-
PDF
- Split View
-
Views
-
Cite
Cite
Cengiz Özcan, Jakob Raunsø, Morten Lamberts, Lars Køber, Tommi Bo Lindhardt, Niels Eske Bruun, Marie Louise Laursen, Christian Torp-Pedersen, Gunnar Hilmar Gislason, Morten Lock Hansen, Infective endocarditis and risk of death after cardiac implantable electronic device implantation: a nationwide cohort study, EP Europace, Volume 19, Issue 6, June 2017, Pages 1007–1014, https://doi.org/10.1093/europace/euw404
Close - Share Icon Share
Abstract
To determine the incidence, risk factors, and mortality of infective endocarditis (IE) following implantation of a first-time, permanent, cardiac implantable electronic device (CIED).
From Danish nationwide administrative registers (beginning in 1996), we identified all de-novo permanent pacemakers (PMs) and implantable cardioverter defibrillators (ICDs) together with the occurrence of post-implantation IE-events in the period from 2000–2012. Included were 43 048 first-time PM/ICD recipients. Total follow-up time was 168 343 person-years (PYs). The incidence rate (per 1000 PYs) of IE in PM was 2.1 (95% confidence interval [CI]: 1.7–2.6) for single chamber devices and 6.2 (95% CI: 4.5–8.7) for cardiac resynchronization therapy (CRT); similarly, the rate of IE in ICD was 3.7 (95% CI: 2.9–4.7) in single chamber devices and 6.3 (95% CI: 4.4–9.0) in CRT. In multivariable analysis, increased PM complexity served as independent risk factor for IE {dual chamber PM [hazard ratio (HR) 1.39; 95% CI: 1.07–1.80] and CRT [HR: 1.84; 95% CI: 1.20–2.84]}. During follow-up, generator replacement (HR: 2.79; 95% CI: 1.87–4.17) and lead revision (HR: 4.33; 95% CI: 3.25–5.78) in PMs were associated with increased risk. Corresponding estimates in ICDs were 2.49 (95% CI: 1.28–4.86) and 6.58 (95% CI: 4.49–9.63). Risk of death after IE was significantly increased in PM and ICD with HRs of 1.56 (95% CI: 1.33–1.82) and 2.63 (95% CI: 2.00–3.48), respectively.
The risk of IE increased with increasing PM complexity. Other important risk factors were subsequent generator replacement and lead revision. IE was associated with an increased risk of mortality in the area of CIED.
Introduction
What’s new?
We sought to identify risk factors for infective endocarditis (IE) and to assess the risk of subsequent mortality in IE among first time cardiac implantable electronic device (CIED) recipients across the country.
The incidence of IE was closely linked to the complexity of CIED and was notably high the first year after implantation.
Multivariable analysis identified several risk factors for IE of which pacemaker complexity and subsequent generator exchange and lead revision were important risk factors.
Irrespective of device type, IE was associated with increased mortality risk.
Methods
Data collection
Since 1968, each Danish citizen has been issued a unique 10-digit number (CPR), enabling cross-linkage of individual-level data from a number of national registries. For this study, data were collected systematically from the Civil Registration System (CRS), the National Patient Registry (NPR), and the Registry of Medicinal Products Statistics.
Established in 1968, the CRS maintains information on citizens regarding their CPR, sex, emigrations, and vital status. The NPR contains data on all somatic inpatient admissions including admission and discharge information since 1978. When discharged from a hospital, each patient is issued one primary, and if appropriate, additional secondary diagnoses, according to International Classification of Diseases, 10th revision (ICD-10) since 1994. This registry also contains data on dates of surgery and procedure codes. Based on national and international classification systems, the NOMESCO classification of surgical procedures (NCSP) has been used to code all surgical procedures since 1996. Since 1995, the Registry of Medicinal Product Statistics has recorded all data concerning prescriptions dispensed from Danish pharmacies, including the date, quantity, strength, and formulation of each therapeutic drug, which is categorized according to the Anatomical Therapeutic Chemical (ATC) classification system.
Study population
Survivors of first time implanted transvenous permanent pacemakers (PM) or implantable cardioverter defibrillators (ICD) were included from 1 January 2000 to 31 December 2012. Patients with a prosthetic heart valve (NCSP codes KFMD, KFKD, KFJF, and KFGE [N = 888]) were excluded. The study population was identified on procedure codes of implanted CIEDs; therefore, additional CIED-related procedures registered on the same day, such as a lead implantation, that could potentially reclassify the type of CIED, were ignored. Details for the identification of patients are found in the supplementary appendix, see Supplementary material online, Table S1.
Comorbidity, concomitant medications, and generator replacement/lead revision
Comorbidity was defined as medical conditions that had occurred and were evident within 2 years before index implantation. Concomitant medications at admission were defined as prescribed medications used within 6 months of index admission. Device manipulations during follow-up were classified into two subgroups: (i) generator exchange and/or downgrade without simultaneous lead revision (hereafter generator replacement) or (ii) lead revisions requiring at least lead manipulation or interim temporary pacing. The rationale behind this approach was to identify procedures that required vascular access. ICD-10 and ATC codes used for identification of comorbidities and concomitant medications are shown in supplementary appendix, see Supplementary material online, Table S1.
Follow-up and outcomes
Follow-up began after hospital discharge until time of emigration, time of death, or the end of the study (31-12-2012), whichever came first. Consequently, patients who eventually underwent device replacement, revision, or removal remained in the study. The clinical endpoints were post-discharge hospitalization with IE, irrespective of localization, and all-cause mortality. Only the first occurrence of IE was accounted for. The ICD-10 codes indicative of IE are listed in the supplementary appendix (see Supplementary material online, Table S1).
Statistical analysis
Categorical variables were presented as frequencies (percentage) and continuous variables were presented as means and standard deviations.
