-
PDF
- Split View
-
Views
-
Cite
Cite
Juan Acosta, Nuno Cabanelas, Diego Penela, Juan Fernández-Armenta, David Andreu, Roger Borràs, Viatcheslav Korshunov, Mario Cabrera, Francesca Vasanelli, Elena Arbelo, Eduard Guasch, Mikel Martínez, Jose M. Tolosana, Lluis Mont, Antonio Berruezo, Long-term benefit of first-line peri-implantable cardioverter–defibrillator implant ventricular tachycardia-substrate ablation in secondary prevention patients, EP Europace, Volume 19, Issue 6, June 2017, Pages 976–982, https://doi.org/10.1093/europace/euw096
Close - Share Icon Share
Abstract
This study assessed the benefit of peri-implantable cardioverter–defibrillator implant ventricular tachycardia (VT)-substrate ablation in patients with structural heart disease (SHD).
Patients with SHD and indication for secondary prevention ICD implant were prospectively included. Patients presenting with incessant and/or slow VT or frequent (≥2) VT episodes who underwent peri-ICD VT-substrate ablation (the scar dechannelling technique) were compared with those who received ICD alone and did not meet ablation criteria. The primary endpoint was any sustained VT/ICD therapy during follow-up. Of 206 patients included (43.2% non-ischaemic), 70 were assigned to ablation and 136 received ICD implant alone. During a mean follow-up of 45.6 ± 24.7 months, the primary endpoint was more frequent in the non-ablation group (47.1 vs. 22.9%; P< 0.0001). Higher VT recurrence-free survival rate [log-rank P= 0.001; HR = 0.42 (0.24–0.73), P= 0.002] and ICD shock-free survival rate [log-rank P= 0.007; HR = 0.36 (0.17–0.78); P = 0.01] were observed in the ablation group. Higher relative risk reduction was observed in ischaemic [HR = 0.38 (0.18–0.83); P = 0.015] vs. non-ischaemic patients [HR = 0.49 (0.23–1.01); P = 0.08]. Patients with left ventricular ejection fraction (LVEF) <35% showed no differences in VT recurrence between treatment groups (log-rank P = 0.213) although VT burden during follow-up was lower in the ablation group [median (interquartile range) 1 (1–3) vs. 4 (1–10) VT episodes; P = 0.05].
First-line peri-ICD implant VT-substrate ablation was associated with decreased VT recurrence and ICD shocks during long-term follow-up in patients with SHD and indication for secondary prevention ICD implant, especially in ischaemic patients. In patients with LVEF <35%, no benefit was observed in terms of VT recurrence-free survival, although VT burden during follow-up was lower in the ablation group.
There is no consensus on the appropriate indication and timing for prophylactic ventricular tachycardia (VT) ablation in patients receiving implantable cardioverter–defibrillator (ICD) for secondary prevention.
In the present study, secondary prevention patients considered at high risk of VT recurrence (those presenting with incessant/slow VT or frequent VT episodes) underwent peri-ICD implant VT-substrate ablation and outcomes were compared with patients who received ICD alone and did not meet ablation criteria.
Peri-ICD VT-substrate ablation is associated with a significant reduction in VT recurrence and ICD shocks during long-term follow-up in patients with structural heart disease and indication for ICD implantation as secondary prevention.
The benefit observed with peri-ICD VT-substrate ablation in non-ischaemic patients was less significant than that observed in the ischaemic subgroup.
No significant benefit was obtained with peri-ICD VT-substrate ablation in patients with LVEF <35%.
Introduction
Patients with a history of structural heart disease who survive a spontaneous episode of ventricular tachycardia (VT) are at high risk of sudden cardiac death from recurrent VT or ventricular fibrillation (VF). Implantable cardioverter–defibrillators (ICDs) decrease mortality and have become the mainstay for treating these patients.1 However, ICDs are not a curative treatment for ventricular arrhythmias. Approximately 38% of patients undergoing ICD implant for secondary prevention receive ICD therapies during the first-year post-implantation.2 Although potentially life-saving, these interventions (especially shocks, even if inappropriately triggered) are associated with decreased quality of life and increased mortality, compared with those who do not receive ICD therapies.3–5 Furthermore, ICDs do not provide absolute protection against death due to arrhythmia when the rate of sudden cardiac death unresponsive to ICD ranges from 3 to 7%.6
Treatment with anti-arrhythmic drugs (AADs)—especially amiodarone in combination with β-blockers—can reduce the number of ICD interventions; however, this is usually a long-term therapy that has been associated with serious adverse events.2 Advances in catheter ablation and mapping technology have made substrate-based catheter ablation, an effective therapeutic option in patients with recurrent episodes of scar-related VT. To date, two randomized trials (SMASH-VT and VTACH) have assessed the benefits of VT-substrate ablation in ischaemic patients receiving ICD for secondary prevention.7,8 The SMASH-VT trial included patients with unstable VT and showed a lower incidence of ICD interventions in those assigned to prophylactic ablation and ICD than those in the group assigned to ICD alone.7 In the VTACH study, prophylactic ablation before ICD implant was associated with a longer time free of any VT/VF recurrence, especially in patients with left ventricular ejection fraction (LVEF) >30%.8 However, it is unknown whether the results of these studies also apply to patients with VT due to other cardiomyopathies or with LVEF in the lower range. Therefore, to date, there is no consensus on the appropriate indication and timing for prophylactic VT ablation in patients receiving ICD for secondary prevention. The aim of this study was to assess the long-term benefit of peri-ICD implant VT-substrate ablation in patients with monomorphic VT due to structural heart disease, regardless of their aetiology and LVEF range. According to this objective, patients presenting with incessant and/or slow VT or frequent (≥2) VT episodes underwent peri-ICD VT-substrate ablation and were compared with those who received ICD alone and did not meet ablation criteria.
