-
PDF
- Split View
-
Views
-
Cite
Cite
Laura Horwood, Anil Attili, Frank Luba, El-Sayed H. Ibrahim, Hemant Parmar, Jadranka Stojanovska, Sharon Gadoth-Goodman, Carey Fette, Hakan Oral, Frank Bogun, Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols, EP Europace, Volume 19, Issue 5, May 2017, Pages 812–817, https://doi.org/10.1093/europace/euw122
Close - Share Icon Share
Magnetic resonance imaging (MRI) has been reported to be safe in patients with cardiac implantable electronic devices (CIED) provided a specific protocol is followed. The objective of this study was to assess whether this is also true for patients excluded from published protocols.
A total of 160 MRIs were obtained in 142 consecutive patients with CIEDs [106 patients had an implantable cardioverter defibrillator (ICD) and 36 had a pacemaker implanted] using an adapted, pre-specified protocol. A cardiac MRI was performed in 95 patients, and a spinal/brain MRI was performed in 47 patients. Forty-six patients (32%) had either abandoned leads (n = 10), and/or were pacemaker dependent with an implanted ICD (n = 19), had recently implanted CIEDs (n = 1), and/or had a CIED device with battery depletion (n = 2), and/or a component of the CIED was recalled or on advisory (n = 32). No major complications occurred. Some device parameters changed slightly, but significantly, right after or at 1-week post-MRI without requiring any reprogramming. In one patient with an ICD on advisory, the pacing rate changed inexplicably during one of his two MRIs from 90 to 50 b.p.m.
Using a pre-specified protocol, cardiac and non-cardiac MRIs were performed in CIED patients with pacemaker dependency, abandoned leads, or depleted batteries without occurrence of major adverse events. Patients with devices on advisory need to be monitored carefully during MRI, especially if they are pacemaker dependent.
Magnetic resonance imaging (MRI) can be safely performed in cardiac implanted electronic device patients with abandoned leads and patients with implanted cardioverter defibrillators who are pacemaker dependent.
Careful monitoring during the MRI of patients with devices who are on advisory is recommended.
Introduction
Magnetic resonance imaging (MRI) is often required for patients with cardiac implanted electronic devices (CIED). Cardiac implanted electronic devices are often viewed as relative contraindications for MRIs. However, there is published evidence that in patients with implanted devices, MRIs can safely be performed when appropriate programming changes are performed prior to the MRI scan.1,2 However, several prior protocols excluded patients with abandoned leads and pacemaker-dependent patients, as well as patients with depleted batteries or recent device implants (<6 weeks).1,2 Furthermore, it is unclear whether MRIs can be safely performed in patients with recalled leads or implanted devices that are on advisory. The purpose of this study was to assess the safety of MRI in consecutive patients with CIEDs excluded from previously published protocols.
Methods
Patient characteristics
| Characteristics . | Patients . |
|---|---|
| Total patients (n) | 142 |
| Gender (m/f) | 117/25 |
| Age (years) | 63 ± 12 |
| Implanted device (n) | 142 |
| ICD | 106 |
| Pacemaker | 36 |
| Implanted devices (n) | 142 |
| Medtronic | 60 |
| Boston Scientific | 35 |
| Biotronik | 12 |
| St. Jude | 35 |
| Pacemaker dependent | 29 |
| PM patients | 10 |
| ICD patients | 19 |
| Abandoned leads (n) | 12 in 10 patients |
| Atrial leads | 1 |
| Ventricular pacing lead | 1 |
| ICD lead dual coil | 4 |
| CRT lead in CVS | 2 |
| Epicardial pacing leads | 3 |
| SVC coil | 1 |
| Implantation <8 weeks | 1 |
| Cardiac MRI | 96 |
| Non-cardiac MRI (n) | 47 |
| Brain | 32 |
| Spinal | 10 |
| Brain and spine together (longer) | 5 |
| Device at ERI prior to MRI | 1 |
| Device at EOL prior to MRI | 1 |
| Total MRIs performed (n = 160) | |
| Single MRI (per patient) | 132 |
| Two MRIs | 8 |
| Five MRIs | 1 |
| Seven MRIs | 1 |
| Characteristics . | Patients . |
|---|---|
| Total patients (n) | 142 |
| Gender (m/f) | 117/25 |
| Age (years) | 63 ± 12 |
| Implanted device (n) | 142 |
| ICD | 106 |
| Pacemaker | 36 |
| Implanted devices (n) | 142 |
| Medtronic | 60 |
| Boston Scientific | 35 |
| Biotronik | 12 |
| St. Jude | 35 |
| Pacemaker dependent | 29 |
| PM patients | 10 |
| ICD patients | 19 |
| Abandoned leads (n) | 12 in 10 patients |
| Atrial leads | 1 |
| Ventricular pacing lead | 1 |
| ICD lead dual coil | 4 |
| CRT lead in CVS | 2 |
| Epicardial pacing leads | 3 |
| SVC coil | 1 |
| Implantation <8 weeks | 1 |
| Cardiac MRI | 96 |
| Non-cardiac MRI (n) | 47 |
| Brain | 32 |
| Spinal | 10 |
| Brain and spine together (longer) | 5 |
| Device at ERI prior to MRI | 1 |
| Device at EOL prior to MRI | 1 |
| Total MRIs performed (n = 160) | |
| Single MRI (per patient) | 132 |
| Two MRIs | 8 |
| Five MRIs | 1 |
| Seven MRIs | 1 |
PM, pacemaker; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; SVC, superior vena cava; ERI, battery: elective replacement indicator; EOL, battery: end of life.