We used two different models to assess the primary outcome of IE and subsequent mortality. First, we estimated the cumulative incidence of IE against time by means of the Aalen-Johansen estimator that accounts for competing risk of death and right-censored data. Crude incidence rates of IE were calculated as the number of events per 1000 person-years (PYs) of follow-up. In addition, early (< 366 days) and late (> 365 days) incidence rates were reported as well. The impact of selected baseline covariates on IE was also reported in univariable and multivariable Cox regression analyses and presented as a hazard ratio (HR) with 95% confidence interval (CI). Additionally, risk stratification by age groups (51–60 years, 61–70 years, 71–80 years, and >80 years), type of CIED, and time period (2005–2008 and 2009–2012) in relation to age 50 or younger, single chamber PM, and implantation between 2000–2004 were also performed by assigning each age group, CIED type, and time period a specific, consecutive value. In the subgroup of patients who underwent subsequent generator replacement or lead revision, four mutually exclusive variables (generator replacement in PM and ICD and lead revision in PM and ICD) were constructed, which were treated as time-dependent covariates. A multivariable analysis without the adjustment for subsequent device replacement/revision was also performed.
Second, we estimated the risk of mortality following IE by means of Cox regression models with post-implantation IE as time-dependent covariate and expressed as HR with 95% CI separately for PMs and ICDs. The mortality analyses were adjusted for the same set of baseline covariates as in the analyses of risk factors. To illustrate differences in the mortality risk of different device systems in relation to a single chamber PM, a subgroup analysis based on patients with IE was performed.
Using a fully adjusted model, we assessed and found no significant interaction between age groups, gender, type of device, or time period related to IE. Furthermore, the assumptions of proportional hazards of age groups, gender, type of device, and time period were tested and found valid. All statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, North Carolina, USA) and R statistic software (version 3.1.1). P values of <0.05 were considered statistically significant.
Ethics
The study was approved by the Danish Data Protection Agency Ref.no 2007-58-0015/ local ref. GEH-2014-015 I-Suite no: 02733, and data were processed without disclosure of personal information. By law, Danish registry studies are exempt from the ethical approval.
Results
Study population
We included 43 048 first-time CIED implantations of which PMs and ICDs constituted 34 801 (80.8%) and 8247 (19.2%), respectively (Table 1). The most commonly implanted PM system was the dual chamber (59.2%), followed by the single chamber (29.0%), and CRT (4.5%). The most commonly implanted ICD system was the single chamber (55.4%), followed by the dual chamber (23.1%) and CRT (21.2%; Tables1and see Supplementary material online, Table S2). During follow-up, generator replacement and lead revision took place in 10.1 and 5.6% of the PM recipients, respectively. The corresponding percentages in ICD recipients were 11.7 and 12.8%.
Baseline characteristics of patients with first-time cardiac implantable electronic device implantations separately for each device type in Denmark between 2000 and 2012
| Patient and procedure related characteristics . | PM . | ICD . |
|---|---|---|
| 34 801(100) | 8247(100) | |
| Age [mean (SD)] years | 75 (13) | 62 (13) |
| Males | 19378 (55.7) | 6622 (80.3) |
| Type of CIED | ||
| Single chamber | 10 097 (29.0) | 4570 (55.4) |
| Dual chamber | 20 597 (59.2) | 1907 (23.1) |
| CRT | 1567 (4.5) | 1750 (21.2) |
| Unclassified | 2540 (7.3) | 20 (0.2) |
| Clinical arrhythmia | ||
| Cardiac arrest | 171(0.5) | 1052 (12.8) |
| Syncope | 5595 (16.1) | 741 (9.0) |
| VT/VF | 547 (1.6) | 2355 (28.6) |
| Advanced atrioventricular block | 6249 (18.0) | 128 (1.6) |
| Atrial fibrillation/flutter | 10 347 (29.7) | 1553 (18.8) |
| Subsequent replacement/revisiona | ||
| Replacement | 3531 (10.1) | 964 (11.7) |
| Generator exchange | 3 511 (10.1) | 963 (11.7) |
| Downgrade | 20 (<0.1) | <3b |
| Lead revisionc | 1961 (5.6) | 1056 (12.8) |
| Extraction | 389 (1.1) | 251 (3.0) |
| Implantation | 449 (1.3) | 373 (4.5) |
| Upgrade | 491 (1.4) | 302 (3.7) |