Methods
Study population
This was a prospective cohort study. From 2009 to July 2015, all patients aged >18 years with structural heart disease and indication for an ICD as secondary prevention after documented monomorphic VT were potentially eligible for participation. Patients were excluded if they met one or more of the following criteria: active on-going ischaemia, polymorphic VT, previous VT-ablation procedure, intraventricular thrombus, or severe heart failure (NYHA class IV). The study complied with the Declaration of Helsinki principles. The local Ethics Committee approved the study protocol, and all included participants signed the informed consent.
According to the study protocol, patients who experienced incessant and/or slow monomorphic VT, frequent episodes (≥2) of monomorphic VT, or multiple shocks despite AAD therapy during the hospitalization were considered at high risk of VT recurrence (and therefore ICD therapy) and underwent peri-ICD implant VT-substrate ablation (ablation group). Patients not meeting any of these three criteria underwent ICD implant alone (non-ablation group; Figure 1).

Study flowchart. ICD, implantable cardioverter defibrillator; PVT, polimorphic ventricular tachycardia; SMVT, sustained monomorphic ventricular tachycardia; VT, ventricular tachycardia.
Study procedures
Implantable cardioverter–defibrillator implant was done in accordance with the standards at our centre. Recommended device programming consisted of a VF zone with a cut-off rate of 200–220 b.p.m. and a VT zone with a cut-off cycle length of 40–60 ms above the slowest documented VT. Anti-tachycardia pacing (ATP) followed by shock was also recommended.
In the ablation group, VT-substrate ablation was performed using the CARTO system (Biosense Webster, Diamond Bar, CA, USA). The scar dechannelling technique was used in all ablation procedures.9 The ablation procedure aimed complete elimination of accessible arrhyhthmogenic substrate and was performed either before ICD implant (i.e. during the hospitalization) or up to 3 months thereafter. In those patients included in the ablation group due to incessant VT or multiple ICD shocks refractory to AADs, the ablation procedure was performed electively once the patient was stabilized by medical treatment.
Successful ablation was defined as non-inducibility of any VT or VF at the end of the procedure. High-density electroanatomical map (EAM) of the right ventricle (RV) or left ventricle (LV) was obtained during sinus rhythm. When endocardial LV EAM was required, transseptal LV access was achieved and the ablation catheter was manipulated using a steerable sheath (Agilis St Jude Medical, St Paul, MN, USA). In patients requiring epicardial access, percutaneous pericardial puncture was performed as described by Sosa et al.10 using the subxiphoid approach. Briefly, standard voltage thresholds (<0.5 mV for the core and <1.5 mV for the border zone) were used to define the scar on the EAM and conducting channels (CCs) were identified as either voltage channels11 or late potential channels.9,12 Electrograms with delayed components (E-DCs) were tagged and dichotomously classified as entrance or inner CC points, depending on delayed component precocity during sinus rhythm. Radiofrequency (RF) energy was delivered at the identified CC entrances, as previously described,9,13 using a Navistar catheter (Biosense Webster). Radiofrequency ablation was controlled by a temperature limit of 45°C with a power limit of 50 W at the endocardium and 40–50 W at the epicardium. The catheter irrigation rate during RF application was 26 mL/min in the endocardium and 17 mL/min in the epicardium. A post-ablation remap was always performed to document the elimination of all the CC-electrograms and to eliminate the remaining E-DCs by back-up RF applications. After substrate ablation, programmed RV stimulation with three basal cycle lengths (600, 500, and 430 ms) and up to three ventricular extrastimuli until refractoriness or 200 ms cycle length was used to induce VT. Clinical and non-clinical VTs induced after scar dechannelling were targeted for ablation.
Drug management during follow-up was determined at the discretion of the physician although the restricted use of AAD was strongly recommended in the ablation group. Most patients in the ablation group were discharged home with a prescription for 4–6 weeks of oral anticoagulant therapy.
