-
PDF
- Split View
-
Views
-
Cite
Cite
Sheldon M Singh, Andre d'Avila, Young-Hoon Kim, Arash Aryana, J Michael Mangrum, Gregory F Michaud, Srinivas R Dukkipati, Conor D Barrett, E Kevin Heist, Michael K Parides, Kevin E Thorpe, Vivek Y Reddy, Termination of persistent atrial fibrillation during pulmonary vein isolation: insight from the MAGIC-AF trial, EP Europace, Volume 19, Issue 10, October 2017, Pages 1657–1663, https://doi.org/10.1093/europace/euw266
Close - Share Icon Share
Abstract
Controversy on the optimal ablation strategy for persistent atrial fibrillation (AF) exists with limited work evaluating a strategy of pulmonary vein isolation (PVI) alone when AF terminates during PVI. Thirty-five patients had AF termination during PVI in the Modified Ablation Guided by Ibutilide Use in Chronic Atrial Fibrillation (MAGIC-AF; ClinicalTrials.gov number: NCT01014741) study. The objective of the current study is to report the 1-year outcome after PVI alone in this unique patient group.
The 1-year single procedure freedom from atrial arrhythmia off anti-arrhythmic drugs was reported for the 35 patients in the MAGIC-AF study with persistent AF termination during or upon completion of PVI.
Freedom from recurrent atrial arrhythmia was achieved in 60% of patients where AF terminated during PVI. Cavotricuspid isthmus flutter was common when AF terminated to a macro re-entrant flutter during PVI, and responsible for 92% of all flutter circuits with AF termination.
Persistent AF termination during PVI may identify a subgroup of patients who experience a similar long-term clinical outcome with PVI ablation alone when compared with other more extensive persistent AF ablation strategies. Pulmonary vein isolation alone may be an appropriate tactic in this subgroup of persistent AF patients.
This study suggests that atrial fibrillation (AF) termination during pulmonary vein isolation (PVI) may identify patients with less advanced AF. This finding provides insight into the diverse mechanisms of persistent AF.
This study highlights the fact that patients with AF termination during PVI experience comparable clinical outcomes with less extensive procedures.
This study encourages further study into novel intra-procedural markers of less severe atrial remodelling to identify persistent AF patients who may benefit from less extensive ablation procedures.
Introduction
Current understanding of the mechanisms and substrate maintaining persistent atrial fibrillation (AF) is rudimentary. As such, controversy exists regarding the optimal ablation strategy for persistent AF.1,2 Objective assessments of the efficacy of a primary pulmonary vein isolation (PVI) strategy for the treatment of persistent AF are limited.3–9 To the best of our knowledge, only one small study specifically evaluated the value of PVI alone10 when AF termination, an endpoint suggested to be associated with lower arrhythmia recurrence,11,12 occurred with PVI. Indeed, empirical placement of left atrial linear lesions in sinus rhythm or AF re-induction to guide additional left atrial ablation in spite of achieving AF termination during PVI is well reported, touted to have improved clinical outcomes, and is consistent with the belief held by many that ablation incremental to PVI is necessary in patients with persistent AF.9,13,14
The Modified Ablation Guided by Ibutilide Use in Chronic Atrial Fibrillation (MAGIC-AF) study (ClinicalTrials.gov number: NCT01014741) was a multicentre international randomized double-blinded trial assessing the utility of intra-procedural administration of intravenous ibutilide before ablation of complex fractionated atrial electrograms (CFAE) in patients with persistent AF undergoing a first-ever catheter ablation procedure.15 Patients with AF termination during or upon completion of PVI were not eligible for randomization as no additional CFAE ablation was performed. These patients were followed in a Registry in order to obtain insight into the value of a primary PVI ablation strategy for persistent AF. Herein, we report the clinical characteristics and 1-year outcomes of these patients.
Methods
Study design and participants
The MAGIC-AF trial design and primary results have been previously published.15 The study was sponsored by St Jude Medical who played no role in the study design, data collection and analysis, or manuscript preparation and submission. The Electrophysiology Clinical Research Group at the Icahn School of Medicine at Mount Sinai served as the study coordinating centre. Ethics approval was obtained from each institution's research ethics board, with adverse events reported to each ethics board as well as the Trial's safety committee.