| Revision | 430 (1.2) | 261 (3.2) |
| Displacement | 437 (1.3) | 98 (1.2) |
| Exchange | 6 (<0.1) | 0 (0.0) |
| Temporary pacingd | 98 (0.3) | 20 (0.2) |
| Generator exchangee | 375 (1.1) | 210 (2.5) |
| Downgradee | <3b | 0 (0.0) |
| Time to replacement [mean (SD)] years | 6.9 (2.4) | 5.6 (2.2) |
| Time to revision [mean (SD)] years | 2.3 (2.8) | 2.2 (2.2) |
| Prior infection | ||
| Infective endocarditis | 120 (0.3) | 23 (0.3) |
| Cardiovascular | ||
| Ischemic heart disease | 8305 (23.9) | 5068 (61.5) |
| Heart failure | 4903 (14.1) | 3730 (45.2) |
| PCI | 1059 (3.0) | 1536 (18.6) |
| CABG | 368 (1.1) | 706 (8.6) |
| Valve disease | 2198 (6.3) | 363 (4.4) |
| Diabetesf | 5090 (14.6) | 1419 (17.2) |
| Dialysis | 212 (0.6) | 42 (0.5) |
| Other | ||
| Cancer | 1956 (5.6) | 272 (3.3) |
| Medication | ||
| VKA | 6663 (19.1) | 1646 (20.0) |
| Patient and procedure related characteristics . | PM . | ICD . |
|---|---|---|
| 34 801(100) | 8247(100) | |
| Age [mean (SD)] years | 75 (13) | 62 (13) |
| Males | 19378 (55.7) | 6622 (80.3) |
| Type of CIED | ||
| Single chamber | 10 097 (29.0) | 4570 (55.4) |
| Dual chamber | 20 597 (59.2) | 1907 (23.1) |
| CRT | 1567 (4.5) | 1750 (21.2) |
| Unclassified | 2540 (7.3) | 20 (0.2) |
| Clinical arrhythmia | ||
| Cardiac arrest | 171(0.5) | 1052 (12.8) |
| Syncope | 5595 (16.1) | 741 (9.0) |
| VT/VF | 547 (1.6) | 2355 (28.6) |
| Advanced atrioventricular block | 6249 (18.0) | 128 (1.6) |
| Atrial fibrillation/flutter | 10 347 (29.7) | 1553 (18.8) |
| Subsequent replacement/revisiona | ||
| Replacement | 3531 (10.1) | 964 (11.7) |
| Generator exchange | 3 511 (10.1) | 963 (11.7) |
| Downgrade | 20 (<0.1) | <3b |
| Lead revisionc | 1961 (5.6) | 1056 (12.8) |
| Extraction | 389 (1.1) | 251 (3.0) |
| Implantation | 449 (1.3) | 373 (4.5) |
| Upgrade | 491 (1.4) | 302 (3.7) |
| Revision | 430 (1.2) | 261 (3.2) |
| Displacement | 437 (1.3) | 98 (1.2) |
| Exchange | 6 (<0.1) | 0 (0.0) |
| Temporary pacingd | 98 (0.3) | 20 (0.2) |
| Generator exchangee | 375 (1.1) | 210 (2.5) |
| Downgradee | <3b | 0 (0.0) |
| Time to replacement [mean (SD)] years | 6.9 (2.4) | 5.6 (2.2) |
| Time to revision [mean (SD)] years | 2.3 (2.8) | 2.2 (2.2) |
| Prior infection | ||
| Infective endocarditis | 120 (0.3) | 23 (0.3) |
| Cardiovascular | ||
| Ischemic heart disease | 8305 (23.9) | 5068 (61.5) |
| Heart failure | 4903 (14.1) | 3730 (45.2) |
| PCI | 1059 (3.0) | 1536 (18.6) |
| CABG | 368 (1.1) | 706 (8.6) |
| Valve disease | 2198 (6.3) | 363 (4.4) |
| Diabetesf | 5090 (14.6) | 1419 (17.2) |
| Dialysis | 212 (0.6) | 42 (0.5) |
| Other | ||
| Cancer | 1956 (5.6) | 272 (3.3) |
| Medication | ||
| VKA | 6663 (19.1) | 1646 (20.0) |
Categorical variables are presented as numbers and percentages in parentheses. Continuous variables are shown as means with standard deviation.
CIED, cardiac implantable electronic device; PM, permanent pacemaker; ICD, implantable cardioverter defibrillator; SD, standard deviation; CRT, cardiac resynchronization therapy; VT/VF, ventricular tachycardia/fibrillation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; VKA, vitamin K antagonist.
Performed during follow-up.
Indicates 1 or 2 observations.
The procedures are not mutually exclusive.
Interim temporary pacing.
Lead manipulations combined with generator exchange or downgrade.
With or without medication.
Baseline characteristics of patients with first-time cardiac implantable electronic device implantations separately for each device type in Denmark between 2000 and 2012
| Patient and procedure related characteristics . | PM . | ICD . |
|---|---|---|
| 34 801(100) | 8247(100) | |
| Age [mean (SD)] years | 75 (13) | 62 (13) |
| Males | 19378 (55.7) | 6622 (80.3) |
| Type of CIED | ||
| Single chamber | 10 097 (29.0) | 4570 (55.4) |
| Dual chamber | 20 597 (59.2) | 1907 (23.1) |
| CRT | 1567 (4.5) | 1750 (21.2) |
| Unclassified | 2540 (7.3) | 20 (0.2) |
| Clinical arrhythmia | ||
| Cardiac arrest | 171(0.5) | 1052 (12.8) |
| Syncope | 5595 (16.1) | 741 (9.0) |
| VT/VF | 547 (1.6) | 2355 (28.6) |
| Advanced atrioventricular block | 6249 (18.0) | 128 (1.6) |
| Atrial fibrillation/flutter | 10 347 (29.7) | 1553 (18.8) |
| Subsequent replacement/revisiona | ||
| Replacement | 3531 (10.1) | 964 (11.7) |
| Generator exchange | 3 511 (10.1) | 963 (11.7) |
| Downgrade | 20 (<0.1) | <3b |
| Lead revisionc | 1961 (5.6) | 1056 (12.8) |
| Extraction | 389 (1.1) | 251 (3.0) |
| Implantation | 449 (1.3) | 373 (4.5) |