Follow-up
Patients were followed up every 6 months. Information was obtained from the ICD memory about the number of events, the number and type of treatments required, and cycle lengths during VT episodes. Additional relevant clinical information such as NYHA class or medication changes was also collected. The primary endpoint was the occurrence of sustained VT. Any sustained VT, whether or not ICD intervention was required, was considered a recurrence during follow-up. The secondary endpoint included freedom from any appropriate ICD shock.
Statistical analysis
Continuous variables are presented as mean ± standard deviation. In bivariate comparisons, Student's t-, Mann–Whitney U, or ANOVA tests were used as appropriate. Categorical variables were expressed as total number (percentages) and compared between groups using the χ2 test. Kaplan–Meier survival analysis was used to analyse time to VT recurrence and redo ablation, and the log-rank test was used to detect significant differences between groups. Baseline clinical characteristics, recurrence, and mortality were analysed by including each patient only once in either group (per-patient analysis). The effect of different variables on event-free survival was investigated using the Cox proportional hazards model. Variables that showed a statistically significant effect on event-free survival in univariate analysis were entered in a multivariate Cox proportional hazards model using a backward stepwise selection to obtain the final model. At each step, the least significant variable was discarded from the model until all variables in the model reached a value of P< 0.10. The number of variables that could enter the multivariate model was limited using the P,m/10 rule to prevent over-fitting. For all tests, a value of P< 0.05 was considered significant. Statistical analysis was performed using R software for Windows version 3.1.2 (R Project for Statistical Computing, Vienna, Austria) and SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Study population
From 2009 to July 2015, 206 patients (63.2 ± 12.6 years, 89.3% male) with structural heart disease and indication for ICD implant as secondary prevention after documented monomorphic VT were included. In 70 patients (33.9%), peri-ICD implant VT-substrate ablation was performed; 41 (58.5%) of these patients were included in the ablation group due to incessant and/or slow VT, 14 (20%) due to recurrent VTs despite AADs, and 15 (21.4%) due to multiple external shocks refractory to medical treatment. The remaining 136 patients (66.1%) underwent ICD implant alone (non-ablation group; Figure 1).
At baseline, the ablation and non-ablation groups were well balanced with respect to demographic and clinical characteristics and had similar underlying drug treatment (Table 1). The only significant differences between groups were longer VT cycle length, more VT episodes, and a higher proportion of patients with arrhythmia storm in the ablation group; all of which indicate increased risk of VT recurrence.
| Characteristicsa . | Non-ablation group (N = 136) . | Ablation group (N = 70) . | P-value . |
|---|---|---|---|
| Age (years) | 63 ± 13 | 63.5 ± 12 | 0.801 |
| Sex (male) | 119 (87%) | 65 (93%) | 0.341 |
| LVEF (%) | 35.7 ± 12.3 | 37.8 ± 11.2 | 0.205 |
| Ischaemic cardiomyopathy | 73 (54%) | 44 (63%) | 0.236 |
| NYHA class | |||
| I or II | 108 (79%) | 60 (86%) | 0.452 |
| III or IV | 28 (20%) | 10 (14%) | |
| Hypertension | 81 (59%) | 45 (64%) | 0.544 |
| Diabetes | 38 (28%) | 12 (17%) | 0.121 |
| Anti-arrhythmic drug treatment | |||
| β-Blocker | 101 (74%) | 54 (78%) | 1 |
| Class III | 78 (57%) | 32 (46%) | 0.074 |
| Basal VT characteristics | |||
| VT cycle length (ms) | 315 ± 58 | 368 ± 75 | <0.0001 |
| No. of VT episodes | 1.3 ± 1.1 | 2.8 ± 8.1 | 0.041 |
| Arrhythmia storm | 9 (6%) | 15 (21%) | <0.0001 |
| Characteristicsa . | Non-ablation group (N = 136) . | Ablation group (N = 70) . | P-value . |
|---|---|---|---|
| Age (years) | 63 ± 13 | 63.5 ± 12 | 0.801 |
| Sex (male) | 119 (87%) | 65 (93%) | 0.341 |
| LVEF (%) | 35.7 ± 12.3 | 37.8 ± 11.2 | 0.205 |
| Ischaemic cardiomyopathy | 73 (54%) | 44 (63%) | 0.236 |
| NYHA class | |||
| I or II | 108 (79%) | 60 (86%) | 0.452 |
| III or IV | 28 (20%) | 10 (14%) | |
| Hypertension | 81 (59%) | 45 (64%) | 0.544 |
| Diabetes | 38 (28%) | 12 (17%) | 0.121 |
| Anti-arrhythmic drug treatment | |||
| β-Blocker | 101 (74%) | 54 (78%) | 1 |
| Class III | 78 (57%) | 32 (46%) | 0.074 |
| Basal VT characteristics | |||
| VT cycle length (ms) | 315 ± 58 | 368 ± 75 | <0.0001 |
| No. of VT episodes | 1.3 ± 1.1 | 2.8 ± 8.1 | 0.041 |
| Arrhythmia storm | 9 (6%) | 15 (21%) | <0.0001 |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VT, ventricular tachycardia.