Patients 18 years of age or older undergoing a first-ever catheter ablation procedure for persistent AF (i.e. continuous AF lasting >7 days) and presenting in AF on the day of the procedure were eligible for enrolment. Patients with a reversible aetiology of AF, hypertrophic cardiomyopathy, rheumatic heart disease, congenital heart disease (including atrial septal defects), and prior left-sided catheter or surgical ablation procedures were excluded. Written consent was obtained from all study participants.
Study procedures
All procedures were performed with the patient in spontaneous AF—AF induction was not performed at any point during the procedure. Open irrigated radiofrequency ablation catheters without contact sensors, a multi-electrode mapping catheter, and an impedance-based electroanatomic mapping system (NavX, St Jude Medical, St Paul, MN, USA) were used in all procedures. All patients underwent circumferential PVI with the requirement for demonstrating bidirectional block when sinus rhythm was restored (Figure 1). Patients who experienced AF termination during or upon completion of PVI were included in the Registry, whereas those with AF continuation for at least 10 min upon completion of PVI were entered in the Trial and thereby eligible for randomization to receive ibutilide or placebo prior to CFAE ablation.
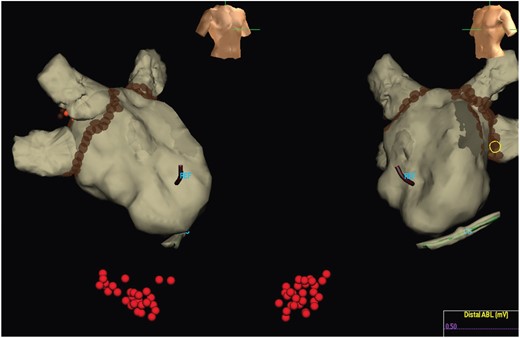
Ablation strategy. All patients in the Registry underwent PVI which resulted in termination of AF. Additional ablation for atrial arrhythmia with AF termination was permitted as was placement of a cavotricuspid isthmus linear lesion in sinus rhythm. In this example, PVI and cavotricuspid isthmus ablation was performed.
For Registry patients, when AF terminated to sinus rhythm with PVI, no additional left atrial ablation (i.e. linear ablation lesions or focal ablation apart from PV re-isolation) was permitted. When AF terminated to an atrial flutter/tachycardia, ablation (including left atrial ablation) was permitted only to target this specific arrhythmia mechanism. Trial patients received ibutilide or placebo prior to undergoing additional left atrial ablation. When AF terminated to sinus rhythm immediately upon completion of the study drug infusion, no additional left atrial ablation was permitted. When AF terminated to an atrial flutter/tachycardia after the study drug, infusion ablation was permitted only to target this specific arrhythmia mechanism. If AF persisted despite completion of the study drug infusion, ablation targeting sites with CFAE was mandated with no additional left atrial ablation performed when sinus rhythm was achieved. When atrial flutter/tachycardia occurred after CFAE ablation, left atrial ablation was permitted only to target this specific arrhythmia. When AF persisted despite exhaustive CFAE ablation, cardioversion to sinus rhythm was performed with no additional left atrial ablation. For all patients, right atrial cavotricuspid isthmus ablation could be performed during sinus rhythm at the discretion of the operator.
Of note, given the potential impact of knowing AF cycle length and its changes during the procedure on blinding (i.e. knowledge of receipt of ibutilide or placebo in the Trial portion of the MAGIC-AF study), operators were not permitted to measure AF cycle length at any point during the procedure.
Anti-arrhythmic drug use was encouraged during the initial 3-month post-ablation blanking period at which point they were discontinued. Clinical follow-up occurred at 1, 3, 6, 9, and 12-months post-ablation with an ECG performed at the 3, 6, and 12-month visit, as well as a 7- to 21-day Holter or multichannel outpatient telemetry recorder performed on at least two of the 3, 6, and 12-month visits.
Efficacy outcomes
The primary analysis reported on the clinical outcomes of patients in the Registry. To provide context as to the relative efficacy of the strategy of PVI alone in persistent AF patients, we compared the clinical outcomes of the Registry patients with that of the Trial regardless of whether the Trial patients were randomized to receive ibutilide or saline placebo.
