-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Müssigbrodt, Silke John, Jedrzej Kosiuk, Sergio Richter, Gerhard Hindricks, Andreas Bollmann, Vernakalant-facilitated electrical cardioversion: comparison of intravenous vernakalant and amiodarone for drug-enhanced electrical cardioversion of atrial fibrillation after failed electrical cardioversion, EP Europace, Volume 18, Issue 1, January 2016, Pages 51–56, https://doi.org/10.1093/europace/euv194
Close - Share Icon Share
Abstract
Electrical cardioversion is one cornerstone for the rhythm control strategy of atrial fibrillation (AF), which is, however, hampered by immediate AF recurrence (IRAF) or failed electrical cardioversion (FECV). We aimed to investigate the potential role of vernakalant for facilitated electrical cardioversion in cardioversion-resistant AF.
The subjects of this study were 63 patients referred to the Heart Centre Leipzig between November 2011 and May 2014 for transthoracic electrical cardioversion of AF. All patients experienced after antiarrhythmic-naïve electrical cardioversion either IRAF (n = 44; 70%) or FECV (n = 19; 30%). After drug infusion, electrical cardioversion was successful in 66.7% of vernakalant-treated as opposed to 46.7% of amiodarone-treated patients (P = 0.109). Multivariate analysis revealed treatment with vernakalant (OR 0.057, 95% CI 0.006–0.540, P = 0.013), treatment with ACEI or ARB (OR 0.101, 95% CI 0.015–0.691 P = 0.019), and IRAF after initial CV (OR 0.047, 95% CI 0.004–0.498, P = 0.011) as predictors for successful, drug-facilitated electrical cardioversion. Subgroup analysis of 18 patients with previous AF ablation revealed a significantly higher success rate of electrical cardioversion after infusion of vernakalant than after infusion of amiodarone (66.7 vs. 11.1%, P = 0.016).
Vernakalant may therefore be considered as a useful agent for facilitated electrical cardioversion in cardioversion-resistant AF.
Vernakalant was similarly efficient as amiodarone for drug-facilitated electrical cardioversion.
Vernakalant was more efficient than amiodarone for drug-facilitated electrical cardioversion in patients with previous AF ablation.
Vernakalant may therefore be considered as a useful agent for facilitated electrical cardioversion in cardioversion-resistant AF.
Introduction
Electrical cardioversion (ECV) is one cornerstone for the rhythm control strategy of atrial fibrillation (AF), which is, however, hampered by immediate AF recurrence (IRAF) or failed electrical cardioversion (FECV) occurring in up to 26%.1–4 Interestingly, current AF management guidelines do not specifically address management in this situation although intravenous amiodarone is considered as the most potent drug for the acute management of cardioversion-resistant AF.5,6
Vernakalant, a new antiarrhythmic multi-channel blocking agent, has demonstrated 50% efficacy in terminating recent-onset AF.7
Vernakalant inhibits a number of potassium channel currents and sodium channels, in detail, the ultra rapid delayed rectified potassium current (IKur), the transient outward potassium current (Ito), the rapidly activating delayed rectified potassium current (IKr), the acetylcholine-regulated inward rectifying potassium current (IK-ACh). Moreover, vernakalant inhibits cardiac sodium channels in a voltage- and frequency-dependent manner.8
However, whether intravenous vernakalant facilitates ECV of cardioversion-resistant AF is unknown and was consequently tested in this non-randomized observational study.
Methods
Characteristics of study population
The subjects of this study were patients referred to the Heart Centre Leipzig for transthoracic electrical antiarrhythmic-naïve CV of AF.
Between November 2011 and May 2014, we consecutively included 63 patients with IRAF (n = 44; 70%) or FECV (n = 19; 30%) after electrical CV. The baseline parameters are shown in Table 1.