| Upgrade | 491 (1.4) | 302 (3.7) |
| Revision | 430 (1.2) | 261 (3.2) |
| Displacement | 437 (1.3) | 98 (1.2) |
| Exchange | 6 (<0.1) | 0 (0.0) |
| Temporary pacingd | 98 (0.3) | 20 (0.2) |
| Generator exchangee | 375 (1.1) | 210 (2.5) |
| Downgradee | <3b | 0 (0.0) |
| Time to replacement [mean (SD)] years | 6.9 (2.4) | 5.6 (2.2) |
| Time to revision [mean (SD)] years | 2.3 (2.8) | 2.2 (2.2) |
| Prior infection | ||
| Infective endocarditis | 120 (0.3) | 23 (0.3) |
| Cardiovascular | ||
| Ischemic heart disease | 8305 (23.9) | 5068 (61.5) |
| Heart failure | 4903 (14.1) | 3730 (45.2) |
| PCI | 1059 (3.0) | 1536 (18.6) |
| CABG | 368 (1.1) | 706 (8.6) |
| Valve disease | 2198 (6.3) | 363 (4.4) |
| Diabetesf | 5090 (14.6) | 1419 (17.2) |
| Dialysis | 212 (0.6) | 42 (0.5) |
| Other | ||
| Cancer | 1956 (5.6) | 272 (3.3) |
| Medication | ||
| VKA | 6663 (19.1) | 1646 (20.0) |
| Patient and procedure related characteristics . | PM . | ICD . |
|---|---|---|
| 34 801(100) | 8247(100) | |
| Age [mean (SD)] years | 75 (13) | 62 (13) |
| Males | 19378 (55.7) | 6622 (80.3) |
| Type of CIED | ||
| Single chamber | 10 097 (29.0) | 4570 (55.4) |
| Dual chamber | 20 597 (59.2) | 1907 (23.1) |
| CRT | 1567 (4.5) | 1750 (21.2) |
| Unclassified | 2540 (7.3) | 20 (0.2) |
| Clinical arrhythmia | ||
| Cardiac arrest | 171(0.5) | 1052 (12.8) |
| Syncope | 5595 (16.1) | 741 (9.0) |
| VT/VF | 547 (1.6) | 2355 (28.6) |
| Advanced atrioventricular block | 6249 (18.0) | 128 (1.6) |
| Atrial fibrillation/flutter | 10 347 (29.7) | 1553 (18.8) |
| Subsequent replacement/revisiona | ||
| Replacement | 3531 (10.1) | 964 (11.7) |
| Generator exchange | 3 511 (10.1) | 963 (11.7) |
| Downgrade | 20 (<0.1) | <3b |
| Lead revisionc | 1961 (5.6) | 1056 (12.8) |
| Extraction | 389 (1.1) | 251 (3.0) |
| Implantation | 449 (1.3) | 373 (4.5) |
| Upgrade | 491 (1.4) | 302 (3.7) |
| Revision | 430 (1.2) | 261 (3.2) |
| Displacement | 437 (1.3) | 98 (1.2) |
| Exchange | 6 (<0.1) | 0 (0.0) |
| Temporary pacingd | 98 (0.3) | 20 (0.2) |
| Generator exchangee | 375 (1.1) | 210 (2.5) |
| Downgradee | <3b | 0 (0.0) |
| Time to replacement [mean (SD)] years | 6.9 (2.4) | 5.6 (2.2) |
| Time to revision [mean (SD)] years | 2.3 (2.8) | 2.2 (2.2) |
| Prior infection | ||
| Infective endocarditis | 120 (0.3) | 23 (0.3) |
| Cardiovascular | ||
| Ischemic heart disease | 8305 (23.9) | 5068 (61.5) |
| Heart failure | 4903 (14.1) | 3730 (45.2) |
| PCI | 1059 (3.0) | 1536 (18.6) |
| CABG | 368 (1.1) | 706 (8.6) |
| Valve disease | 2198 (6.3) | 363 (4.4) |
| Diabetesf | 5090 (14.6) | 1419 (17.2) |
| Dialysis | 212 (0.6) | 42 (0.5) |
| Other | ||
| Cancer | 1956 (5.6) | 272 (3.3) |
| Medication | ||
| VKA | 6663 (19.1) | 1646 (20.0) |
Categorical variables are presented as numbers and percentages in parentheses. Continuous variables are shown as means with standard deviation.
CIED, cardiac implantable electronic device; PM, permanent pacemaker; ICD, implantable cardioverter defibrillator; SD, standard deviation; CRT, cardiac resynchronization therapy; VT/VF, ventricular tachycardia/fibrillation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; VKA, vitamin K antagonist.
Performed during follow-up.
Indicates 1 or 2 observations.
The procedures are not mutually exclusive.
Interim temporary pacing.
Lead manipulations combined with generator exchange or downgrade.
With or without medication.
Incidence of IE
Total follow-up time was 168 343 PYs, during which 519 (1.2%) cases of IE were identified (Table 2). The crude incidence rate of IE was greater for ICDs [4.3 per 1000 PYs (95%: 3.6–5.1)] than PMs [2.8 per 1000 PYs (95%: 2.5–3.1)]. When accounting for competing risk of death, the 5-year risk of IE was higher in ICDs [1.7% (1.3–2.0)] than in PMs [1.0% (0.9–1.2)]; Figure1A and B and Table2). The incidence rate per 1000 PYs in PM recipients increased from 2.1 (95%: 1.7–2.6) in single chamber devices to 6.2 (95%: 4.5–8.7) in CRT, while in ICD recipients the rate increased from 3.7 (95%: 2.9–4.7) in single chamber devices to 6.3 (95%: 4.4–9.0) in CRT. The cumulative incidence showed similar trends. The incidence rate was generally higher in early onset IE than in late onset IE.
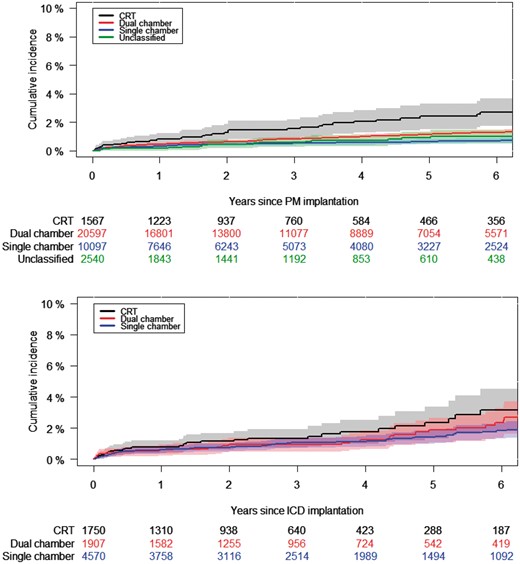
Cumulative incidence of IE following first-time pacemaker (A) and ICD (B) systems with 95% CIs. Cumulative incidence by means of Aalen–Johansen estimator. PM, pacemaker; ICD, implantable cardioverter defibrillator.