an (%) unless otherwise indicated.
| Characteristicsa . | Non-ablation group (N = 136) . | Ablation group (N = 70) . | P-value . |
|---|---|---|---|
| Age (years) | 63 ± 13 | 63.5 ± 12 | 0.801 |
| Sex (male) | 119 (87%) | 65 (93%) | 0.341 |
| LVEF (%) | 35.7 ± 12.3 | 37.8 ± 11.2 | 0.205 |
| Ischaemic cardiomyopathy | 73 (54%) | 44 (63%) | 0.236 |
| NYHA class | |||
| I or II | 108 (79%) | 60 (86%) | 0.452 |
| III or IV | 28 (20%) | 10 (14%) | |
| Hypertension | 81 (59%) | 45 (64%) | 0.544 |
| Diabetes | 38 (28%) | 12 (17%) | 0.121 |
| Anti-arrhythmic drug treatment | |||
| β-Blocker | 101 (74%) | 54 (78%) | 1 |
| Class III | 78 (57%) | 32 (46%) | 0.074 |
| Basal VT characteristics | |||
| VT cycle length (ms) | 315 ± 58 | 368 ± 75 | <0.0001 |
| No. of VT episodes | 1.3 ± 1.1 | 2.8 ± 8.1 | 0.041 |
| Arrhythmia storm | 9 (6%) | 15 (21%) | <0.0001 |
| Characteristicsa . | Non-ablation group (N = 136) . | Ablation group (N = 70) . | P-value . |
|---|---|---|---|
| Age (years) | 63 ± 13 | 63.5 ± 12 | 0.801 |
| Sex (male) | 119 (87%) | 65 (93%) | 0.341 |
| LVEF (%) | 35.7 ± 12.3 | 37.8 ± 11.2 | 0.205 |
| Ischaemic cardiomyopathy | 73 (54%) | 44 (63%) | 0.236 |
| NYHA class | |||
| I or II | 108 (79%) | 60 (86%) | 0.452 |
| III or IV | 28 (20%) | 10 (14%) | |
| Hypertension | 81 (59%) | 45 (64%) | 0.544 |
| Diabetes | 38 (28%) | 12 (17%) | 0.121 |
| Anti-arrhythmic drug treatment | |||
| β-Blocker | 101 (74%) | 54 (78%) | 1 |
| Class III | 78 (57%) | 32 (46%) | 0.074 |
| Basal VT characteristics | |||
| VT cycle length (ms) | 315 ± 58 | 368 ± 75 | <0.0001 |
| No. of VT episodes | 1.3 ± 1.1 | 2.8 ± 8.1 | 0.041 |
| Arrhythmia storm | 9 (6%) | 15 (21%) | <0.0001 |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VT, ventricular tachycardia.
an (%) unless otherwise indicated.
Catheter ablation
For logistical reasons, 35 patients (50%) received post-ICD ablation and pre-ICD implant ablation was done in 35 patients (50%). The approach used was endocardial in the majority of patients [50 (71.4%)], endo-epicardial in 17 (24.2%), and epicardial in 3 (4.2%). The mean procedure duration and fluoroscopy time were 234 ± 49 min (range, 155–350 min) and 23 ± 10 min (range, 8–49 min), respectively. The mean number of points acquired to build the endocardial and epicardial EAMs was 497 ± 192 and 512 ± 235, respectively. A fill threshold within the scar area of <6 mm was obtained in all cases. The mean RF time was 24.1 ± 10.8 min. Acute success (i.e. non-inducibility of any VT or VF) was achieved in 51 patients (72.8%); a sustained monomorphic VT remained inducible in 12 patients (17.1%) and polymorphic VT or VF in 7 patients (10%). Relevant ablation-related complications occurred in 7 patients (10%): 2 had pericardial effusion (one requiring urgent drainage and one managed conservatively), 2 had phrenic nerve palsy, 2 had pericarditis, and 1 patient had complete auriculoventricular block.
Follow-up
Mean duration (±SD) of follow-up was 45.6 ± 24.7 months. Recurrences of sustained VT were observed in 80 patients (38.8%). Of these, 45 (56.2%) received ICD shock and 34 (42.5%) had VT episodes that were terminated by ATP. One patient (1.2%) had VT episodes that were monitored in the VT detection zone and did not receive any therapy. Anti-arrhythmic treatment was maintained during follow-up in 16 patients (22.8%) in the ablation group vs. 65 patients (47.9%) in the non-ablation group (P < 0.0001).