A sensitivity analysis combined Trial patients who experienced AF termination during the study drug infusion (either ibutilide or saline placebo) with Registry patients who experienced AF termination upon completion of PVI alone. This combined group facilitated reporting on the clinical outcome of all patients undergoing a primary PVI strategy without CFAE ablation regardless if AF termination was achieved with PVI alone or with PVI alone and the use of the study drug. Again, to provide some context as to the relative efficacy of the strategy of PVI alone in persistent AF patients, we compared the clinical outcomes of these patients with those where CFAE ablation was mandatory.
Consistent with current guidelines, any atrial arrhythmia >30 s in duration occurring after a 3-month blanking period indicated arrhythmia recurrence.14 We report: (i) the freedom from recurrent atrial arrhythmia after a single ablation procedure without the use of anti-arrhythmic drugs; and (ii) the freedom from recurrent atrial arrhythmia after a single ablation procedure with or without the use of anti-arrhythmic drugs. As the ablation strategy at the time of redo procedures were not pre-specified (i.e. patients who initially received a primary PVI strategy at their initial procedure were not mandated to simply have PV re-isolation at the redo procedure), and our intention is to describe the long-term outcomes after a primary PVI strategy, outcomes after a second ablation procedure are not reported.
Statistical analysis
The analysis of patient characteristics as well as clinical outcomes of the Registry and Trial patients was pre-specified; however, sample size calculations for this analysis were not possible due to the unpredictable rate of AF termination with PVI and hence enrolment in the Registry. Continuous variables were reported as mean and standard deviation and compared using the Student's t-test. Categorical variables were compared using the χ2 test or Fisher's exact test. When multiple testing was performed, P-values were adjusted using the Hochberg procedure. An adjusted two-sided P-value of <0.05 indicated statistical significance. Statistical analysis was performed using R, version 3.0.2 (R Core Team, Vienna, Austria, 2014).
Results
Patients
Three hundred and fifty-five patients were screened for eligibility between November 2009 and December 2012 at seven centres (five in the USA, one in South Korea, and one in Canada). Two hundred and thirty-five patients met the study eligibility criteria, 35 of whom had AF termination with PVI and were included in the Registry and 200 of whom remained in AF after PVI and were eligible for the Trial (Figure 2). Complete follow-up at 12 months was obtained for 90% of the patients.
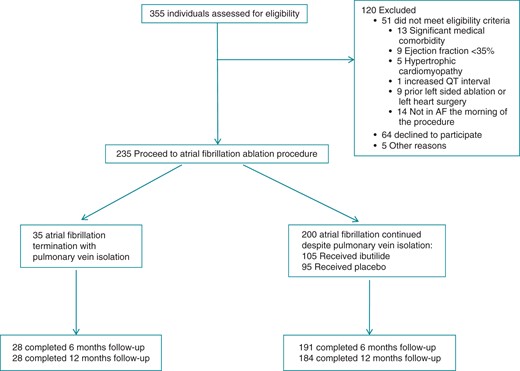
Table 1 summarizes the characteristics of patients with AF termination during or upon completion of PVI (Registry patients) and patients with continuation of AF despite PVI (Trial patients). Patients with AF termination during or upon completion of PVI had a trend towards older age (64 vs. 60 years), smaller left atrium (43 vs. 46 mm), prior stroke (20 vs. 6%), and statin use (53 vs. 36%); however, none of these baseline differences were statistically significant after adjustment with the Hochberg procedure. Symptoms of AF, stroke risk, and duration of current and longest AF episodes were similar between the two groups.