Crude incidence rate and cumulative incidence of infective endocarditis after first time cardiac implantable electronic device implantation
| Device . | PYs . | Events (%) . | Incidence rate pr. 1000 PYs (95% CI) . | Five years cumulative incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early-onset . | Late-onset . | Overall . | ||||
| CIED | 168 343 | 519 (1.2) | 5.0 (4.3–5.7) | 1.9 (1.7–2.1) | 3.1 (2.8–3.4) | 1.1 (1.0–1.3) |
| PM | ||||||
| Overall | 138 037 | 388 (1.1) | 4.5 (3.8–5.3) | 1.8 (1.6–2.0) | 2.8 (2.5–3.1) | 1.0 (0.9–1.2) |
| Single chamber | 38 777 | 80 (0.8) | 3.6 (2.5–5.1) | 1.2 (0.9–1.6) | 2.1 (1.7–2.6) | 0.7 (0.5–0.8) |
| Dual chamber | 85 519 | 253 (1.2) | 4.9 (4.0-6.0) | 1.8 (1.6–2.2) | 3.0 (2.6–3.3) | 1.2 (1.0–1.3) |
| CRT | 5607 | 35 (2.2) | 8.7 (4-9–15.3) | 4.0 (2.6-6.0) | 6.2 (4.5–8.7) | 2.4 (1.5–3.3) |
| Unclassified | 8133 | 20 (0.8) | 1.9 (0.7–5.0) | 1.9 (1.1–3.0) | 2.5 (1.6–3.8) | 0.9 (0.4–1.3) |
| ICD | ||||||
| Overall | 30 306 | 131 (1.6) | 6.9 (5.2–9.0) | 2.6 (2.1–3.2) | 4.3 (3.6–5.1) | 1.7 (1.3–2.0) |
| Single chamber | 18 263 | 67 (1.5) | 6.5 (4.5–9.4) | 2.1 (1.6–2.9) | 3.7 (2.9–4.7) | 1.4 (1.0–1.8) |
| Dual chamber | 7241 | 34 (1.8) | 6.3 (3.5–11.3) | 3.1 (2.1–4.7) | 4.7 (3.4–6.6) | 1.9 (1.1–2.7) |
| CRT | 4782 | 30 (1.7) | 8.5 (4.9–14.7) | 3.4 (2.1-5.5) | 6.3 (4.4–9.0) | 2.4 (1.4–3.4) |
| Unclassified | 19 | 0 (0) | N.A. | N.A. | N.A. | N.A. |
| Device . | PYs . | Events (%) . | Incidence rate pr. 1000 PYs (95% CI) . | Five years cumulative incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early-onset . | Late-onset . | Overall . | ||||
| CIED | 168 343 | 519 (1.2) | 5.0 (4.3–5.7) | 1.9 (1.7–2.1) | 3.1 (2.8–3.4) | 1.1 (1.0–1.3) |
| PM | ||||||
| Overall | 138 037 | 388 (1.1) | 4.5 (3.8–5.3) | 1.8 (1.6–2.0) | 2.8 (2.5–3.1) | 1.0 (0.9–1.2) |
| Single chamber | 38 777 | 80 (0.8) | 3.6 (2.5–5.1) | 1.2 (0.9–1.6) | 2.1 (1.7–2.6) | 0.7 (0.5–0.8) |
| Dual chamber | 85 519 | 253 (1.2) | 4.9 (4.0-6.0) | 1.8 (1.6–2.2) | 3.0 (2.6–3.3) | 1.2 (1.0–1.3) |
| CRT | 5607 | 35 (2.2) | 8.7 (4-9–15.3) | 4.0 (2.6-6.0) | 6.2 (4.5–8.7) | 2.4 (1.5–3.3) |
| Unclassified | 8133 | 20 (0.8) | 1.9 (0.7–5.0) | 1.9 (1.1–3.0) | 2.5 (1.6–3.8) | 0.9 (0.4–1.3) |
| ICD | ||||||
| Overall | 30 306 | 131 (1.6) | 6.9 (5.2–9.0) | 2.6 (2.1–3.2) | 4.3 (3.6–5.1) | 1.7 (1.3–2.0) |
| Single chamber | 18 263 | 67 (1.5) | 6.5 (4.5–9.4) | 2.1 (1.6–2.9) | 3.7 (2.9–4.7) | 1.4 (1.0–1.8) |
| Dual chamber | 7241 | 34 (1.8) | 6.3 (3.5–11.3) | 3.1 (2.1–4.7) | 4.7 (3.4–6.6) | 1.9 (1.1–2.7) |
| CRT | 4782 | 30 (1.7) | 8.5 (4.9–14.7) | 3.4 (2.1-5.5) | 6.3 (4.4–9.0) | 2.4 (1.4–3.4) |
| Unclassified | 19 | 0 (0) | N.A. | N.A. | N.A. | N.A. |
Early- and late-onset infective endocarditis are defined as the occurrence of infective endocarditis within < 366 days and > 365 days after implantation, respectively. Five-year cumulative incidence is calculated using the Aalen-Johansen estimator.
PYs, person-years; CI, confidence interval; CIED, cardiac implantable electronic defibrillator; PM, pacemaker; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; N.A., not available.