| Characteristics . | Patients . |
|---|---|
| Total patients (n) | 142 |
| Gender (m/f) | 117/25 |
| Age (years) | 63 ± 12 |
| Implanted device (n) | 142 |
| ICD | 106 |
| Pacemaker | 36 |
| Implanted devices (n) | 142 |
| Medtronic | 60 |
| Boston Scientific | 35 |
| Biotronik | 12 |
| St. Jude | 35 |
| Pacemaker dependent | 29 |
| PM patients | 10 |
| ICD patients | 19 |
| Abandoned leads (n) | 12 in 10 patients |
| Atrial leads | 1 |
| Ventricular pacing lead | 1 |
| ICD lead dual coil | 4 |
| CRT lead in CVS | 2 |
| Epicardial pacing leads | 3 |
| SVC coil | 1 |
| Implantation <8 weeks | 1 |
| Cardiac MRI | 96 |
| Non-cardiac MRI (n) | 47 |
| Brain | 32 |
| Spinal | 10 |
| Brain and spine together (longer) | 5 |
| Device at ERI prior to MRI | 1 |
| Device at EOL prior to MRI | 1 |
| Total MRIs performed (n = 160) | |
| Single MRI (per patient) | 132 |
| Two MRIs | 8 |
| Five MRIs | 1 |
| Seven MRIs | 1 |
| Characteristics . | Patients . |
|---|---|
| Total patients (n) | 142 |
| Gender (m/f) | 117/25 |
| Age (years) | 63 ± 12 |
| Implanted device (n) | 142 |
| ICD | 106 |
| Pacemaker | 36 |
| Implanted devices (n) | 142 |
| Medtronic | 60 |
| Boston Scientific | 35 |
| Biotronik | 12 |
| St. Jude | 35 |
| Pacemaker dependent | 29 |
| PM patients | 10 |
| ICD patients | 19 |
| Abandoned leads (n) | 12 in 10 patients |
| Atrial leads | 1 |
| Ventricular pacing lead | 1 |
| ICD lead dual coil | 4 |
| CRT lead in CVS | 2 |
| Epicardial pacing leads | 3 |
| SVC coil | 1 |
| Implantation <8 weeks | 1 |
| Cardiac MRI | 96 |
| Non-cardiac MRI (n) | 47 |
| Brain | 32 |
| Spinal | 10 |
| Brain and spine together (longer) | 5 |
| Device at ERI prior to MRI | 1 |
| Device at EOL prior to MRI | 1 |
| Total MRIs performed (n = 160) | |
| Single MRI (per patient) | 132 |
| Two MRIs | 8 |
| Five MRIs | 1 |
| Seven MRIs | 1 |
PM, pacemaker; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; SVC, superior vena cava; ERI, battery: elective replacement indicator; EOL, battery: end of life.
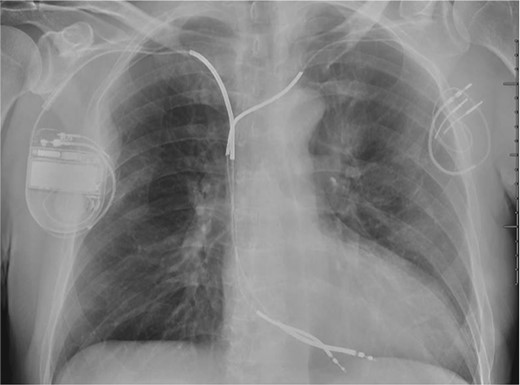
Implantable cardioverter defibrillator generator implant on the right side with abandoned ICD lead on the left side.