| Characteristic . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | Unadjusted P-values . | Adjusted P-values . |
|---|---|---|---|---|
| Age (years) | 64 ± 10 | 60 ± 11 | 0.03 | 0.60 |
| Male sex [no. (%)] | 26 (74) | 156 (78) | 0.63 | 0.90 |
| Body mass index | 29 ± 6 | 29 ± 6 | 0.53 | 0.90 |
| Ejection fraction (%) | 55 ± 8 | 55 ± 8 | 0.69 | 0.90 |
| Left atrial diameter (mm) | 43 ± 6 | 46 ± 8 | 0.006 | 0.15 |
| Medical history | ||||
| Hypertension [no. (%)] | 20 (57) | 116 (58) | 0.89 | 0.90 |
| Diabetes [no. (%)] | 9 (26) | 32 (16) | 0.17 | 0.90 |
| Coronary artery disease [no. (%)] | 5 (14) | 30 (15) | 0.90 | 0.90 |
| Stroke or transient ischaemic attack [no. (%)] | 7 (20) | 11 (6) | 0.003 | 0.07 |
| Congestive heart failure [no. (%)] | 2 (6) | 23 (12) | 0.30 | 0.90 |
| CHA2DS2VASc score | 2.0 ± 1.8 | 1.5 ± 1.3 | 0.13 | 0.90 |
| Atrial fibrillation history | ||||
| Baseline CCS SAF score | 2.2 ± 0.8 | 2.1 ± 1.0 | 0.44 | 0.90 |
| Duration of current AF episode (months) | 8 ± 13 | 10 ± 21 | 0.56 | 0.90 |
| Duration of longest AF episode (months) | 12 ± 21 | 11 ± 22 | 0.67 | 0.90 |
| Long-lasting AF [no. (%)] | 8 (23) | 38 (19) | 0.62 | 0.90 |
| Prior cardioversion [no. (%)] | 21 (60) | 155 (78) | 0.02 | 0.52 |
| Medication use | ||||
| Statin [no. (%)] | 18 (53) | 72 (36) | 0.06 | 0.90 |
| ACE inhibitor/ARB [no. (%)] | 12 (35) | 80 (40) | 0.59 | 0.90 |
| Current or prior anti-arrhythmic drug use [no. (%)] | 25 (74) | 147 (75) | 0.89 | 0.90 |
| Current amiodarone use [no. (%)] | 4 (12) | 16 (8) | 0.47 | 0.90 |
| Characteristic . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | Unadjusted P-values . | Adjusted P-values . |
|---|---|---|---|---|
| Age (years) | 64 ± 10 | 60 ± 11 | 0.03 | 0.60 |
| Male sex [no. (%)] | 26 (74) | 156 (78) | 0.63 | 0.90 |
| Body mass index | 29 ± 6 | 29 ± 6 | 0.53 | 0.90 |
| Ejection fraction (%) | 55 ± 8 | 55 ± 8 | 0.69 | 0.90 |
| Left atrial diameter (mm) | 43 ± 6 | 46 ± 8 | 0.006 | 0.15 |
| Medical history | ||||
| Hypertension [no. (%)] | 20 (57) | 116 (58) | 0.89 | 0.90 |
| Diabetes [no. (%)] | 9 (26) | 32 (16) | 0.17 | 0.90 |
| Coronary artery disease [no. (%)] | 5 (14) | 30 (15) | 0.90 | 0.90 |
| Stroke or transient ischaemic attack [no. (%)] | 7 (20) | 11 (6) | 0.003 | 0.07 |
| Congestive heart failure [no. (%)] | 2 (6) | 23 (12) | 0.30 | 0.90 |
| CHA2DS2VASc score | 2.0 ± 1.8 | 1.5 ± 1.3 | 0.13 | 0.90 |
| Atrial fibrillation history | ||||
| Baseline CCS SAF score | 2.2 ± 0.8 | 2.1 ± 1.0 | 0.44 | 0.90 |
| Duration of current AF episode (months) | 8 ± 13 | 10 ± 21 | 0.56 | 0.90 |
| Duration of longest AF episode (months) | 12 ± 21 | 11 ± 22 | 0.67 | 0.90 |
| Long-lasting AF [no. (%)] | 8 (23) | 38 (19) | 0.62 | 0.90 |
| Prior cardioversion [no. (%)] | 21 (60) | 155 (78) | 0.02 | 0.52 |
| Medication use | ||||
| Statin [no. (%)] | 18 (53) | 72 (36) | 0.06 | 0.90 |
| ACE inhibitor/ARB [no. (%)] | 12 (35) | 80 (40) | 0.59 | 0.90 |
| Current or prior anti-arrhythmic drug use [no. (%)] | 25 (74) | 147 (75) | 0.89 | 0.90 |
| Current amiodarone use [no. (%)] | 4 (12) | 16 (8) | 0.47 | 0.90 |
The plus and minus values indicate mean ± standard deviation. CCS SAF, Canadian Cardiovascular Society Severity of Atrial Fibrillation scale; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker. Long-lasting AF is defined as AF >1 year in duration. Unadjusted P-values and P-values adjusted for multiple testing using the Hochberg procedure are reported.