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 33 | 30 | |
| Female, n (%) | 11 (33.3) | 10 (33.3) | 1.00 |
| Age (years) | 63.9 ± 10.3 | 66.5 ± 9.8 | 0.299 |
| Weight (kg) | 92.3 ± 24.1 | 85.8 ± 20.6 | 0.256 |
| Height (cm) | 173 ± 8 | 174 ± 10 | 0.978 |
| BMI (kg/m2) | 30.4 ± 7.3 | 28.3 ± 5.5 | 0.208 |
| Coronary heart disease, n (%) | 6 (18.2) | 2 (6.7) | 0.170 |
| Dilative cardiomyopathy, n (%) | 1 (3.0) | 1 (3.3) | 0.945 |
| Hypertension, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| Diabetes mellitus, n (%) | 6 (18.2) | 4 (13.3) | 0.599 |
| COPD, n (%) | 1 (3) | 3 (10) | 0.257 |
| LVEF, (%) | 50 ± 11 | 54 ± 13 | 0.277 |
| LA diameter (mm) | 44 ± 7 | 46 ± 6 | 0.236 |
| Mitral insufficiency | 22 (66.7) | 22 (73.3) | 0.565 |
| Beta blockers, n (%) | 27 (81.8) | 23 (76.7) | 0.614 |
| Digitalis, n (%) | 3 (9.1) | 5 (16.7) | 0.367 |
| ACEI or ARB, n (%) | 19 (57.6) | 17 (56.7) | 0.942 |
| Previous AF ablation, n (%) | 9 (27.3) | 9 (30.0) | 0.811 |
| AF ablation within 30 days, n (%) | 7 (21.2) | 7 (23.3) | 0.840 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.2 ± 0.4 | 0.216 |
| AF duration >48 h, n (%) | 30 (90.9) | 26 (86.6) | 0.700 |
| AF duration >7 days, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| IRAF, n (%) | 23 (69.7) | 21 (70.0) | 0.979 |
| FECV, n (%) | 10 (30.3) | 9 (30.0) |
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 33 | 30 | |
| Female, n (%) | 11 (33.3) | 10 (33.3) | 1.00 |
| Age (years) | 63.9 ± 10.3 | 66.5 ± 9.8 | 0.299 |
| Weight (kg) | 92.3 ± 24.1 | 85.8 ± 20.6 | 0.256 |
| Height (cm) | 173 ± 8 | 174 ± 10 | 0.978 |
| BMI (kg/m2) | 30.4 ± 7.3 | 28.3 ± 5.5 | 0.208 |
| Coronary heart disease, n (%) | 6 (18.2) | 2 (6.7) | 0.170 |
| Dilative cardiomyopathy, n (%) | 1 (3.0) | 1 (3.3) | 0.945 |
| Hypertension, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| Diabetes mellitus, n (%) | 6 (18.2) | 4 (13.3) | 0.599 |
| COPD, n (%) | 1 (3) | 3 (10) | 0.257 |
| LVEF, (%) | 50 ± 11 | 54 ± 13 | 0.277 |
| LA diameter (mm) | 44 ± 7 | 46 ± 6 | 0.236 |
| Mitral insufficiency | 22 (66.7) | 22 (73.3) | 0.565 |
| Beta blockers, n (%) | 27 (81.8) | 23 (76.7) | 0.614 |
| Digitalis, n (%) | 3 (9.1) | 5 (16.7) | 0.367 |
| ACEI or ARB, n (%) | 19 (57.6) | 17 (56.7) | 0.942 |
| Previous AF ablation, n (%) | 9 (27.3) | 9 (30.0) | 0.811 |
| AF ablation within 30 days, n (%) | 7 (21.2) | 7 (23.3) | 0.840 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.2 ± 0.4 | 0.216 |
| AF duration >48 h, n (%) | 30 (90.9) | 26 (86.6) | 0.700 |
| AF duration >7 days, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| IRAF, n (%) | 23 (69.7) | 21 (70.0) | 0.979 |
| FECV, n (%) | 10 (30.3) | 9 (30.0) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 33 | 30 | |
| Female, n (%) | 11 (33.3) | 10 (33.3) | 1.00 |
| Age (years) | 63.9 ± 10.3 | 66.5 ± 9.8 | 0.299 |
| Weight (kg) | 92.3 ± 24.1 | 85.8 ± 20.6 | 0.256 |
| Height (cm) | 173 ± 8 | 174 ± 10 | 0.978 |
| BMI (kg/m2) | 30.4 ± 7.3 | 28.3 ± 5.5 | 0.208 |
| Coronary heart disease, n (%) | 6 (18.2) | 2 (6.7) | 0.170 |
| Dilative cardiomyopathy, n (%) | 1 (3.0) | 1 (3.3) | 0.945 |
| Hypertension, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| Diabetes mellitus, n (%) | 6 (18.2) | 4 (13.3) | 0.599 |
| COPD, n (%) | 1 (3) | 3 (10) | 0.257 |
| LVEF, (%) | 50 ± 11 | 54 ± 13 | 0.277 |
| LA diameter (mm) | 44 ± 7 | 46 ± 6 | 0.236 |
| Mitral insufficiency | 22 (66.7) | 22 (73.3) | 0.565 |
| Beta blockers, n (%) | 27 (81.8) | 23 (76.7) | 0.614 |
| Digitalis, n (%) | 3 (9.1) | 5 (16.7) | 0.367 |
| ACEI or ARB, n (%) | 19 (57.6) | 17 (56.7) | 0.942 |
| Previous AF ablation, n (%) | 9 (27.3) | 9 (30.0) | 0.811 |
| AF ablation within 30 days, n (%) | 7 (21.2) | 7 (23.3) | 0.840 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.2 ± 0.4 | 0.216 |
| AF duration >48 h, n (%) | 30 (90.9) | 26 (86.6) | 0.700 |
| AF duration >7 days, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| IRAF, n (%) | 23 (69.7) | 21 (70.0) | 0.979 |
| FECV, n (%) | 10 (30.3) | 9 (30.0) |
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 33 | 30 | |