Pre- and intra-procedural magnetic resonance imaging protocol
If the indication for an MRI was established by a radiologist and an electrophysiologist, an MRI was planned. The MRI protocol from Johns Hopkins University2 was initially used. All patients signed an informed consent form after a discussion about potential risks. The protocol was limited to 1.5 Tesla scanners, and the pre-MRI assessment included interrogation of the baseline device characteristics, including threshold testing, sensing, battery voltage, and impedance as well as dependency on pacing. A chest X-ray was obtained to assess for abandoned leads. During the MRI, the tachycardia therapy for the ICDs was programmed off to avoid inappropriate device therapy. The magnet mode, noise reversion mode, rate response, premature ventricular contraction response, ventricular sense response, atrial aflutter response, and rate smoothing feature were turned off. If the patient was pacemaker dependent, the pacing mode was programmed to an asynchronous pacing mode (DOO for dual-chamber devices and VOO for single-chamber devices). The patients' rhythm and vital signs were monitored while the patient was in the scanner. A device nurse who was trained in advanced cardiac life support was present throughout the scan of the patient.
After the MRI, the device was interrogated again and reprogrammed to its original settings. The device was interrogated again 1 week and 3 months after the MRI.
The imaging quality and the diagnostic value of the MRI were determined by the attending radiologist, depending on the specific question asked by the ordering physician. Reasons for inability to answer the diagnostic questions were documented.
Cardiac magnetic resonance imaging
Cardiac MRIs were performed to assess the existence of late gadolinium enhancement (LGE) prior to VT ablation procedures. In these patients, the MRI was limited to the LGE sequence and limited to ≤2.0 W/kg specific absorption rate (SAR). The LGE MRI studies were performed on a 1.5 Tesla MRI scanner (Signa Excite CV/i, General Electric, Milwaukee, WI, USA) with an eight-element phased-array coil placed over the chest of patients in the supine position. Ten to twenty minutes after the administration of 0.1–0.15 mmol/kg of intravenous gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Princeton, NJ, USA), two-dimensional (2D) LGE imaging was performed using an inversion-recovery-prepared sequence3 (TR = 6.7 ms, TE = 3.2 ms, spatial resolution = 1.4 × 2.2 mm2, slice thickness = 8 mm) in the short-axis and long-axis views of the left ventricle (LV). The dB/dT level was set to first level. The inversion time (TI: 200–300 ms) was optimized to null normal myocardium. Total scan time for cardiac MRI was ∼30 min.
Non-cardiac magnetic resonance imaging
Brain MRI studies were performed on a 1.5 Tesla magnet (Signa Excite CV/i, General Electric, Milwaukee, WI, USA) with a bird-cage coil placed over the head. The sequences obtained include axial T2-weighted, axial fluid attenuation inversion recovery (FLAIR), axial T1-weighted, and post-gadolinium enhanced T1-weighted images in the coronal and axial planes. All images were obtained using 5-mm slice thickness and 1-mm slice gap. Total scan time for brain MRI was ∼16 min.
The spine MRI studies were performed using an eight-channel spine coil on the same 1.5 Tesla scanner. Sequences of spine imaging include sagittal and axial T2-weighted fast spin-echo sagittal and axial T1-weighted spin-echo and post-contrast T1-weighted spin-echo in the sagittal and axial planes. The sagittal images were acquired using 4 mm slice thickness with 0.4 mm slice gap, while the axial images were obtained using 5 mm slice thickness and 2 mm slice gap. Total time for the spine MRIs was ∼15 min.
Statistical analysis
Variables are expressed as the mean ± SD. Variables were compared by the Student's t-test, Fisher's exact test, or χ2 analysis, as appropriate. If sample size was <5, the Fisher's exact test was used. A P-value of <0.05 was considered statistically significant.