| Characteristic . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | Unadjusted P-values . | Adjusted P-values . |
|---|---|---|---|---|
| Age (years) | 64 ± 10 | 60 ± 11 | 0.03 | 0.60 |
| Male sex [no. (%)] | 26 (74) | 156 (78) | 0.63 | 0.90 |
| Body mass index | 29 ± 6 | 29 ± 6 | 0.53 | 0.90 |
| Ejection fraction (%) | 55 ± 8 | 55 ± 8 | 0.69 | 0.90 |
| Left atrial diameter (mm) | 43 ± 6 | 46 ± 8 | 0.006 | 0.15 |
| Medical history | ||||
| Hypertension [no. (%)] | 20 (57) | 116 (58) | 0.89 | 0.90 |
| Diabetes [no. (%)] | 9 (26) | 32 (16) | 0.17 | 0.90 |
| Coronary artery disease [no. (%)] | 5 (14) | 30 (15) | 0.90 | 0.90 |
| Stroke or transient ischaemic attack [no. (%)] | 7 (20) | 11 (6) | 0.003 | 0.07 |
| Congestive heart failure [no. (%)] | 2 (6) | 23 (12) | 0.30 | 0.90 |
| CHA2DS2VASc score | 2.0 ± 1.8 | 1.5 ± 1.3 | 0.13 | 0.90 |
| Atrial fibrillation history | ||||
| Baseline CCS SAF score | 2.2 ± 0.8 | 2.1 ± 1.0 | 0.44 | 0.90 |
| Duration of current AF episode (months) | 8 ± 13 | 10 ± 21 | 0.56 | 0.90 |
| Duration of longest AF episode (months) | 12 ± 21 | 11 ± 22 | 0.67 | 0.90 |
| Long-lasting AF [no. (%)] | 8 (23) | 38 (19) | 0.62 | 0.90 |
| Prior cardioversion [no. (%)] | 21 (60) | 155 (78) | 0.02 | 0.52 |
| Medication use | ||||
| Statin [no. (%)] | 18 (53) | 72 (36) | 0.06 | 0.90 |
| ACE inhibitor/ARB [no. (%)] | 12 (35) | 80 (40) | 0.59 | 0.90 |
| Current or prior anti-arrhythmic drug use [no. (%)] | 25 (74) | 147 (75) | 0.89 | 0.90 |
| Current amiodarone use [no. (%)] | 4 (12) | 16 (8) | 0.47 | 0.90 |
| Characteristic . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | Unadjusted P-values . | Adjusted P-values . |
|---|---|---|---|---|
| Age (years) | 64 ± 10 | 60 ± 11 | 0.03 | 0.60 |
| Male sex [no. (%)] | 26 (74) | 156 (78) | 0.63 | 0.90 |
| Body mass index | 29 ± 6 | 29 ± 6 | 0.53 | 0.90 |
| Ejection fraction (%) | 55 ± 8 | 55 ± 8 | 0.69 | 0.90 |
| Left atrial diameter (mm) | 43 ± 6 | 46 ± 8 | 0.006 | 0.15 |
| Medical history | ||||
| Hypertension [no. (%)] | 20 (57) | 116 (58) | 0.89 | 0.90 |
| Diabetes [no. (%)] | 9 (26) | 32 (16) | 0.17 | 0.90 |
| Coronary artery disease [no. (%)] | 5 (14) | 30 (15) | 0.90 | 0.90 |
| Stroke or transient ischaemic attack [no. (%)] | 7 (20) | 11 (6) | 0.003 | 0.07 |
| Congestive heart failure [no. (%)] | 2 (6) | 23 (12) | 0.30 | 0.90 |
| CHA2DS2VASc score | 2.0 ± 1.8 | 1.5 ± 1.3 | 0.13 | 0.90 |
| Atrial fibrillation history | ||||
| Baseline CCS SAF score | 2.2 ± 0.8 | 2.1 ± 1.0 | 0.44 | 0.90 |
| Duration of current AF episode (months) | 8 ± 13 | 10 ± 21 | 0.56 | 0.90 |
| Duration of longest AF episode (months) | 12 ± 21 | 11 ± 22 | 0.67 | 0.90 |
| Long-lasting AF [no. (%)] | 8 (23) | 38 (19) | 0.62 | 0.90 |
| Prior cardioversion [no. (%)] | 21 (60) | 155 (78) | 0.02 | 0.52 |
| Medication use | ||||
| Statin [no. (%)] | 18 (53) | 72 (36) | 0.06 | 0.90 |
| ACE inhibitor/ARB [no. (%)] | 12 (35) | 80 (40) | 0.59 | 0.90 |
| Current or prior anti-arrhythmic drug use [no. (%)] | 25 (74) | 147 (75) | 0.89 | 0.90 |
| Current amiodarone use [no. (%)] | 4 (12) | 16 (8) | 0.47 | 0.90 |
The plus and minus values indicate mean ± standard deviation. CCS SAF, Canadian Cardiovascular Society Severity of Atrial Fibrillation scale; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker. Long-lasting AF is defined as AF >1 year in duration. Unadjusted P-values and P-values adjusted for multiple testing using the Hochberg procedure are reported.