Patients in the non-ablation group had a significantly higher event rate for any kind of VT recurrence (47.1 vs. 22.9%; P< 0.0001), and Kaplan–Meier analysis showed a significantly higher VT recurrence-free survival rate in the ablation group (log-rank P= 0.001; Figure 2). Implantable cardioverter–defibrillator shocks occurred in 8 patients in the ablation group and in 37 patients in the non-ablation group (11.4 vs. 27.2%, respectively; P = 0.009). Additionally, Kaplan–Meier analysis showed a significantly higher ICD shock-free survival rate in the ablation group (log-rank P= 0.007; Figure 3). Twenty-seven patients (19.9%) in the non-ablation group required VT-substrate ablation during follow-up, due to frequent drug-refractory VT episodes in 15 patients (55.5%), arrhythmia storm in 6 (22.2%), incessant VT in 3 (11.1%), and isolated symptomatic VT episode in 3 patients (11.1%). In contrast (P = 0.026), 6 patients (8.6%) in the ablation group required redo ablation: 3 had arrhythmia storm, 2 had frequent drug-refractory VT episodes, and 1 had incessant VT.
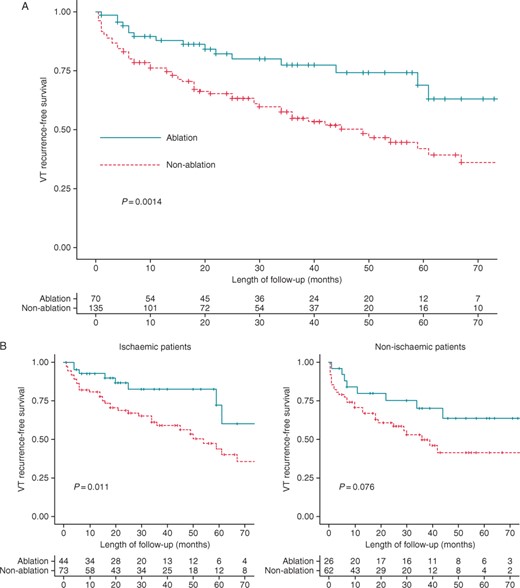
(A) Kaplan–Meier curve for the endpoint of ventricular tachycardia recurrence. (B) Kaplan–Meier curve for the endpoint of ventricular tachycardia recurrence in ablation and non-ablation groups by the type of cardiomyopathy.
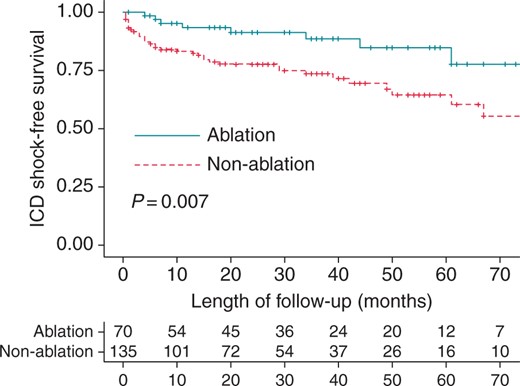
Table 2 summarizes univariate Cox regression analysis results for the endpoint of VT recurrence. Univariate analysis identified a lower risk of VT recurrence in patients who underwent peri-ICD implant ablation [HR = 0.42 (0.24–0.73), P= 0.002]. The rest of the variables analysed were not significantly associated with VT recurrence; thus, multivariate analysis was not necessary. It should be noted that neither the number of VT episodes nor the VT cycle length was predictors of VT recurrence, due to the inclusion of all patients with frequent episodes of VT and/or long VT cycle length in the ablation group. Additionally, no significant association was observed between the presence of non-ischaemic cardiomyopathy and VT recurrence rate (Table 2). Higher benefit of peri-ICD VT-substrate ablation was observed in ischaemic patients, achieving a greater relative risk reduction for the primary endpoint [HR = 0.38 (0.18–0.83); P = 0.015] compared with non-ischaemic patients [HR = 0.49 (0.22–1.01); P = 0.08] (Figure 2 and Table 3).