Results
Comparison of device data before and after magnetic resonance imaging
Tables2 and 3 compare the device data before and after the MRI scans. Significant changes of battery voltage, and lead impedance were observed initially, but they went back to baseline level 3 months after the MRI, none of them required programming changes. This was also the case whether an MRI contraindication was present (abandoned leads, pacemaker-dependency with implanted ICD, battery depletion, or recent CIED implants, but also including recalled leads or CIEDs on advisory) or not. Table 3 indicates the number of patients in whom changes of impedance, capture threshold and sensing (>30%, 50%, and 40%, respectively) were observed before and after the MRI.1
| Parameters . | Pre-MRI . | Post-MRI . | 1 week post-MRI . | 3 months post-MRI . | P-value (pre/post) . |
|---|---|---|---|---|---|
| Patient number | 142 | 142 | 93 | 80 | |
| Battery | |||||
| (mV), n = 82 | 2.96 ± 0.31 | 2.94 ± 0.32 | 2.92 ± 0.41 | 2.91 ± 0.34 | ns |
| %, n = 17 | 86 ± 27 | 85 ± 13 | 78 ± 13a | 73 ± 15a | <0.05 |
| Years, n = 43 | 8.5 ± 2.7 | 8.3 ± 2.6 | 8.1 ± 2.7a | 8.1 ± 2.8 | <0.05 |
| P-wave amplitude (mV) | 3.38 ± 2.1 | 3.43 ± 2.1 | 3.1 ± 1.83 | 3.47 ± 2.2 | ns |
| R-wave amplitude (mV) | 12.92 ± 9.7 | 12.9 ± 9.8 | 11.38 ± 5.44 | 11.55 ± 5.26 | ns |
| R-wave amplitude LV lead (mV) | 19.94 ± 5.5 | 19.1 ± 6.96 | 20.87 ± .52 | 13.17 ± 7.55 | ns |
| Atrial lead impedance (Ω) | 477 ± 159 | 474 ± 159 | 480 ± 183a | 479 ± 127 | <0.05 |
| Ventricular lead impedance (Ω) | 492 ± 143 | 490 ± 143 | 471 ± 141 | 485 ± 146 | ns |
| LV lead impedance (Ω) | 708 ± 338 | 703 ± 339 | 712 ± 327a | 671 ± 307 | <0.05 |
| Shock lead impedance (Ω) | 57 ± 17 | 58 ± 17 | 54 ± 14a | 57 ± 18 | <0.05 |
| SVC shock impedance (Ω) | 55 ± 9 | 54 ± 9 | 54 ± 9 | 56 ± 10 | ns |
| Atrial lead capture (V) | 0.79 ± 0.48 | 0.79 ± 0.5 | 0.75 ± 0.31 | 0.71 ± 0.33 | ns |
| Right ventricular lead capture (V) | 0.89 ± 0.32 | 0.92 ± 0.33 | 0.91 ± 0.41 | 0.92 ± 0.36 | ns |
| Left ventricular lead capture (V) | 1.48 ± 0.8 | 1.55 ± 0.85 | 1.48 ± 0.77 | 1.52 ± 0.76 | ns |
| Parameters . | Pre-MRI . | Post-MRI . | 1 week post-MRI . | 3 months post-MRI . | P-value (pre/post) . |
|---|---|---|---|---|---|
| Patient number | 142 | 142 | 93 | 80 | |
| Battery | |||||
| (mV), n = 82 | 2.96 ± 0.31 | 2.94 ± 0.32 | 2.92 ± 0.41 | 2.91 ± 0.34 | ns |
| %, n = 17 | 86 ± 27 | 85 ± 13 | 78 ± 13a | 73 ± 15a | <0.05 |
| Years, n = 43 | 8.5 ± 2.7 | 8.3 ± 2.6 | 8.1 ± 2.7a | 8.1 ± 2.8 | <0.05 |
| P-wave amplitude (mV) | 3.38 ± 2.1 | 3.43 ± 2.1 | 3.1 ± 1.83 | 3.47 ± 2.2 | ns |
| R-wave amplitude (mV) | 12.92 ± 9.7 | 12.9 ± 9.8 | 11.38 ± 5.44 | 11.55 ± 5.26 | ns |
| R-wave amplitude LV lead (mV) | 19.94 ± 5.5 | 19.1 ± 6.96 | 20.87 ± .52 | 13.17 ± 7.55 | ns |
| Atrial lead impedance (Ω) | 477 ± 159 | 474 ± 159 | 480 ± 183a | 479 ± 127 | <0.05 |
| Ventricular lead impedance (Ω) | 492 ± 143 | 490 ± 143 | 471 ± 141 | 485 ± 146 | ns |
| LV lead impedance (Ω) | 708 ± 338 | 703 ± 339 | 712 ± 327a | 671 ± 307 | <0.05 |
| Shock lead impedance (Ω) | 57 ± 17 | 58 ± 17 | 54 ± 14a | 57 ± 18 | <0.05 |
| SVC shock impedance (Ω) | 55 ± 9 | 54 ± 9 | 54 ± 9 | 56 ± 10 | ns |
| Atrial lead capture (V) | 0.79 ± 0.48 | 0.79 ± 0.5 | 0.75 ± 0.31 | 0.71 ± 0.33 | ns |
| Right ventricular lead capture (V) | 0.89 ± 0.32 | 0.92 ± 0.33 | 0.91 ± 0.41 | 0.92 ± 0.36 | ns |
| Left ventricular lead capture (V) | 1.48 ± 0.8 | 1.55 ± 0.85 | 1.48 ± 0.77 | 1.52 ± 0.76 | ns |
aComparison of pre-MRI data to 1-week or 3-month post-MRI data with a P-value of <0.05.