Crude incidence rate and cumulative incidence of infective endocarditis after first time cardiac implantable electronic device implantation
| Device . | PYs . | Events (%) . | Incidence rate pr. 1000 PYs (95% CI) . | Five years cumulative incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early-onset . | Late-onset . | Overall . | ||||
| CIED | 168 343 | 519 (1.2) | 5.0 (4.3–5.7) | 1.9 (1.7–2.1) | 3.1 (2.8–3.4) | 1.1 (1.0–1.3) |
| PM | ||||||
| Overall | 138 037 | 388 (1.1) | 4.5 (3.8–5.3) | 1.8 (1.6–2.0) | 2.8 (2.5–3.1) | 1.0 (0.9–1.2) |
| Single chamber | 38 777 | 80 (0.8) | 3.6 (2.5–5.1) | 1.2 (0.9–1.6) | 2.1 (1.7–2.6) | 0.7 (0.5–0.8) |
| Dual chamber | 85 519 | 253 (1.2) | 4.9 (4.0-6.0) | 1.8 (1.6–2.2) | 3.0 (2.6–3.3) | 1.2 (1.0–1.3) |
| CRT | 5607 | 35 (2.2) | 8.7 (4-9–15.3) | 4.0 (2.6-6.0) | 6.2 (4.5–8.7) | 2.4 (1.5–3.3) |
| Unclassified | 8133 | 20 (0.8) | 1.9 (0.7–5.0) | 1.9 (1.1–3.0) | 2.5 (1.6–3.8) | 0.9 (0.4–1.3) |
| ICD | ||||||
| Overall | 30 306 | 131 (1.6) | 6.9 (5.2–9.0) | 2.6 (2.1–3.2) | 4.3 (3.6–5.1) | 1.7 (1.3–2.0) |
| Single chamber | 18 263 | 67 (1.5) | 6.5 (4.5–9.4) | 2.1 (1.6–2.9) | 3.7 (2.9–4.7) | 1.4 (1.0–1.8) |
| Dual chamber | 7241 | 34 (1.8) | 6.3 (3.5–11.3) | 3.1 (2.1–4.7) | 4.7 (3.4–6.6) | 1.9 (1.1–2.7) |
| CRT | 4782 | 30 (1.7) | 8.5 (4.9–14.7) | 3.4 (2.1-5.5) | 6.3 (4.4–9.0) | 2.4 (1.4–3.4) |
| Unclassified | 19 | 0 (0) | N.A. | N.A. | N.A. | N.A. |
| Device . | PYs . | Events (%) . | Incidence rate pr. 1000 PYs (95% CI) . | Five years cumulative incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early-onset . | Late-onset . | Overall . | ||||
| CIED | 168 343 | 519 (1.2) | 5.0 (4.3–5.7) | 1.9 (1.7–2.1) | 3.1 (2.8–3.4) | 1.1 (1.0–1.3) |
| PM | ||||||
| Overall | 138 037 | 388 (1.1) | 4.5 (3.8–5.3) | 1.8 (1.6–2.0) | 2.8 (2.5–3.1) | 1.0 (0.9–1.2) |
| Single chamber | 38 777 | 80 (0.8) | 3.6 (2.5–5.1) | 1.2 (0.9–1.6) | 2.1 (1.7–2.6) | 0.7 (0.5–0.8) |
| Dual chamber | 85 519 | 253 (1.2) | 4.9 (4.0-6.0) | 1.8 (1.6–2.2) | 3.0 (2.6–3.3) | 1.2 (1.0–1.3) |
| CRT | 5607 | 35 (2.2) | 8.7 (4-9–15.3) | 4.0 (2.6-6.0) | 6.2 (4.5–8.7) | 2.4 (1.5–3.3) |
| Unclassified | 8133 | 20 (0.8) | 1.9 (0.7–5.0) | 1.9 (1.1–3.0) | 2.5 (1.6–3.8) | 0.9 (0.4–1.3) |
| ICD | ||||||
| Overall | 30 306 | 131 (1.6) | 6.9 (5.2–9.0) | 2.6 (2.1–3.2) | 4.3 (3.6–5.1) | 1.7 (1.3–2.0) |
| Single chamber | 18 263 | 67 (1.5) | 6.5 (4.5–9.4) | 2.1 (1.6–2.9) | 3.7 (2.9–4.7) | 1.4 (1.0–1.8) |
| Dual chamber | 7241 | 34 (1.8) | 6.3 (3.5–11.3) | 3.1 (2.1–4.7) | 4.7 (3.4–6.6) | 1.9 (1.1–2.7) |
| CRT | 4782 | 30 (1.7) | 8.5 (4.9–14.7) | 3.4 (2.1-5.5) | 6.3 (4.4–9.0) | 2.4 (1.4–3.4) |
| Unclassified | 19 | 0 (0) | N.A. | N.A. | N.A. | N.A. |
Early- and late-onset infective endocarditis are defined as the occurrence of infective endocarditis within < 366 days and > 365 days after implantation, respectively. Five-year cumulative incidence is calculated using the Aalen-Johansen estimator.
PYs, person-years; CI, confidence interval; CIED, cardiac implantable electronic defibrillator; PM, pacemaker; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; N.A., not available.
Risk factors for IE after cardiac implantable electronic device implantation
Unadjusted risk factors are presented in the supplementary appendix (see Supplementary material online, Figure S1). In a fully adjusted model with age ≤ 50 years, single PM, and the time period of 2000–2004 serving as reference variables, several important risk factors for IE after CIED implantation were identified (Figure 2). Increasing PM complexity was associated with an increased risk of IE with a nearly doubled risk increase with CRT. In terms of subsequent device-related procedures, generator replacement, and in particular lead revision, were by far the strongest risk factors for IE. Male gender, comorbidity, and vitamin K antagonist (VKA) treatment were also associated with increased risk. Particularly, prior IE, dialysis, valve disease, and diabetes mellitus (DM) were all significantly associated with high risk of IE. In contrast, coronary artery bypass grafting (CABG) was associated with a lower risk of IE. In a multivariable analysis without the adjustment of subsequent device manipulations, a similar risk factor pattern was noted apart from ICD, for which the dual chamber device and CRT were significantly associated with increased risk (see Supplementary material online, Figure S2).