Univariate and multivariate Cox regression analyses for the association between clinical variables and the study endpoint (VT recurrence)
| . | Univariate HR (95% CI) . | P-value . |
|---|---|---|
| Age | 0.99 (0.98–1.01) | 0.883 |
| LVEF <35% | 0.70 (0.45–1.09) | 0.118 |
| Heart failure | 0.80 (0.45–1.41) | 0.442 |
| AAD class III | 1.08 (0.69–1.69) | 0.733 |
| VT episodes (>2) | 0.94 (0.79–1.11) | 0.469 |
| Clinical VT CL | 1 (0.99–1.01) | 0.157 |
| Non-ischaemic cardiomyopathy | 1.32 (0.85–2.05) | 0.212 |
| Hypertension | 1.04 (0.66–1.65) | 0.848 |
| Diabetes | 1.13 (0.68–1.91) | 0.626 |
| Peri-ICD ablation | 0.42 (0.24–0.73) | 0.002 |
| . | Univariate HR (95% CI) . | P-value . |
|---|---|---|
| Age | 0.99 (0.98–1.01) | 0.883 |
| LVEF <35% | 0.70 (0.45–1.09) | 0.118 |
| Heart failure | 0.80 (0.45–1.41) | 0.442 |
| AAD class III | 1.08 (0.69–1.69) | 0.733 |
| VT episodes (>2) | 0.94 (0.79–1.11) | 0.469 |
| Clinical VT CL | 1 (0.99–1.01) | 0.157 |
| Non-ischaemic cardiomyopathy | 1.32 (0.85–2.05) | 0.212 |
| Hypertension | 1.04 (0.66–1.65) | 0.848 |
| Diabetes | 1.13 (0.68–1.91) | 0.626 |
| Peri-ICD ablation | 0.42 (0.24–0.73) | 0.002 |
AAD, anti-arrhythmic drugs; CL, cycle length; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia; ICD, implantable cardioverter–defibrillator.
Univariate and multivariate Cox regression analyses for the association between clinical variables and the study endpoint (VT recurrence)
| . | Univariate HR (95% CI) . | P-value . |
|---|---|---|
| Age | 0.99 (0.98–1.01) | 0.883 |
| LVEF <35% | 0.70 (0.45–1.09) | 0.118 |
| Heart failure | 0.80 (0.45–1.41) | 0.442 |
| AAD class III | 1.08 (0.69–1.69) | 0.733 |
| VT episodes (>2) | 0.94 (0.79–1.11) | 0.469 |
| Clinical VT CL | 1 (0.99–1.01) | 0.157 |
| Non-ischaemic cardiomyopathy | 1.32 (0.85–2.05) | 0.212 |
| Hypertension | 1.04 (0.66–1.65) | 0.848 |
| Diabetes | 1.13 (0.68–1.91) | 0.626 |
| Peri-ICD ablation | 0.42 (0.24–0.73) | 0.002 |
| . | Univariate HR (95% CI) . | P-value . |
|---|---|---|
| Age | 0.99 (0.98–1.01) | 0.883 |
| LVEF <35% | 0.70 (0.45–1.09) | 0.118 |
| Heart failure | 0.80 (0.45–1.41) | 0.442 |
| AAD class III | 1.08 (0.69–1.69) | 0.733 |
| VT episodes (>2) | 0.94 (0.79–1.11) | 0.469 |
| Clinical VT CL | 1 (0.99–1.01) | 0.157 |
| Non-ischaemic cardiomyopathy | 1.32 (0.85–2.05) | 0.212 |
| Hypertension | 1.04 (0.66–1.65) | 0.848 |
| Diabetes | 1.13 (0.68–1.91) | 0.626 |
| Peri-ICD ablation | 0.42 (0.24–0.73) | 0.002 |
AAD, anti-arrhythmic drugs; CL, cycle length; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia; ICD, implantable cardioverter–defibrillator.
Benefit of peri-ICD implant VT-substrate ablation according to the type of cardiomyopathy
| . | Ischaemic (N = 117) . | Non-ischaemic (N = 89) . | ||||
|---|---|---|---|---|---|---|
| Ablation group (n = 44) . | Non-ablation group (n = 73) . | P-value . | Ablation group (n = 26) . | Non-ablation group (n = 63) . | P-value . | |
| Any kind of VT recurrence | 8 (18%) | 35 (48%) | 0.001 | 8 (31%) | 29 (46%) | 0.239 |
| ICD shock recurrence | 3 (7%) | 20 (27%) | 0.008 | 5 (19%) | 17 (27%) | 0.591 |
| . | Ischaemic (N = 117) . | Non-ischaemic (N = 89) . | ||||
|---|---|---|---|---|---|---|
| Ablation group (n = 44) . | Non-ablation group (n = 73) . | P-value . | Ablation group (n = 26) . | Non-ablation group (n = 63) . | P-value . | |
| Any kind of VT recurrence | 8 (18%) | 35 (48%) | 0.001 | 8 (31%) | 29 (46%) | 0.239 |
| ICD shock recurrence | 3 (7%) | 20 (27%) | 0.008 | 5 (19%) | 17 (27%) | 0.591 |
VT, ventricular tachycardia; ICD, implantable cardioverter–defibrillator.