| Female, n (%) | 11 (33.3) | 10 (33.3) | 1.00 |
| Age (years) | 63.9 ± 10.3 | 66.5 ± 9.8 | 0.299 |
| Weight (kg) | 92.3 ± 24.1 | 85.8 ± 20.6 | 0.256 |
| Height (cm) | 173 ± 8 | 174 ± 10 | 0.978 |
| BMI (kg/m2) | 30.4 ± 7.3 | 28.3 ± 5.5 | 0.208 |
| Coronary heart disease, n (%) | 6 (18.2) | 2 (6.7) | 0.170 |
| Dilative cardiomyopathy, n (%) | 1 (3.0) | 1 (3.3) | 0.945 |
| Hypertension, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| Diabetes mellitus, n (%) | 6 (18.2) | 4 (13.3) | 0.599 |
| COPD, n (%) | 1 (3) | 3 (10) | 0.257 |
| LVEF, (%) | 50 ± 11 | 54 ± 13 | 0.277 |
| LA diameter (mm) | 44 ± 7 | 46 ± 6 | 0.236 |
| Mitral insufficiency | 22 (66.7) | 22 (73.3) | 0.565 |
| Beta blockers, n (%) | 27 (81.8) | 23 (76.7) | 0.614 |
| Digitalis, n (%) | 3 (9.1) | 5 (16.7) | 0.367 |
| ACEI or ARB, n (%) | 19 (57.6) | 17 (56.7) | 0.942 |
| Previous AF ablation, n (%) | 9 (27.3) | 9 (30.0) | 0.811 |
| AF ablation within 30 days, n (%) | 7 (21.2) | 7 (23.3) | 0.840 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.2 ± 0.4 | 0.216 |
| AF duration >48 h, n (%) | 30 (90.9) | 26 (86.6) | 0.700 |
| AF duration >7 days, n (%) | 28 (84.8) | 25 (83.3) | 0.869 |
| IRAF, n (%) | 23 (69.7) | 21 (70.0) | 0.979 |
| FECV, n (%) | 10 (30.3) | 9 (30.0) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Patients were excluded if they had a known hypersensitivity to vernakalant or amiodarone, thyroid disease, allergy to iod or low potassium (≤3.6 mmol/L). We also excluded patients under treatment with MAO (l-monoamine oxidases)-inhibitors and/or antiarrhythmic therapy class I or III, pregnant women and women in lacation period, patients with severe aortic stenosis, patients with systolic blood pressure <100 mmHg, patients with decompensated heart failure, patients with QT interval prolongation >440 ms (uncorrected), patients with acute coronary syndrome within the last 30 days, and patients with sinus node dysfunction or second-degree or third-degree heart block in the absence of a pacemaker. In order to facilitate electrical cardioversion, our patients were assigned to receive either a single dose of vernakalant (n = 33; 52%) or amiodarone (n = 30; 48%) prior to another attempt with electrical CV at the discretion of the treating physician. In contrast to current recommendations, we administered vernakalant off-label for patients with AF > 7 days as well. Patients did not differ significantly in their demographic or clinical characteristics (Table 1). All patients provided informed consent for electrical and drug-facilitated cardioversion as well as data collection.
Protocol
We aimed to investigate the potential role of vernakalant for facilitated electrical cardioversion in cardioversion-resistant AF. The primary endpoint was acute successful electrical cardioversion into sinus rhythm after drug-facilitated electrical cardioversion.
If AF had been present for more than 48 h, electrical CV was preceded by either a transoesophageal echocardiogram to rule out left atrial thrombi, or by therapeutic anticoagulation (international normalized ratio 2–3) with phenprocoumon for at least 3 weeks. Electrical CV was performed with a rectilinear biphasic waveform (Medtronic LIFEPAK® 20, Redmond, WA, USA). After sedation with intravenous midazolam hydrochloride and etomidate, transthoracic electrical CV was performed with biphasic shock using 200 J, 300 J, or 360 J. Successful electrical CV was defined as the presence of at least two clearly identifiable sinus complexes after delivery of a shock. Immediate atrial fibrillation recurrence was defined as a recurrence of AF within 5 min after successful electrical CV.
If IRAF occurred, drug enhanced electrical CV was planned.
If AF persisted after electrical CV, a second attempt was undertaken with increased energy (360 J, if 200 or 300 J were used before) and modified patch position (anterior–posterior instead of sternal–apical). If no sinus rhythm could be achieved after this attempt, drug-enhanced electrical CV was scheduled after the second electrical CV (Figure 1 ).