Procedural characteristics
Of the 35 Registry patients where AF terminated during PVI, AF terminated to sinus rhythm in 22 and a macro-reentrant flutter in 13 (12 cavotricuspid isthmus flutter and 1 mitral annular flutter). Of the 200 Trial patients where AF persisted despite PVI, 15 had AF termination upon completion of infusion of the study drug [13 with ibutilide (11 sinus rhythm, 1 mitral annular flutter, and 1 roof flutter) and 2 with saline placebo (both to sinus rhythm)]. Complex fractionated atrial electrograms ablation was performed in 185 Trial patients where AF persisted despite infusion of the study drug. Atrial fibrillation termination occurred in 117 of these 185 patients, 49 to sinus rhythm and 68 to a macro-reentrant flutter (26 cavotricuspid isthmus flutter, 17 roof flutter, 14 mitral annular flutter, and 9 focal or other flutter). Cavotricuspid isthmus flutter was more common when AF terminated to a macro-reentrant flutter when CFAE ablation was not performed (80% with PVI ± study drug vs. 40% during CFAE ablation; P = 0.008).
Table 2 summarizes the procedural characteristics of patients with AF termination during PVI and patients with continuation of AF despite PVI. No differences in ablation time, fluoroscopy time, and procedure time to perform PVI were noted in the two groups. As to be expected, overall ablation, fluoroscopy, and procedure times were longer when AF persisted despite PVI.
| . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Total ablation time for pulmonary vein isolation (min) | 62 ± 28 | 64 ± 26 | 0.80 |
| Total ablation time (min) | 80 ± 29 | 105 ± 43 | <0.001 |
| Total fluoroscopy time for pulmonary vein isolation (min) | 9 ± 9 | 9 ± 9 | 0.81 |
| Total fluoroscopy time (min) | 10 ± 9 | 36 ± 25 | <0.001 |
| Total procedure time for pulmonary vein isolation (min) | 117 ± 53 | 107 ± 45 | 0.26 |
| Total procedure time (min) | 265 ± 85 | 319 ± 80 | <0.001 |
| . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Total ablation time for pulmonary vein isolation (min) | 62 ± 28 | 64 ± 26 | 0.80 |
| Total ablation time (min) | 80 ± 29 | 105 ± 43 | <0.001 |
| Total fluoroscopy time for pulmonary vein isolation (min) | 9 ± 9 | 9 ± 9 | 0.81 |
| Total fluoroscopy time (min) | 10 ± 9 | 36 ± 25 | <0.001 |
| Total procedure time for pulmonary vein isolation (min) | 117 ± 53 | 107 ± 45 | 0.26 |
| Total procedure time (min) | 265 ± 85 | 319 ± 80 | <0.001 |
Data available for 30 patients with termination of AF with PVI and 197 patients where AF continued despite PVI. The plus and minus values indicate means ± standard deviation.
| . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Total ablation time for pulmonary vein isolation (min) | 62 ± 28 | 64 ± 26 | 0.80 |
| Total ablation time (min) | 80 ± 29 | 105 ± 43 | <0.001 |
| Total fluoroscopy time for pulmonary vein isolation (min) | 9 ± 9 | 9 ± 9 | 0.81 |
| Total fluoroscopy time (min) | 10 ± 9 | 36 ± 25 | <0.001 |
| Total procedure time for pulmonary vein isolation (min) | 117 ± 53 | 107 ± 45 | 0.26 |
| Total procedure time (min) | 265 ± 85 | 319 ± 80 | <0.001 |
| . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Total ablation time for pulmonary vein isolation (min) | 62 ± 28 | 64 ± 26 | 0.80 |
| Total ablation time (min) | 80 ± 29 | 105 ± 43 | <0.001 |
| Total fluoroscopy time for pulmonary vein isolation (min) | 9 ± 9 | 9 ± 9 | 0.81 |
| Total fluoroscopy time (min) | 10 ± 9 | 36 ± 25 | <0.001 |
| Total procedure time for pulmonary vein isolation (min) | 117 ± 53 | 107 ± 45 | 0.26 |
| Total procedure time (min) | 265 ± 85 | 319 ± 80 | <0.001 |
Data available for 30 patients with termination of AF with PVI and 197 patients where AF continued despite PVI. The plus and minus values indicate means ± standard deviation.