Benefit of peri-ICD implant VT-substrate ablation according to the type of cardiomyopathy
| . | Ischaemic (N = 117) . | Non-ischaemic (N = 89) . | ||||
|---|---|---|---|---|---|---|
| Ablation group (n = 44) . | Non-ablation group (n = 73) . | P-value . | Ablation group (n = 26) . | Non-ablation group (n = 63) . | P-value . | |
| Any kind of VT recurrence | 8 (18%) | 35 (48%) | 0.001 | 8 (31%) | 29 (46%) | 0.239 |
| ICD shock recurrence | 3 (7%) | 20 (27%) | 0.008 | 5 (19%) | 17 (27%) | 0.591 |
| . | Ischaemic (N = 117) . | Non-ischaemic (N = 89) . | ||||
|---|---|---|---|---|---|---|
| Ablation group (n = 44) . | Non-ablation group (n = 73) . | P-value . | Ablation group (n = 26) . | Non-ablation group (n = 63) . | P-value . | |
| Any kind of VT recurrence | 8 (18%) | 35 (48%) | 0.001 | 8 (31%) | 29 (46%) | 0.239 |
| ICD shock recurrence | 3 (7%) | 20 (27%) | 0.008 | 5 (19%) | 17 (27%) | 0.591 |
VT, ventricular tachycardia; ICD, implantable cardioverter–defibrillator.
Benefit according to left ventricular ejection fraction
The Kaplan–Meier analysis of the primary endpoint was repeated for patients with LVEF <35% (n = 94) and LVEF >35% (n = 112). Baseline clinical characteristics of both groups are summarized in Supplementary material online. No differences were observed in the rate of patients undergoing peri-ICD VT-substrate ablation according to LVEF (30.8% in the LVEF <35% group vs. 36.6% in the LVEF >35% group; P = 0.104). Although acute results of peri-ICD VT-substrate ablation were similar in patients with LVEF <35% and those with LVEF >35% (non-inducibility of VT/VF in 69.2 vs. 75%, respectively; P = 0.781); when patients with LVEF <35% were analysed separately, VT recurrence-free survival did not differ between treatment groups although VT burden during follow-up was significantly lower in those who underwent peri-ICD substrate ablation [median (interquartile range) number of VT episodes 1 (1–3) vs. 4 (1–10); P = 0.05]. However, the difference between ablation and non-ablation groups was greater in the subgroup of patients with LVEF >35% than that observed in the entire study population (Figure 4).
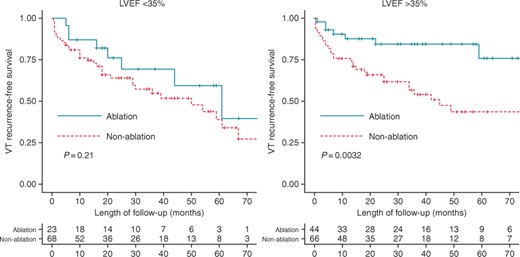
Kaplan–Meier curves for VT recurrence by left ventricular ejection fraction.
Mortality
Sixteen patients (7.7%) died during follow-up: 8 (50%) due to advanced heart failure, 3 (18.7%) due to arrhythmic storm or sudden cardiac death, and 5 (31.2%) due to non-cardiac causes. No significant differences in all-cause mortality were observed between groups: 3 patients died in the ablation group (4.3%) and 13 in the non-ablation group (9.6%; P= 0.272).
Discussion
The present study explored the benefits that could be expected from peri-ICD VT-substrate ablation in selected patients with structural heart disease and indication for ICD as secondary prevention due to monomorphic VT. The main findings of this study are: (i) peri-ICD VT-substrate ablation was associated with a lower rate of VT/ICD therapy recurrence during long-term follow-up, (ii) this benefit was especially significant in patients with ischaemic cardiomyopathy, and (iii) no benefit was observed in patients with LVEF <35% in terms of VT recurrence-free survival although a significantly lower VT burden was observed during follow-up in ablation groups.
To date, there is no consensus on the appropriate timing of VT-substrate ablation in secondary prevention patients. Two randomized trials (SMASH-VT and VTACH) have shown the benefit of prophylactic VT ablation in ischaemic patients7,8 although the long-term results of VT ablation are not as good in non-ischaemic patients, a significant reduction in VT burden is achieved in the majority of these patients.14 Despite these data, patients with structural heart disease and VT continue to be referred for ablation late in the course of the disease.15 Unlike the SMASH-VT and VTACH trials, the present study included both ischaemic and non-ischaemic patients. Furthermore, patients were assigned to ablation according to clinical criteria16 associated with a potential higher risk of VT recurrence: drug-refractory frequent VT episodes, incessant VT, or arrhythmia storm.17,18 Therefore, patients in the ablation group had longer VT cycle length, a higher number of VT episodes, and a higher proportion of arrhythmia storm as a referring episode (Table 1), potentially putting them at higher risk of VT/ICD shock recurrence. Nonetheless, a significant reduction of any kind of VT recurrence rate during long-term follow-up was achieved in this group by peri-ICD ablation (24.2% absolute risk reduction and 52.4% relative risk reduction). The benefit was also observed in the ∼15% reduced incidence rate of ICD shock in the ablation group (52.1% relative risk reduction). This could be particularly important to patient outcomes as ICD shocks are associated with increased mortality despite termination of the acute event.3,19,20 The results obtained in the present study in the reduction of any VT recurrence (24.2% absolute risk reduction) in the ablation group exceed those observed in SMASH-VT (11%)7 and VTACH trials (18%),8 despite our inclusion of non-ischaemic patients and a significantly higher proportion of patients under anti-arrhythmic treatment during follow-up in the non-ablation group. This difference in results could be explained by a more extensive elimination of the arrhythmic substrate achieved by the scar dechannelling technique in the present study (when compared with the elimination of only the substrate responsible for the clinical VT), as has been described in an earlier study by our group.9