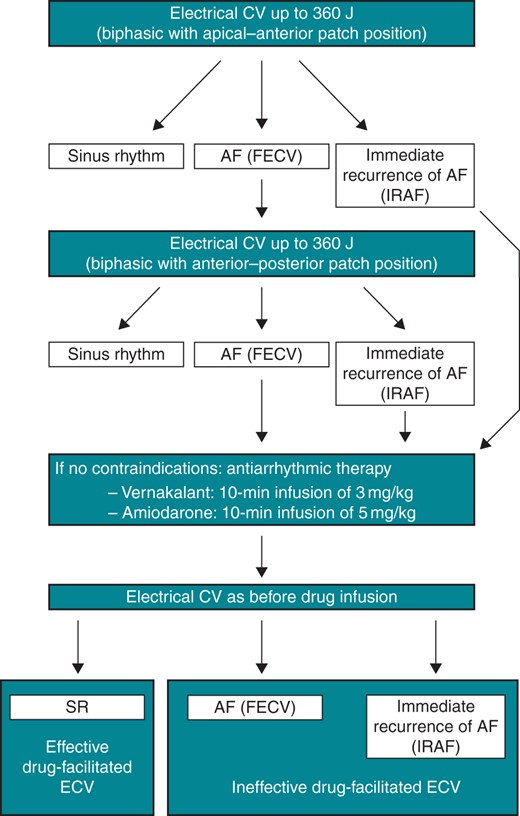
Algorithm for drug-facilitated electrical cardioversion. CV, cardioversion; SR, sinus rhythm; AF, atrial fibrillation; FECV, failed electrical cardioversion; IRAF, immediate recurrence of atrial fibrillation; J, Joules; ECV, electrical cardioversion.
For drug-enhanced electrical CV, patients were assigned to receive an infusion of either 5 mg/kg bodyweight (BW) of amiodarone over 10 min or 3 mg/kg BW of vernakalant over 10 min.
Ten minutes after completion of the drug infusion, transthoracic electrical CV was attempted again with a shock that had the same energy as the previous shock. In the event of another episode of IRAF, no more attempts of electrical CV were repeated. A 12-lead electrocardiogram was recorded continuously for 15 min after initiation of drug therapy in order to monitor the prevailing rhythm.
Follow-up
Amiodarone or vernakalant were administered as single-dose antiarrhythmic drug at the time of electrical CV. If electrical CV failed or recurrences occurred during the time of hospitalization, antiarrhythmic drug prescribed at the discretion of the treating physicians. Anticoagulation therapy was maintained for at least 1 month after electrical CV in patients in whom AF had been present for more than 48 h. Rhythm was analysed before and after each electrical CV and at 1 h after last electrical CV.
Statistical analysis
Categorical variables are provided as total numbers and frequencies (%) and continuous variables as mean ± standard deviation. Continuous variables were compared using unpaired Student's t-test or Mann–Whitney U test, according to normality, and paired data using paired Student's t-test or Wilcoxon analysis. Categorical variables were compared using χ2 analysis. Multivariate regression analysis including variables identified in univariate analysis (P < 0.15) was used to identify independent predictors for successful cardioversion. P-values ≤0.05 were considered statistically significant. Multivariate analysis was additionally adjusted for AF duration up to 48 and >48 h.
All data management and analysis were performed using SPSS 20.0 software (SPSS, IL, USA).
Results
Results after antiarrhythmic drug-facilitated electrical cardioversion
Drug-infusion was completed in all patients. No spontaneous cardioversion occurred before electrical cardioversion. After drug infusion, electrical cardioversion was successful in 66.7% of vernakalant-treated as opposed to 46.7% of amiodarone-treated patients (P = 0.109). Immediate atrial fibrillation recurrence was observed in 24.2% of vernakalant-treated when compared with 36.7% of amiodarone-treated patients (P = 0.283). Failed electrical cardioversion occurred in 9.1% of vernakalant-treated when compared with 16.7% of amiodarone-treated patients (P = 0.271). Successful cardioversion into SR could be achieved in six of 10 patients (60%) with AF up to 7 days and 30 of 53 patients (56%) with AF > 7 days. There was no significant difference in respect of successful drug-facilitated electrical cardioversion between both groups (P = 0.842).
The results after 1 h of rhythm monitoring did not differ from the acute results. Characteristics of patients with successful restoration of sinus rhythm are compared with those with IRAF or FECV after drug-administration in Table 2.