| Parameters . | Pre-MRI . | Post-MRI . | 1 week post-MRI . | 3 months post-MRI . | P-value (pre/post) . |
|---|---|---|---|---|---|
| Patient number | 142 | 142 | 93 | 80 | |
| Battery | |||||
| (mV), n = 82 | 2.96 ± 0.31 | 2.94 ± 0.32 | 2.92 ± 0.41 | 2.91 ± 0.34 | ns |
| %, n = 17 | 86 ± 27 | 85 ± 13 | 78 ± 13a | 73 ± 15a | <0.05 |
| Years, n = 43 | 8.5 ± 2.7 | 8.3 ± 2.6 | 8.1 ± 2.7a | 8.1 ± 2.8 | <0.05 |
| P-wave amplitude (mV) | 3.38 ± 2.1 | 3.43 ± 2.1 | 3.1 ± 1.83 | 3.47 ± 2.2 | ns |
| R-wave amplitude (mV) | 12.92 ± 9.7 | 12.9 ± 9.8 | 11.38 ± 5.44 | 11.55 ± 5.26 | ns |
| R-wave amplitude LV lead (mV) | 19.94 ± 5.5 | 19.1 ± 6.96 | 20.87 ± .52 | 13.17 ± 7.55 | ns |
| Atrial lead impedance (Ω) | 477 ± 159 | 474 ± 159 | 480 ± 183a | 479 ± 127 | <0.05 |
| Ventricular lead impedance (Ω) | 492 ± 143 | 490 ± 143 | 471 ± 141 | 485 ± 146 | ns |
| LV lead impedance (Ω) | 708 ± 338 | 703 ± 339 | 712 ± 327a | 671 ± 307 | <0.05 |
| Shock lead impedance (Ω) | 57 ± 17 | 58 ± 17 | 54 ± 14a | 57 ± 18 | <0.05 |
| SVC shock impedance (Ω) | 55 ± 9 | 54 ± 9 | 54 ± 9 | 56 ± 10 | ns |
| Atrial lead capture (V) | 0.79 ± 0.48 | 0.79 ± 0.5 | 0.75 ± 0.31 | 0.71 ± 0.33 | ns |
| Right ventricular lead capture (V) | 0.89 ± 0.32 | 0.92 ± 0.33 | 0.91 ± 0.41 | 0.92 ± 0.36 | ns |
| Left ventricular lead capture (V) | 1.48 ± 0.8 | 1.55 ± 0.85 | 1.48 ± 0.77 | 1.52 ± 0.76 | ns |
| Parameters . | Pre-MRI . | Post-MRI . | 1 week post-MRI . | 3 months post-MRI . | P-value (pre/post) . |
|---|---|---|---|---|---|
| Patient number | 142 | 142 | 93 | 80 | |
| Battery | |||||
| (mV), n = 82 | 2.96 ± 0.31 | 2.94 ± 0.32 | 2.92 ± 0.41 | 2.91 ± 0.34 | ns |
| %, n = 17 | 86 ± 27 | 85 ± 13 | 78 ± 13a | 73 ± 15a | <0.05 |
| Years, n = 43 | 8.5 ± 2.7 | 8.3 ± 2.6 | 8.1 ± 2.7a | 8.1 ± 2.8 | <0.05 |
| P-wave amplitude (mV) | 3.38 ± 2.1 | 3.43 ± 2.1 | 3.1 ± 1.83 | 3.47 ± 2.2 | ns |
| R-wave amplitude (mV) | 12.92 ± 9.7 | 12.9 ± 9.8 | 11.38 ± 5.44 | 11.55 ± 5.26 | ns |
| R-wave amplitude LV lead (mV) | 19.94 ± 5.5 | 19.1 ± 6.96 | 20.87 ± .52 | 13.17 ± 7.55 | ns |
| Atrial lead impedance (Ω) | 477 ± 159 | 474 ± 159 | 480 ± 183a | 479 ± 127 | <0.05 |
| Ventricular lead impedance (Ω) | 492 ± 143 | 490 ± 143 | 471 ± 141 | 485 ± 146 | ns |
| LV lead impedance (Ω) | 708 ± 338 | 703 ± 339 | 712 ± 327a | 671 ± 307 | <0.05 |
| Shock lead impedance (Ω) | 57 ± 17 | 58 ± 17 | 54 ± 14a | 57 ± 18 | <0.05 |
| SVC shock impedance (Ω) | 55 ± 9 | 54 ± 9 | 54 ± 9 | 56 ± 10 | ns |
| Atrial lead capture (V) | 0.79 ± 0.48 | 0.79 ± 0.5 | 0.75 ± 0.31 | 0.71 ± 0.33 | ns |
| Right ventricular lead capture (V) | 0.89 ± 0.32 | 0.92 ± 0.33 | 0.91 ± 0.41 | 0.92 ± 0.36 | ns |
| Left ventricular lead capture (V) | 1.48 ± 0.8 | 1.55 ± 0.85 | 1.48 ± 0.77 | 1.52 ± 0.76 | ns |
aComparison of pre-MRI data to 1-week or 3-month post-MRI data with a P-value of <0.05.