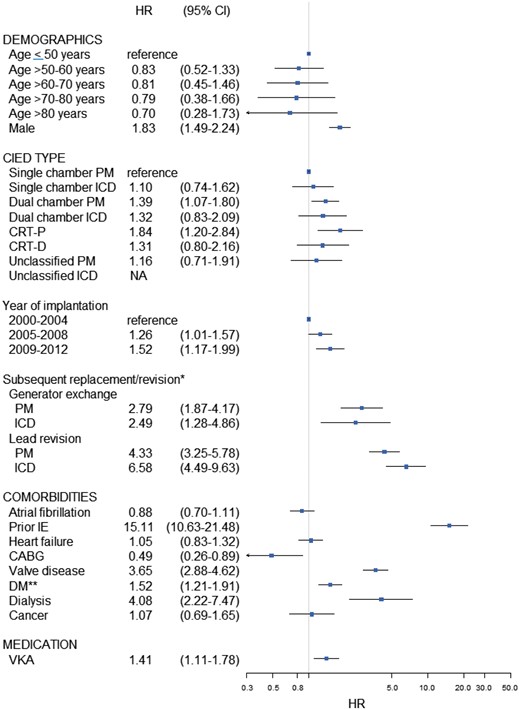
Risk factors of IE with hazard ratios and 95% CI—multivariable analysis. Multivariable analysis adjusted for age groups, gender, type of CIED, calendar time subgroups, atrial fibrillation, prior IE, heart failure, CABG, valve disease, diabetes mellitus, dialysis, cancer, VKA treatment, and subsequent device manipulations as time-dependent covariates. *Performed during follow-up; **With and without medication. HR, hazard ratio; CI, confidence interval; CIED, cardiac implantable electronic device; PM, pacemaker; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; IE, infective endocarditis; CABG, coronary artery bypass grafting; DM, diabetes mellitus; VKA, vitamin K antagonist.
Mortality
The total number of deaths in PMs and ICDs were 14 707 (42.3%) and 1708 (20.7%), respectively. Compared to patients without IE, patients with IE had higher mortality, particularly ICD recipients [HR 2.63 (95% CI, 2.00–3.48)] but also PM recipients [HR 1.56 (95% CI, 1.33–1.82)]. A subgroup analysis limited to patients with IE showed that dual-chamber ICDs and CRT had a greater mortality risk in comparison to single chamber PMs (see Supplementary material online, Table S3).
Discussion
Our study examined the risk of IE and the subsequent mortality risk in more than 40 000 first time Danish CIED patients over a 13-year period. The main results were as follows: (i) The incidence of IE increased with increasing device complexity and was particularly high during the first year; (ii) The risk of IE increased with PM complexity; (iii) Subsequent device manipulations and comorbidities were associated with risk of IE; and (iv) IE was associated with an up to three-fold increased risk of death.
Incidence
We found that the incidence rates of IE in first time PM and ICD recipients were 2.8 and 4.3 per 1000 PYs, respectively. In fact, a dose-response relationship between the number of leads and the incidence of IE was noted, trends that were also confirmed by means of a competing risks approach. In comparison, a French study on IE among de-novo and replacement PM recipients in six regions reported an incidence rate of 0.55/1000 PM recipients per year.8 A similar study reported an incidence rate of 1.14/1000 device years for IE or pocket infections with bacteraemia among patients with CIEDs in Minnesota.10 In terms of CIED infection (pocket infection and/or systemic infection/endocarditis), a Danish study using nationwide data from the DPIR showed that the incidence rate of PM infection following de-novo placement and replacement of CIEDs was 1.82/1000 and 5.32/1000 device-years, respectively5; an even higher incidence rate of ICD infection (8.9/1000 device-years) was observed in Minnesota.10 However, the lack of uniform study design across the study populations, the fact that IE only constitutes 21–22% of CIED infection,11,12 and the lack of information on pocket infections and bacteraemia in the current study prevent a meaningful comparison. Nevertheless, providing an overall incidence rate is clearly insufficient, as the estimates of prevalence vary considerably within each device subtype. A similar pattern was observed in relation to the onset of IE. Notably, the incidence rate of early-onset IE was higher than late-onset IE, which is consistent with other studies on CIED infection.5,13–15 This finding suggests that particularly early infection could be, to a certain extent, a result of contamination at the time of implantation, because it has been demonstrated that perioperative contamination can cause delayed CIED infection.16
Independent risk factors
We demonstrated that PM complexity was a key risk factor for IE, which is supported by previous studies.17–19 Implantation of complex devices leads to increased procedure time, which has been linked to increased risk of CIED related infection.20 Additionally, other factors might also contribute to this finding, including the number of leads and patient comorbidities. Also of importance in this regards is the presence of PM or ICD, since studies have found a greater risk for IE with ICDs.20,21 This contradicts our finding that there was no increased risk of IE with ICDs. Our results suggested that the likely reason for this difference was a loss of statistical power after adjustment for subsequent device manipulations, especially lead revisions, which occurred more frequently in ICDs. Nonetheless, special consideration should be given before selecting a given device class.
In terms of device-related procedures, we found that generator replacement and lead revision were the strongest risk factors for IE. Replacement procedures, including both generator and lead manipulations, have been linked to an increased risk of CIED infections.5,13,15,18,22,23 Interestingly, others have suggested that generator replacement-induced risk is primarily driven by pocket infection rather than systemic infection.23 This might explain why generator replacement had a lower risk estimate than procedures that required lead revision or interim temporary pacing, which are well-known risk factors for infection.22 Another potential contributing factor is that generator replacement and lead revision most likely represent two different clinical settings. A generator exchange is most likely anticipated and elective, while lead revision is more likely to be unanticipated and emergent with little time to optimize the patient and procedure-related factors prior to implantation. Our findings highlight the need for further efforts to reduce infections associated with lead revisions.
Several host-related risk factors were identified. Similar to other studies,5,15 we found a strong gender disparity with males having a greater risk of infection. The underlying mechanism for this disparity is most likely unmeasured confounding. This study also demonstrated that comorbidities have a significant impact on the risk for IE. Prior heart valve disease, including prior IE, was associated with an increased risk of IE, a finding that is in agreement with other studies that have found prosthetic valves and prior CIED infection to be risk factors for CIED infection.15,21,24 Additionally, chronic conditions with impaired immune response (dialysis and diabetes mellitus) were associated with an increased risk of IE, and this confirms previous studies.12,13,20,24–27 The low risk of IE observed in CABG patients may be ascribed to patient selection for the procedure, as frail patients are less likely to be selected to undergo CABG. Given that the number of patients with significant comorbidities is rising, the assessment of comorbidities will become more important in the future.