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 36 | 27 | |
| Female, n (%) | 17 (81.0) | 4 (19.0) | 0.007 |
| Age (years) | 67.5 ± 9.4 | 62.0 ± 10.3 | 0.030 |
| Weight (kg) | 89 ± 22 | 90 ± 24 | 0.909 |
| Height (cm) | 172 ± 9 | 176 ± 8 | 0.139 |
| BMI (kg/m2) | 29.8 ± 6.3 | 28.9 ± 7.0 | 0.599 |
| Coronary heart disease, n (%) | 6 (75.0) | 2 (25.0) | 0.448 |
| Dilative cardiomyopathy, n (%) | 1 (50.0) | 1 (50.0) | 0.836 |
| Hypertension, n (%) | 32 (60.4) | 21 (39.6) | 0.232 |
| Diabetes mellitus, n (%) | 6 (60) | 4 (40) | 0.842 |
| COPD, n (%) | 4 (100) | 0 (0) | 0.128 |
| LVEF, (%) | 49 ± 12 | 56 ± 11 | 0.039 |
| LA diameter (mm) | 44 ± 6 | 45 ± 7 | 0.661 |
| Mitral insufficiency | 26 (59.1) | 18 (40.9) | 0.634 |
| Beta-blocker, n (%) | 32 (64.0) | 18 (36.0) | 0.031 |
| Digitalis, n (%) | 4 (50.0) | 4 (50.0) | 0.715 |
| ACEI or ARB, n (%) | 26 (72.2) | 10 (27.8) | 0.005 |
| Previous AF ablation, n (%) | 7 (38.9) | 11 (61.1) | 0.064 |
| AF Ablation within 30 days, n (%) | 7 (50.0) | 7 (50.0) | 0.540 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.4 ± 0.4 | 0.432 |
| AF >48 h | 33 (58.9) | 23 (41.1) | 0.449 |
| AF >7 days | 30 (56.6) | 23 (43.4) | 0.842 |
| IRAF after initial CV, n (%) | 30 (68.2) | 14 (31.8) | 0.007 |
| FECV after initial CV, n (%) | 6 (31.6) | 13 (68.4) | |
| Vernakalant, n (%) | 22 (66.7) | 11 (33.3) | 0.109 |
| Amiodarone, n (%) | 14 (46.7) | 16 (53.3) |
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 36 | 27 | |
| Female, n (%) | 17 (81.0) | 4 (19.0) | 0.007 |
| Age (years) | 67.5 ± 9.4 | 62.0 ± 10.3 | 0.030 |
| Weight (kg) | 89 ± 22 | 90 ± 24 | 0.909 |
| Height (cm) | 172 ± 9 | 176 ± 8 | 0.139 |
| BMI (kg/m2) | 29.8 ± 6.3 | 28.9 ± 7.0 | 0.599 |
| Coronary heart disease, n (%) | 6 (75.0) | 2 (25.0) | 0.448 |
| Dilative cardiomyopathy, n (%) | 1 (50.0) | 1 (50.0) | 0.836 |
| Hypertension, n (%) | 32 (60.4) | 21 (39.6) | 0.232 |
| Diabetes mellitus, n (%) | 6 (60) | 4 (40) | 0.842 |
| COPD, n (%) | 4 (100) | 0 (0) | 0.128 |
| LVEF, (%) | 49 ± 12 | 56 ± 11 | 0.039 |
| LA diameter (mm) | 44 ± 6 | 45 ± 7 | 0.661 |
| Mitral insufficiency | 26 (59.1) | 18 (40.9) | 0.634 |
| Beta-blocker, n (%) | 32 (64.0) | 18 (36.0) | 0.031 |
| Digitalis, n (%) | 4 (50.0) | 4 (50.0) | 0.715 |
| ACEI or ARB, n (%) | 26 (72.2) | 10 (27.8) | 0.005 |
| Previous AF ablation, n (%) | 7 (38.9) | 11 (61.1) | 0.064 |
| AF Ablation within 30 days, n (%) | 7 (50.0) | 7 (50.0) | 0.540 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.4 ± 0.4 | 0.432 |
| AF >48 h | 33 (58.9) | 23 (41.1) | 0.449 |
| AF >7 days | 30 (56.6) | 23 (43.4) | 0.842 |
| IRAF after initial CV, n (%) | 30 (68.2) | 14 (31.8) | 0.007 |
| FECV after initial CV, n (%) | 6 (31.6) | 13 (68.4) | |
| Vernakalant, n (%) | 22 (66.7) | 11 (33.3) | 0.109 |
| Amiodarone, n (%) | 14 (46.7) | 16 (53.3) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 36 | 27 | |
| Female, n (%) | 17 (81.0) | 4 (19.0) | 0.007 |
| Age (years) | 67.5 ± 9.4 | 62.0 ± 10.3 | 0.030 |
| Weight (kg) | 89 ± 22 | 90 ± 24 | 0.909 |
| Height (cm) | 172 ± 9 | 176 ± 8 | 0.139 |
| BMI (kg/m2) | 29.8 ± 6.3 | 28.9 ± 7.0 | 0.599 |
| Coronary heart disease, n (%) | 6 (75.0) | 2 (25.0) | 0.448 |
| Dilative cardiomyopathy, n (%) | 1 (50.0) | 1 (50.0) | 0.836 |
| Hypertension, n (%) | 32 (60.4) | 21 (39.6) | 0.232 |
| Diabetes mellitus, n (%) | 6 (60) | 4 (40) | 0.842 |
| COPD, n (%) | 4 (100) | 0 (0) | 0.128 |
| LVEF, (%) | 49 ± 12 | 56 ± 11 | 0.039 |
| LA diameter (mm) | 44 ± 6 | 45 ± 7 | 0.661 |
| Mitral insufficiency | 26 (59.1) | 18 (40.9) | 0.634 |
| Beta-blocker, n (%) | 32 (64.0) | 18 (36.0) | 0.031 |