| . | Number of patients . |
|---|---|
| Impedance change >30% | |
| Atrial lead | 0/142 |
| Right ventricular lead | 0/142 |
| Left ventricular lead | 0/142 |
| Shocking lead | 0/142 |
| Capture threshold change >50% | |
| Atrium | 2/142 |
| Right ventricle | 5/142 |
| Left ventricle | 1/142 |
| Sensing change >40% | |
| P-wave | 11/142 |
| R-wave right ventricle | 6/142 |
| R-wave left ventricle | 0/142 |
| . | Number of patients . |
|---|---|
| Impedance change >30% | |
| Atrial lead | 0/142 |
| Right ventricular lead | 0/142 |
| Left ventricular lead | 0/142 |
| Shocking lead | 0/142 |
| Capture threshold change >50% | |
| Atrium | 2/142 |
| Right ventricle | 5/142 |
| Left ventricle | 1/142 |
| Sensing change >40% | |
| P-wave | 11/142 |
| R-wave right ventricle | 6/142 |
| R-wave left ventricle | 0/142 |
| . | Number of patients . |
|---|---|
| Impedance change >30% | |
| Atrial lead | 0/142 |
| Right ventricular lead | 0/142 |
| Left ventricular lead | 0/142 |
| Shocking lead | 0/142 |
| Capture threshold change >50% | |
| Atrium | 2/142 |
| Right ventricle | 5/142 |
| Left ventricle | 1/142 |
| Sensing change >40% | |
| P-wave | 11/142 |
| R-wave right ventricle | 6/142 |
| R-wave left ventricle | 0/142 |
| . | Number of patients . |
|---|---|
| Impedance change >30% | |
| Atrial lead | 0/142 |
| Right ventricular lead | 0/142 |
| Left ventricular lead | 0/142 |
| Shocking lead | 0/142 |
| Capture threshold change >50% | |
| Atrium | 2/142 |
| Right ventricle | 5/142 |
| Left ventricle | 1/142 |
| Sensing change >40% | |
| P-wave | 11/142 |
| R-wave right ventricle | 6/142 |
| R-wave left ventricle | 0/142 |
Contraindications to other protocols
No adverse events occurred during the MRI in the patients with abandoned leads. None of the patients with abandoned leads had chest discomfort during the scanning procedure. There was a total of 12 abandoned leads in 10 patients, including 7 pacing leads located in the coronary venous system (n = 2), epicardium (n = 3), right atrium (n = 1), and right ventricular apex (n = 1). There were 5 abandoned ICD leads, including dual coil leads (n = 4), and a coil in the superior vena cava (n = 1).
No major adverse events occurred in the patients who were pacemaker dependent. There were 18 patients with cardiac resynchronization therapy. Twenty-nine patients were pacemaker dependent, including 10 patients with implanted pacemakers and 19 patients with implanted ICDs. Power-on reset was not observed in any patient.
In the two patients with CIEDs approaching end of battery life, the battery status did not change after the MRI scan. The ICDs were changed during the same hospital admission as the MRI was performed, and therefore the battery status ≥1 week was not determined. In one patient who underwent MRI within 6 weeks after ICD implantation, no complications occurred.
Thirty-two patients (22%) had either a recalled lead (Riata, St. Jude Medical, St. Paul, MN, USA: n = 4; Quickflex, St. Jude Medial: n = 3; Sprint Fidelis, Medtronic, Minneapolis, MN, USA: n = 10) or a device that was on advisory (Cognis, Boston Scientific, Marlborough, MA, USA: n = 7; Teligen, Boston Scientific: n = 2; Ellipse, St. Jude Medical: n = 5; Enrhythm, Medtronic: n = 1). Nine of the recalled leads were still functional, whereas eight leads were abandoned. In one pacemaker-dependent patient with an ICD that was placed on advisory (Ellipse, St. Jude Medical), the heart rate changed suddenly from 90 to 50 b.p.m. during a spinal MRI when a 2D fast spin-echo pulse sequence was used. This is the pacing rate of the noise reversion mode of this device. The SAR level for this pulse sequence was 1.89 W/kg. Parallel to the decrease in heart rate, the patient's blood pressure dropped to the low 80s and the scan was terminated without sequelae to the patient. The patient was asymptomatic. When the sequence was terminated, the patient's heart rate increased back to 90 b.p.m. The same patient had a cardiac MRI 1 week earlier and no adverse events occurred during that scan. In the patient's cardiac MRI, the maximal SAR was 0.11 W/kg.