Efficacy outcomes
Freedom from atrial arrhythmias >30 s in duration after a single ablation procedure and without the use of anti-arrhythmic drugs occurred in 60% of the Registry patients. This long-term clinical outcome was similar to the outcome in the Trial patients (52%) (Table 3). The secondary endpoint of either freedom from atrial arrhythmia with or without anti-arrhythmic drugs was 64% in the Registry patients, which was similar to that in the Trial patients (56%).
| Outcome . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Freedom from atrial arrhythmia after one procedure without drugs [no. (%)] | 17 (60) | 99 (52) | 0.42 |
| Freedom from atrial arrhythmia after one procedure with or without drugs [no. (%)] | 18 (64) | 107 (56) | 0.54 |
| Outcome . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Freedom from atrial arrhythmia after one procedure without drugs [no. (%)] | 17 (60) | 99 (52) | 0.42 |
| Freedom from atrial arrhythmia after one procedure with or without drugs [no. (%)] | 18 (64) | 107 (56) | 0.54 |
Data available for 28 patients with termination of AF with PVI and 189 patients where AF continued despite PVI. The plus and minus values indicate means ± standard deviation.
| Outcome . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Freedom from atrial arrhythmia after one procedure without drugs [no. (%)] | 17 (60) | 99 (52) | 0.42 |
| Freedom from atrial arrhythmia after one procedure with or without drugs [no. (%)] | 18 (64) | 107 (56) | 0.54 |
| Outcome . | Termination of AF with pulmonary vein isolation (n = 35) . | Continuation of AF despite pulmonary vein isolation (n = 200) . | P-value . |
|---|---|---|---|
| Freedom from atrial arrhythmia after one procedure without drugs [no. (%)] | 17 (60) | 99 (52) | 0.42 |
| Freedom from atrial arrhythmia after one procedure with or without drugs [no. (%)] | 18 (64) | 107 (56) | 0.54 |
Data available for 28 patients with termination of AF with PVI and 189 patients where AF continued despite PVI. The plus and minus values indicate means ± standard deviation.
The results of the sensitivity analysis combining the 35 Registry patients with the 15 Trial patients where AF terminated upon completion of the study drug infusion (n = 50) were similar to the primary analysis. The freedom from atrial arrhythmias >30 s in duration after a single ablation procedure and without the use of anti-arrhythmic drugs was 50% in the group of patient with a primary PVI strategy. This finding was similar to that observed in the group of patients where CFAE ablation was mandatory (49%).
Complications
The most common peri-ablation complication was symptomatic congestive heart failure requiring initiation/escalation of diuretics or hospitalization (Registry: 3% vs. Trial: 10%). Pericarditis or pericardial effusion occurred in no patients in the Registry but 16 (8%) patients in the Trial. Three deaths occurred, all in the Trial patients—one death was due to a left atrial-oesophageal fistula, one due to the consequences of aspiration pneumonia likely related to non-cardiac co-morbidities, and one of unknown causes.
Discussion
There is considerable controversy surrounding the optimal approach to catheter ablation of persistent AF.1,2 Our study suggests that PVI without additional empirical left atrial ablation may provide acceptable long-term clinical outcomes in patients where AF terminates with PVI. Our finding is important and provides insight into the diverse mechanisms of, and the need for individualized ablation strategies in, persistent AF patients.