| Digitalis, n (%) | 4 (50.0) | 4 (50.0) | 0.715 |
| ACEI or ARB, n (%) | 26 (72.2) | 10 (27.8) | 0.005 |
| Previous AF ablation, n (%) | 7 (38.9) | 11 (61.1) | 0.064 |
| AF Ablation within 30 days, n (%) | 7 (50.0) | 7 (50.0) | 0.540 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.4 ± 0.4 | 0.432 |
| AF >48 h | 33 (58.9) | 23 (41.1) | 0.449 |
| AF >7 days | 30 (56.6) | 23 (43.4) | 0.842 |
| IRAF after initial CV, n (%) | 30 (68.2) | 14 (31.8) | 0.007 |
| FECV after initial CV, n (%) | 6 (31.6) | 13 (68.4) | |
| Vernakalant, n (%) | 22 (66.7) | 11 (33.3) | 0.109 |
| Amiodarone, n (%) | 14 (46.7) | 16 (53.3) |
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 36 | 27 | |
| Female, n (%) | 17 (81.0) | 4 (19.0) | 0.007 |
| Age (years) | 67.5 ± 9.4 | 62.0 ± 10.3 | 0.030 |
| Weight (kg) | 89 ± 22 | 90 ± 24 | 0.909 |
| Height (cm) | 172 ± 9 | 176 ± 8 | 0.139 |
| BMI (kg/m2) | 29.8 ± 6.3 | 28.9 ± 7.0 | 0.599 |
| Coronary heart disease, n (%) | 6 (75.0) | 2 (25.0) | 0.448 |
| Dilative cardiomyopathy, n (%) | 1 (50.0) | 1 (50.0) | 0.836 |
| Hypertension, n (%) | 32 (60.4) | 21 (39.6) | 0.232 |
| Diabetes mellitus, n (%) | 6 (60) | 4 (40) | 0.842 |
| COPD, n (%) | 4 (100) | 0 (0) | 0.128 |
| LVEF, (%) | 49 ± 12 | 56 ± 11 | 0.039 |
| LA diameter (mm) | 44 ± 6 | 45 ± 7 | 0.661 |
| Mitral insufficiency | 26 (59.1) | 18 (40.9) | 0.634 |
| Beta-blocker, n (%) | 32 (64.0) | 18 (36.0) | 0.031 |
| Digitalis, n (%) | 4 (50.0) | 4 (50.0) | 0.715 |
| ACEI or ARB, n (%) | 26 (72.2) | 10 (27.8) | 0.005 |
| Previous AF ablation, n (%) | 7 (38.9) | 11 (61.1) | 0.064 |
| AF Ablation within 30 days, n (%) | 7 (50.0) | 7 (50.0) | 0.540 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.4 ± 0.4 | 0.432 |
| AF >48 h | 33 (58.9) | 23 (41.1) | 0.449 |
| AF >7 days | 30 (56.6) | 23 (43.4) | 0.842 |
| IRAF after initial CV, n (%) | 30 (68.2) | 14 (31.8) | 0.007 |
| FECV after initial CV, n (%) | 6 (31.6) | 13 (68.4) | |
| Vernakalant, n (%) | 22 (66.7) | 11 (33.3) | 0.109 |
| Amiodarone, n (%) | 14 (46.7) | 16 (53.3) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Multivariate analysis revealed treatment with vernakalant (OR 0.057, 95% CI 0.006–0.540, P = 0.013), treatment with ACEI or ARB (OR 0.101, 95% CI 0.015–0.691 P = 0.019) and IRAF after initial CV (OR 0.047, 95% CI 0.004–0.498, P = 0.011) as predictors for successful, drug-facilitated electrical cardioversion. Complete multivariate model is presented in Table 3.
Parameters associated with successful cardioversion in uni- and multivariate analysis
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Female | 0.194 (0.056–0.677) | 0.010 | ||
| Age, per year | 1.060 (1.004–1.118) | 0.035 | ||
| AF duration >48 h | 1.913 (0.391–9.370) | 0.424 | ||
| COPD | 1.153 (0.913–1.956) | 0.128 | ||
| Previous AF ablation | 0.351 (0.114–1.084) | 0.069 | ||
| LVEF, per % increase | 0.951 (0.906–0.999) | 0.045 | ||
| Beta-blocker use | 0.250 (0.067–0.928) | 0.038 | ||
| IRAF after initial CV | 0.215 (0.068–0.685) | 0.009 | 0.047 (0.004–0.498) | 0.011 |
| ACEI or ARB use | 0.226 (0.078–0.659) | 0.006 | 0.101 (0.015–0.691) | 0.019 |
| Vernakalant use | 0.438 (0.158–1.212) | 0.112 | 0.057 (0.006–0.540) | 0.013 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Female | 0.194 (0.056–0.677) | 0.010 | ||
| Age, per year | 1.060 (1.004–1.118) | 0.035 | ||
| AF duration >48 h | 1.913 (0.391–9.370) | 0.424 | ||
| COPD | 1.153 (0.913–1.956) | 0.128 | ||
| Previous AF ablation | 0.351 (0.114–1.084) | 0.069 | ||
| LVEF, per % increase | 0.951 (0.906–0.999) | 0.045 | ||