Quality of magnetic resonance imaging
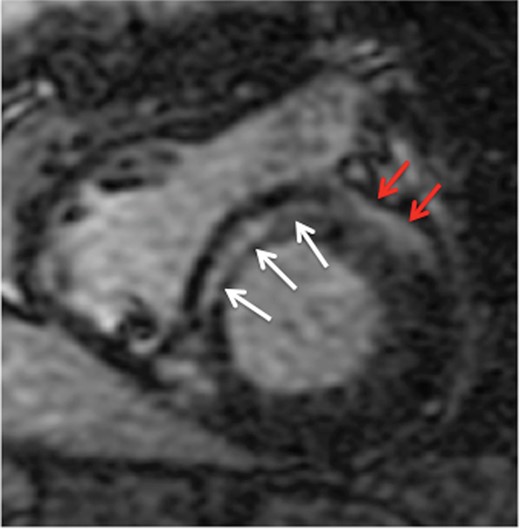
Short-axis view of the basal left and right ventricles of a patient with non-ischaemic cardiomyopathy. White arrows indicate the location of an intramural septal scar. Red arrows indicate artefact that is projected on the anterior left ventricular free wall.
Forty-six patients underwent brain/spinal MRIs. Eight patients underwent multiple studies and one patient underwent seven MRIs. All, except one brain/spinal MRI, were diagnostic without artefacts compromising the analysis.
Device parameters
Table 2 summarizes the baseline device parameters and data from subsequent interrogations. No changes in the device parameters occurred that required reprogramming of any device. Some significant differences in lead impedances were detected when the baseline impedances were compared with the impedance 1 week after the MRI in the atrial leads, the defibrillation lead, and coronary sinus pacing leads. These changes could not be detected at 3 months after the MRI. The case was the same for patients with contraindications/recalled leads/devices on advisory when compared with patients without these issues. Changes in battery status were seen at 1 week and 3 months, but only in devices reporting battery life as ‘battery %’ and as ‘years of life’ remaining (n = 60). No change of battery voltage was seen in devices (n = 82) where battery life was reported in millivolt. Furthermore, the battery status of patients with devices that were on advisory was not significantly different after the MRI when compared with the battery status prior to the MRI.
Adverse events
One patient went into VT in the MRI suite after the LGE image acquisition was completed. The patient was moved out of the scanner and the patient's ICD was programmed back on. The ICD subsequently terminated the VT without any sequelae for the patient. No further image acquisition was performed. This patient was initially admitted with VT storm and the patient had recurrent VT despite antiarrhythmic therapy with amiodarone and lidocaine. The MRI was performed to identify and localize the scar tissue for a VT ablation procedure. The VT observed in the MRI suite was not triggered by programming of the ICD, and based on ICD interrogation, this was the clinical VT of the patient, which was successfully ablated subsequently.
One patient had worsening shortness of breath during the MRI and the study was terminated. The patient had a cardiac resynchronization device and was programmed into a VOO mode.
One patient with recurrent seizures was found to have a fractured lead 1 month after a brain MRI. Sensing and capture threshold were unchanged immediately after the MRI and 1 week after the MRI was done. The fractured lead was a Medtronic lead 5076 that is currently approved for MRI.
Follow-up
Immediate follow-up comparing pre- and post-MRI data were available in all 142 patients. At 1 week and 3 months 93 (65%) and 80 (56%) patients, respectively, had their follow-up completed. Follow-up data were incomplete at 1 week and 3 months because these patients were followed elsewhere. Device data were not available from these patients; however, adequate device function was confirmed by contacting the device clinics following these patients.
Discussion
The present study demonstrates that cardiac and non-cardiac MRIs can be performed in patients with abandoned pacemaker and defibrillator leads as well as in pacemaker-dependent patients. No major adverse events occurred in these patients. Caution, however, needs to be exerted in patients with CIEDs that are on advisory due to the potential of inexplicable changes in the pacing rate during the MRI.
Data from Johns Hopkins University2 demonstrated that MRIs are safe if certain precautions are taken prior and during scanning. The safety data were updated and reported in 2011.1 The MagnaSafe registry used a similar protocol and aimed to prospectively assess the safety of MRI examination in patients with CIEDs. However, only non-thoracic MRIs were included in the MagnaSafe registry. No major adverse events occurred in the studied 1500 patients.4 In the present study, the majority of scans were cardiac MRIs, thereby further extending the safety data to include these patients. It is important to note that the used protocol was limited to a 1.5 Tesla scanner with the SAR of the LGE sequence <2.0 W/kg.