This present study reported that VKA was associated with an increased risk of IE, which is consistent with prior studies.13,24,25 It is believed that VKA increases the risk of post-operative haematoma, which is associated with an increased risk of infection.28 Thus, it is paramount to medically optimize patients prior to implantation.
Mortality
Mortality after CIED infection is high, and mortality following IE appears to be greater than following pocket infection.29 Even after guideline-recommended explantation,30 the mortality associated with CIED-related IE remains high.9,11,31 We found an almost two- and three-fold risk of mortality after IE in PMs and ICDs, respectively. In comparison, a previous study found that CIED infection complicated by IE was associated with a 1.7 fold increased risk for long-term death, although no separate estimate for each device system was provided.32 Others have found a difference in mortality depending on the type of device system infected, which includes pocket infections, systemic infection/endocarditis, or a combination. In Medicare beneficiaries, long-term mortality was higher in PM-related infections as compared to ICD and CRT-D.33 Clearly direct comparison with our nationwide study on IE after CIED is difficult, but we also found that mortality varies depending on the type of device infected. Although no clear trend was observed, mortality was higher in dual chamber ICDs and CRT than in single chamber PMs. Several explanations for the variability in mortality risk have been provided, including differences in infection severity, management, and incomplete risk adjustment,33 which we think are likely underlying reasons for the observed results in our study.
Clinical implications
Measuring the burden of subsequent infection is essential and ought to be understood before undergoing implantation. Moreover, tools should be readily available to facilitate the identification of patients at risk for experiencing an adverse event, so that necessary precautions can be taken to reduce this risk. The present study has provided knowledge that can be useful in clinical practice in order to optimize patient selection and health before implantation and to facilitate close monitoring of at risk patients, especially the first year following implantation.
Prevention of cardiac implantable electronic device infection
Preventing infectious complications starts before the implantation of a CIED. The first step is to optimize the risk profile of CIED candidates. This includes achieving a favourable international normalized ratio level in patients receiving OAC treatment and treating ongoing infection, since clinical evidence of infection in the form of fever prior to implantation has been linked to an increased risk.22 Next step involves the use of prophylactic antibiotics, which has proven to reduce the risk of infection.28,35 In addition, evidence supports the use of adjunct skin preparation with antiseptic antibacterial agent to prophylactic antibiotics.34,35 However, the latest studies have not shown any difference between different antiseptic antibacterial agents (chlorhexidine-alcohol vs. povidone-iodine and aqueous povidone-iodine vs. alcoholic povidone-iodine).36,37 Equally, if not more important, aseptic technic should be applied during the implantation as infectious complications are directly linked to local contamination during the procedure.16 In high-risk patients, the placement of the generator in an envelope impregnated with minocycline and rifampin is associated with a lower risk of infection.38–41 Another important part of the prevention strategy is the experience of the clinician performing the procedure, as centres with high volume and experienced operators are noted to have lower complications.42–44
Limitations
Registry studies are inherently limited by the lack of clinical information and rely heavily on the quality of coding, which has been explained in a Danish study by likely under-reporting.45 Additionally, it is probable that patients with underlying and potentially unrecognised IE are a frequent occurrence. Altogether, it seems fair to state that the present estimate presumably understates the true number of IE. Furthermore, data on the distribution of lead IE, pocket infection, and causative pathogens remain unknown. A sizeable group of high-risk patients with AVR were excluded, as we wanted to focus on one cardiac electronic device only. Follow-up time was measured without accounting for subsequent generator replacement, lead revision, or device removal; these factors would certainly alter our incidence rate estimates, as generator exchange and lead manipulations are well-established risk factors of CIED infection. Finally, the CIED data used for this project have not been validated against the DPIR, which is a clinical quality registry maintained by manual reporting by each operator with a standardized data collection form. The DPIR has registered all device-related procedures since 1982, as opposed to 1996 in the NPR. Ultimately, we cannot rule out that misclassification might be present (de-novo patients who were actually non-naïve patients or misidentification of the type of implanted device), which would certainly affect our study findings. Nevertheless, we believe that the data collected from the NPR are highly reliable because of the Danish reimbursement policy since 2002, which required that all implantation centres also report each type of CIED procedure to the NPR.
Conclusion
Our study showed that the incidence of IE increased with increasing CIED complexity and was particularly high the first year after implantation. In terms of device-related risk factors, generator exchange and lead revision were the strongest risk factors for IE. Only PM complexity was significantly associated with increased risk of IE, although that was mainly due to more frequent lead revision in ICDs than in PMs. More importantly, regardless of the type of CIED, the mortality risk was increased after IE. This current study confirms the findings of previous studies on CIED-related infection and illustrates the importance of identifying high-risk patients before procedures, as this is a crucial step towards an effective prevention strategy.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: M.L has received lecture fees from Bristol-Myers Squibb, outside the submitted work. L.K has received speaker fees at symposia, outside the submitted work. N.E.B has received speaker fees from Biotronik, outside the submitted work. C.T.P has received research grants and personal fees from Bayer and grants from Biotronik, outside the submitted work. G.H.G. has received grants from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer , and AstraZenaca, outside the submitted work. C.O, J. R, M.L.L, T.B.L, and M.L.H have no conflict of interest to declare.
Funding
This study was conducted without external funding.
References
Marianne Jespersen, Jens Haarbo, Gunnar Jensen, Ulrik Hintze, Jens Cosedis Nielsen, Søren Pihlkjær Hjorthøj et al. Pacemakere, ICD’er og andre avancerede pacemakersystemer: Sundhedsstyrelsen (National Board of Health, Denmark);
Author notes
Study conducted at Department of Cardiology, Copenhagen University Hospital Gentofte, 2900 Hellerup, Denmark.