| Beta-blocker use | 0.250 (0.067–0.928) | 0.038 | ||
| IRAF after initial CV | 0.215 (0.068–0.685) | 0.009 | 0.047 (0.004–0.498) | 0.011 |
| ACEI or ARB use | 0.226 (0.078–0.659) | 0.006 | 0.101 (0.015–0.691) | 0.019 |
| Vernakalant use | 0.438 (0.158–1.212) | 0.112 | 0.057 (0.006–0.540) | 0.013 |
AF, atrial fibrillation; IRAF, immediate recurrence of AF; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Parameters associated with successful cardioversion in uni- and multivariate analysis
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Female | 0.194 (0.056–0.677) | 0.010 | ||
| Age, per year | 1.060 (1.004–1.118) | 0.035 | ||
| AF duration >48 h | 1.913 (0.391–9.370) | 0.424 | ||
| COPD | 1.153 (0.913–1.956) | 0.128 | ||
| Previous AF ablation | 0.351 (0.114–1.084) | 0.069 | ||
| LVEF, per % increase | 0.951 (0.906–0.999) | 0.045 | ||
| Beta-blocker use | 0.250 (0.067–0.928) | 0.038 | ||
| IRAF after initial CV | 0.215 (0.068–0.685) | 0.009 | 0.047 (0.004–0.498) | 0.011 |
| ACEI or ARB use | 0.226 (0.078–0.659) | 0.006 | 0.101 (0.015–0.691) | 0.019 |
| Vernakalant use | 0.438 (0.158–1.212) | 0.112 | 0.057 (0.006–0.540) | 0.013 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Female | 0.194 (0.056–0.677) | 0.010 | ||
| Age, per year | 1.060 (1.004–1.118) | 0.035 | ||
| AF duration >48 h | 1.913 (0.391–9.370) | 0.424 | ||
| COPD | 1.153 (0.913–1.956) | 0.128 | ||
| Previous AF ablation | 0.351 (0.114–1.084) | 0.069 | ||
| LVEF, per % increase | 0.951 (0.906–0.999) | 0.045 | ||
| Beta-blocker use | 0.250 (0.067–0.928) | 0.038 | ||
| IRAF after initial CV | 0.215 (0.068–0.685) | 0.009 | 0.047 (0.004–0.498) | 0.011 |
| ACEI or ARB use | 0.226 (0.078–0.659) | 0.006 | 0.101 (0.015–0.691) | 0.019 |
| Vernakalant use | 0.438 (0.158–1.212) | 0.112 | 0.057 (0.006–0.540) | 0.013 |
AF, atrial fibrillation; IRAF, immediate recurrence of AF; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Subgroup analysis of patients with previous AF ablation revealed a significantly higher success rate of electrical cardioversion after infusion of vernakalant than after infusion of amiodarone (Tables 4 and 5). 66.7% of previously not convertible patients could be successfully converted after vernakalant infusion, whereas only one amiodarone-treated patient (11.1%) could be converted into sinus rhythm (P = 0.016).
Baseline parameters of patients with previous AF ablation prior to drug-facilitated electrical cardioversion
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 9 | 9 | |
| Female, n (%) | 2 (22.2) | 2 (22.2) | 1.00 |
| Age (years) | 61.4 ± 7.8 | 65.0 ± 10.6 | 0.431 |
| Weight (kg) | 92.4 ± 21.1 | 84.6 ± 16.8 | 0.393 |
| Height (cm) | 175 ± 9 | 173 ± 10 | 0.672 |
| BMI (kg/m2) | 29.4 ± 5.2 | 28.0 ± 4.6 | 0.454 |
| Coronary heart disease, n (%) | 0 (0) | 2 (22.2) | 0.134 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | |
| Hypertension, n (%) | 9 (100.0) | 7 (77.8) | 0.134 |
| Diabetes mellitus, n (%) | 1 (11.1) | 2 (22.2) | 0.527 |
| COPD, n (%) | 0 (0) | 1 (11.1) | 0.303 |
| LVEF (%) | 50 ± 10 | 57 ± 16 | 0.307 |
| LA diameter (mm) | 45 ± 7 | 46 ± 5 | 0.900 |
| Mitral insufficiency | 6 (66.7) | 7 (77.8) | 0.599 |
| Beta-blockers, n (%) | 9 (100) | 7 (77.7) | 0.614 |
| Digitalis, n (%) | 2 (22.2) | 2 (22.2) | 1.000 |
| ACEI or ARB, n (%) | 6 (66.7) | 4 (44.4) | 0.343 |
| AF Ablation within last 30 days, n (%) | 7 (77.8) | 7 (77.8) | 1.000 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.869 |
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 9 | 9 | |
| Female, n (%) | 2 (22.2) | 2 (22.2) | 1.00 |