Protocols from both Johns Hopkins University and the MagnaSafe registry excluded patients with abandoned leads. The concern of abandoned leads is heating of the abandoned lead at the lead tip.5 This has been demonstrated in animal and in vitro studies.6 Higgins et al.7 showed that scanning can be done without major adverse events in a study in which devices were explanted prior to the MRI. It is not clear how valid these data are for patients with implanted devices and abandoned leads because the majority of patients had their devices explanted for the MRI and had their devices re-implanted subsequently. Furthermore, the scans were non-thoracic, and none of the patients were pacemaker dependent. Our data supplement and extend the findings by Higgins et al. by demonstrating that cardiac MRIs are also feasible without major adverse events in patients with abandoned leads and implanted devices whether or not they are pacemaker dependent.
Patients with ICDs who are pacemaker dependent were excluded in the Johns Hopkins protocol and in the MagnaSafe registry.2,8 Power-on reset has been observed and can lead to rapid battery depletion, potentially jeopardizing adequate pacing in pacemaker-dependent patients. This condition occurred only in devices released prior to 2002 that were implanted from 1999 to 2004 in one study.9 No power-on reset occurred in any of the patients described in this patient cohort, but it may be prudent not to proceed with MRI scanning if the device was manufactured prior to 2002.
To the best of our knowledge, no data about MRIs and safety have been reported regarding recalled leads or CIEDs on advisory. In one pacemaker-dependent patient, the heart rate dropped during a fast spin-echo pulse sequence from 90 to 50 b.p.m. corresponding to the noise reversion rate of the ICD (Ellipse, St. Jude Medical). Interestingly, this did not occur during a cardiac MRI that the patient had a week earlier where no adverse events occurred. The pulse sequence during which this occurred resulted in a SAR close to 2.0 W/kg. It is possible that this sequence resulted in an electromagnetic interference that was detected by the device, switching the asynchronous pacing mode to a noise reversion mode despite the noise reversion being programmed off prior to the MRI. This might have been prevented by modifying imaging parameters resulting in a lower SAR, including increasing the slice thickness, using a smaller matrix, increasing the echo train separation, using a lower flip angle, or reducing the readout bandwidth.10 It should be also noted that some institutions use lower SAR limit (e.g. 1.5 W/kg) when imaging patients with ICDs to provide a larger safety margin. Nevertheless, this patient had a device that was placed on advisory and it is possible that the unexpected pacing behaviour was secondary to malfunction of this type of device in the magnetic field. Caution is therefore necessary when MRIs are performed in patients with implanted Ellipse ICDs, especially if the patient is pacemaker dependent.
The majority of the scans in this study were cardiac MRIs that were performed prior to VT ablation procedures to assess for scar location. It is not surprising that in one of the patients VT occurred while the patient was inside the MRI scanner. The VT was terminated uneventfully after the patient was removed from the scanner and the patient's tachycardia therapies were turned back on. The VT was not triggered by programming the ICD, but corresponded to the patient's clinical VT. This VT was eventually ablated during the subsequent ablation procedure. Cardiac MRIs are helpful in identifying the scar tissue that harbours the substrate for VTs,11 and therefore MRIs are routinely performed at our institution in patients undergoing VT ablation procedures. Magnetic resonance imaging is particularly helpful in patients with non-ischaemic cardiomyopathies11 and can prevent unnecessary epicardial mapping/ablation procedures, e.g. by demonstrating the absence of epicardial scarring or scarring in the interventricular septum. Demonstration that MRIs are safe in patients with abandoned leads and pacemaker-dependent patients with implanted ICDs will contribute to render this technology available to more patients who can benefit from MRIs.
Limitations
One limitation of this study is that the 1-week and 3-month post-ablation follow-up was not achieved in all patients, and therefore it is possible that some changes in parameters might have been detected if complete follow-up data would have been available. However, all patients had baseline and immediate post-MRI data assessment without changes in parameters requiring programming changes. This study reflects a single-centre experience in patients with CIEDs including a small number of patients with contraindications to MRIs. The study will need to be confirmed in larger patient series.
Conclusion
This study demonstrates that if appropriate precautions are taken, MRIs can be performed without major adverse events in patients with CIEDs even in the presence of abandoned leads or if patients are pacemaker dependent. In patients with devices on advisory, careful monitoring is required if the patients are pacemaker dependent. Extending safety data to patients with CIEDs not currently included in MRI protocols may render these patients eligible for the diagnostic benefit of MRIs.
Conflict of interest: none declared.