Current definitions of persistent AF do not account for the broad spectrum of patients and diverse mechanisms of this condition. Termination of persistent AF with PVI may identify patients with less advanced structural and electrical remodelling. This is suggested by the trend towards smaller left atrial diameters and greater rates of AF termination to cavotricuspid isthmus flutter, an arrhythmia mechanism related to functional block between the vena cava rather than the presence of atrial scar.16 Such patients may require less extensive ablation in order to achieve an optimal outcome. Our theory is consistent with the observations of others who have also evaluated the merits of PVI alone for persistent AF, albeit without the intra-procedure endpoint of AF termination during PVI. Markers of less severe electrical and structural atrial remodelling, including duration of AF,7 left atrial volume,12 and regression of P wave duration with the use of anti-arrhythmic drugs,8 and AF cycle length prior to ablation10 have also been shown to predict freedom from atrial arrhythmia with a strategy of PVI alone, with or without AF termination, in persistent AF patients. Thus, efforts to identify pre- and intra-procedural characteristics associated with less advanced persistent AF should be encouraged as this may allow for a less extensive ablation strategy.
It is likely that the benefits of PVI for persistent AF extend beyond simply eliminating PV triggers. Pulmonary vein ablation has also been shown to reduce total left atrial CFAE area,17,18 injure and reduce ganglionic plexi,18 Marshall's ligament,19 and Bachman's bundle.20 This collateral injury may provide sufficient left atrial substrate modification necessary to maintain sinus rhythm. Avoiding additional ablation in this group of patients may prevent atypical atrial flutters, and minimize procedure and fluoroscopy times during the index procedure—an important consideration, given the frequent need for repeat AF ablation procedures in this population. While the findings of our work are important, it is necessary to appreciate that this strategy may not be applicable to the broad spectrum of persistent AF patients. Specifically, persistent AF termination during PVI occurred in only 15% of our cohort, suggesting that it may be applicable to the minority of patients with persistent AF. It is possible that the low rate of persistent AF termination with PVI observed in our cohort may be related to our ostial approach to PVI. Wide antral isolation may provide further collateral injury to modify the substrate of persistent AF with PVI alone and may result in higher rates of persistent AF termination during PVI.
Our work has significant limitations. First, although pre-specified, our analysis is limited by the small number of patients with AF termination during PVI. Our sample size was not within our control as it was dependent on the rate of AF termination with PVI, and the duration of the study. Secondly, we did not specifically test the value of additional empirical ablation after AF termination with PVI but rather compared clinical outcomes in this group of patients with those where AF persisted, despite PVI resulting in additional left atrial ablation. Thus, we cannot definitively state that additional empirical left atrial ablation in patients where AF terminates during PVI has no added value. Furthermore, it must be appreciated that the current comparison group (Trial patients) is inherently different from the group with a primary PVI strategy (Registry patients). The comparison in this case was selected simply to provide some context to the long-term outcomes of other persistent AF patients undergoing catheter ablation. Thirdly, due to the lack of randomization (which is of course not possible as one cannot randomly assign patients to AF termination during PVI), it is possible that other factors associated with AF termination during PVI may be present in our study; thus, we cannot definitively eliminate an alternative link between AF termination with PVI and adequate long-term clinical results. Of note, we specifically did not perform multivariate logistic regression analysis to determine the predictors of arrhythmia recurrence with a primary PVI strategy as, from a methodological standpoint, we did not believe this was appropriate as the small number of Registry patients (n = 35) would permit the inclusion of at most three variables in any model (assuming each results in a single coefficient). Such data-driven approaches often yield invalid inference and as such were not considered. Despite this, our work provides insight into the diverse mechanisms of persistent AF and highlights the importance of a personalized approach to AF ablation in this patient population. Furthermore, our work compliments the growing body of work evaluating the role of PVI alone in persistent AF patients3,4,8 and suggests that additional empirical left atrial ablation and AF re-induction upon cessation of persistent AF with catheter ablation may not provide additional benefits when AF terminates during or upon completion of PVI.
Conclusions
In conclusion, PVI without additional left atrial substrate modification may provide acceptable long-term clinical outcomes in patients where AF terminates during PVI. These findings highlight that a single ablation strategy may not be appropriate for all patients with persistent AF, and should serves as a stimulus to better define ablation strategies among the diverse groups of patients with persistent AF.
Funding
This work was supported by St Jude Medical.
Conflict of interest: A.A., Ar.A., J.M.M., G.F.M., C.D.B., E.K.H., and V.Y.R. have received grant support from St Jude Medical. A.A., Ar.A., J.M.M, S.R.D., E.K.H., and V.Y.R. have served as consultants to/received honorarium from St Jude Medical. S.M.S., Y.-H.K., M.K.P., and K.E.T. have no conflict of interest to disclose.



