrillation or flutter were not associated with elevated risk of VTE. This may reflect that comparisons were made with ICD recipients treated for other types of heart disease, rather than with healthy individuals. Use of antithrombotic therapy did not explain our finding,16 but use of angiotensin-converting enzyme inhibitors17 and statins11 may have decreased risk in these patients, as well as in patients with diabetes. While our high risk estimates for ICD recipients with mild liver disease and/or moderate-to-severe renal disease relied on few VTE cases and had relatively broad CIs, they are in line with results derived from larger numbers in studies of non-ICD recipients.18,19
Pathophysiological mechanisms
Both external and endogenous factors have been proposed as underlying the increased risk of VTE among patients with liver and renal disease. Immobilization, which is a known risk factor for VTE,3 is associated with renal disease19 and with mild liver disease through obesity.18 Moreover, inflammation in renal disease induces a prothrombotic state through upregulation of thrombin levels and downregulation of anticoagulant pathways.3,19 Altered levels of serum oestrogen and anticoagulants may facilitate development of VTE in patients with some types of liver disease.18 Our results indicate that liver disease in particular facilitates the development of PE. It cannot be ruled out that the complex coagulopathy associated with liver disease increases the risk of PE.
Among ICD recipients categorized as having no comorbidity according to their CCI score, VTE risk may have been elevated secondary to conditions not included in the CCI. For instance, one genetic predisposition to VTE is caused by the lamin A/C mutation,20 which is also associated with the development of cardiomyopathy.
Study strengths and limitations
The major strengths of this nationwide study lie in its population-based design and virtually complete follow-up within the setting of a national healthcare system providing free care at the point of delivery.8,9 However, medical databases may not be entirely accurate. Positive predictive values of 71, 82, and 98% have been reported for diagnoses of DVT,14 PE,14 and the CCI conditions,12 respectively, in the DNPR. Although not validated, ICD implantation codes in the DNPR are thought to have a high positive predictive value because they serve as the basis for payment of public and private hospitals.9 Despite the large size of our study population, the number of VTE events was low in certain patient subgroups, and the resulting wide CIs should be taken into consideration when interpreting our results. As well, our study would have been strengthened by inclusion of data on clinical characteristics such as obesity3 and smoking.3 However, these data are incomplete in the DNPR. Our study includes data about prescription redemption for antithrombotic therapy within 90 days prior to ICD implantation for 85.9% of the study cohort, but does not include data about medication adherence. Finally, this observational study allows us to report associations only and no causality can be derived.
Conclusions
This nationwide cohort study with up to 13 years of follow-up showed that the 3-month risk of VTE following ICD implantation was 0.3% regardless of comorbidity level. The 5-year risk of VTE following ICD implantation was 1.9% and more than twice as high for patients with severe comorbidity as for patients without comorbidity. Our findings underscore the impact of comorbidity on long-term risk of VTE among ICD recipients, but suggest that its impact on short-term risk of VTE is surpassed by other risk factors.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by grants from the Danish Heart Foundation, Denmark (grant number 12-04-R90-A3919-22728 to S.B.P.); Aarhus University, Denmark (to S.B.P.); the Health Research Foundation of the Central Denmark Region, Denmark (to S.B.P.), the Clinical Epidemiological Research Foundation, Aarhus University Hospital, Aarhus, Denmark; and the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck and the Novo Nordisk Foundations. None of the funding sources had any role in the design, data collection, analysis, interpretation, or reporting of the study, nor in the decision to submit the article for publication.
Conflict of interest: J.C.N. has received speakers' fees from Biotronik and Biosense Webster, consultant fees from Boston Scientific, and a research grant from Biosense Webster. The other authors report no conflicts of interest.