Consistent with other studies reporting VT ablation results in non-ischaemic cardiomyopathy,14,21 the benefit observed in non-ischaemic patients was less significant than that in the ischaemic subgroup in the present study. Indeed, in the subgroup of non-ischaemic patients, no statistically significant difference was observed in the rate of VT recurrence between ablation and non-ablation groups (Table 3) although a trend towards fewer VT recurrences or ICD shocks was observed in the ablation group (Figure 2B). This could be explained by the higher number of non-ischaemic patients requiring ablation in order to observe a significant reduction in VT recurrence, given the worse results obtained in comparison with ischaemic patients. Additionally, no differences were observed between treatment groups in terms of VT recurrence among patients with LVEF <35%. This was mainly due to a higher recurrence rate among those patients in the ablation group, similar to the results described in the VTACH study, in which a difference in the recurrence rate between the ablation and non-ablation group was only seen in patients with an LVEF of >30%.8 The worse results obtained with ablation in patients with non-ischaemic cardiomyopathy and those with LVEF <35% may be due to more extensive substrate and/or incomplete substrate accessibility, resulting in incomplete substrate elimination. However, despite VT recurrence-free survival did not differ between treatment groups in patients with LVEF <35%, it should be noted that VT burden during follow-up was significantly lower in the peri-ICD ablation group.
A recent epidemiological study by Bunch et al. compared long-term outcomes in patients with ICD shocks who underwent VT ablation vs. those with ICD and no prior shocks or who received shocks, but did not undergo ablation. As in the present study, they report fewer appropriate ICD shocks in patients treated with ablation than in those treated with medication only.22 Furthermore, patients who underwent ablation had a significantly lower risk of cardiac death and heart failure hospitalization than those managed only medically.22 An important limitation of their study, however, is that patients were assigned to ablation according to each individual physician's criteria; given the higher presence of comorbidities in the non-ablation group, this could reflect a selection bias.22 In the present study, the ablation and non-ablation groups were comparable in terms of comorbidities and ejection fraction. Furthermore, the ablation group could be identified as having the sickest patients, given the higher number of VT episodes, the longer VT cycle length, and the higher proportion of arrhythmia storm and incessant VT as referring episodes. This makes the benefits observed in the ablation group particularly notable and validates the clinical criteria used in this study to select patients for peri-ICD VT-substrate ablation.
Limitations
The present study was a prospective cohort study reporting the results of the clinical practice at a single centre, using the inclusion criteria described for performing peri-ICD VT-substrate ablation. As it was not randomized, it could not be determined whether the lower incidence of VT recurrence observed in patients in the ablation group is exclusively due to peri-ICD VT-substrate ablation or might involve other uncontrolled conditions not taken into account. However, it should be noted that the criteria used to select patients to be included in the ablation group are known to be associated with a higher risk of VT recurrence; a randomized controlled trial comparing peri-ICD ablation with medical treatment in patients with the proposed criteria would be needed to address this question. Secondly, this study cannot address the potential additional benefit of AAD usage in some patients, as anti-arrhythmic therapy during follow-up was decided at the discretion of the treating physician; in turn, however, this makes the study results more applicable to clinical practice. Furthermore, the proportion of patients under anti-arrhythmic treatment during follow-up was significantly lower in the ablation group, which highlights the benefit of peri-ICD ablation. Thirdly, this study cannot address the higher recurrence rate observed in non-ischaemic patients and patients with LVEF <35% undergoing VT-substrate ablation, and it remains unknown whether more extensive ablation approaches may yield better results in these patients. However, VT recurrence rates after scar dechannelling are in the lower range of those reported for other VT-substrate ablation strategies. To answer this question, a randomized study comparing scar dechannelling and more extensive ablation approaches would be required. Finally, it remains unknown whether ablation techniques systematically using multielectrode mapping could improve long-term outcomes of VT-substrate ablation, due to better substrate identification. Again, in order to address this issue, a randomized trial comparing point-by-pint and multielectrode mapping is required.
Conclusion
Peri-ICD ablation is associated with a significant reduction in VT recurrence and ICD shocks during long-term follow-up in patients with structural heart disease and indication for ICD implantation as secondary prevention. This benefit was especially evident in patients with ischaemic heart disease. In patients with LVEF <35%, no benefit was observed in terms of VT recurrence-free survival although VT burden during follow-up was significantly lower in the peri-ICD ablation group.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.