| Age (years) | 61.4 ± 7.8 | 65.0 ± 10.6 | 0.431 |
| Weight (kg) | 92.4 ± 21.1 | 84.6 ± 16.8 | 0.393 |
| Height (cm) | 175 ± 9 | 173 ± 10 | 0.672 |
| BMI (kg/m2) | 29.4 ± 5.2 | 28.0 ± 4.6 | 0.454 |
| Coronary heart disease, n (%) | 0 (0) | 2 (22.2) | 0.134 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | |
| Hypertension, n (%) | 9 (100.0) | 7 (77.8) | 0.134 |
| Diabetes mellitus, n (%) | 1 (11.1) | 2 (22.2) | 0.527 |
| COPD, n (%) | 0 (0) | 1 (11.1) | 0.303 |
| LVEF (%) | 50 ± 10 | 57 ± 16 | 0.307 |
| LA diameter (mm) | 45 ± 7 | 46 ± 5 | 0.900 |
| Mitral insufficiency | 6 (66.7) | 7 (77.8) | 0.599 |
| Beta-blockers, n (%) | 9 (100) | 7 (77.7) | 0.614 |
| Digitalis, n (%) | 2 (22.2) | 2 (22.2) | 1.000 |
| ACEI or ARB, n (%) | 6 (66.7) | 4 (44.4) | 0.343 |
| AF Ablation within last 30 days, n (%) | 7 (77.8) | 7 (77.8) | 1.000 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.869 |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Baseline parameters of patients with previous AF ablation prior to drug-facilitated electrical cardioversion
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 9 | 9 | |
| Female, n (%) | 2 (22.2) | 2 (22.2) | 1.00 |
| Age (years) | 61.4 ± 7.8 | 65.0 ± 10.6 | 0.431 |
| Weight (kg) | 92.4 ± 21.1 | 84.6 ± 16.8 | 0.393 |
| Height (cm) | 175 ± 9 | 173 ± 10 | 0.672 |
| BMI (kg/m2) | 29.4 ± 5.2 | 28.0 ± 4.6 | 0.454 |
| Coronary heart disease, n (%) | 0 (0) | 2 (22.2) | 0.134 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | |
| Hypertension, n (%) | 9 (100.0) | 7 (77.8) | 0.134 |
| Diabetes mellitus, n (%) | 1 (11.1) | 2 (22.2) | 0.527 |
| COPD, n (%) | 0 (0) | 1 (11.1) | 0.303 |
| LVEF (%) | 50 ± 10 | 57 ± 16 | 0.307 |
| LA diameter (mm) | 45 ± 7 | 46 ± 5 | 0.900 |
| Mitral insufficiency | 6 (66.7) | 7 (77.8) | 0.599 |
| Beta-blockers, n (%) | 9 (100) | 7 (77.7) | 0.614 |
| Digitalis, n (%) | 2 (22.2) | 2 (22.2) | 1.000 |
| ACEI or ARB, n (%) | 6 (66.7) | 4 (44.4) | 0.343 |
| AF Ablation within last 30 days, n (%) | 7 (77.8) | 7 (77.8) | 1.000 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.869 |
| . | Vernakalant . | Amiodarone . | P-value . |
|---|---|---|---|
| Number of patients, n | 9 | 9 | |
| Female, n (%) | 2 (22.2) | 2 (22.2) | 1.00 |
| Age (years) | 61.4 ± 7.8 | 65.0 ± 10.6 | 0.431 |
| Weight (kg) | 92.4 ± 21.1 | 84.6 ± 16.8 | 0.393 |
| Height (cm) | 175 ± 9 | 173 ± 10 | 0.672 |
| BMI (kg/m2) | 29.4 ± 5.2 | 28.0 ± 4.6 | 0.454 |
| Coronary heart disease, n (%) | 0 (0) | 2 (22.2) | 0.134 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | |
| Hypertension, n (%) | 9 (100.0) | 7 (77.8) | 0.134 |
| Diabetes mellitus, n (%) | 1 (11.1) | 2 (22.2) | 0.527 |
| COPD, n (%) | 0 (0) | 1 (11.1) | 0.303 |
| LVEF (%) | 50 ± 10 | 57 ± 16 | 0.307 |
| LA diameter (mm) | 45 ± 7 | 46 ± 5 | 0.900 |
| Mitral insufficiency | 6 (66.7) | 7 (77.8) | 0.599 |
| Beta-blockers, n (%) | 9 (100) | 7 (77.7) | 0.614 |
| Digitalis, n (%) | 2 (22.2) | 2 (22.2) | 1.000 |
| ACEI or ARB, n (%) | 6 (66.7) | 4 (44.4) | 0.343 |
| AF Ablation within last 30 days, n (%) | 7 (77.8) | 7 (77.8) | 1.000 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.3 ± 0.5 | 0.869 |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Baseline parameters of patients with previous AF ablation after drug-facilitated electrical cardioversion
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 7 | 11 | |
| Female, n (%) | 2 (28.6) | 2 (18.2) | 0.605 |
| Age (years) | 64.6 ± 8.4 | 62.3 ± 10.0 | 0.632 |
| Weight (kg) | 93 ± 18 | 85 ± 20 | 0.408 |
| Height (cm) | 175 ± 11 | 174 ± 8 | 0.827 |
| BMI (kg/m2) | 29.6 ± 4.6 | 28.0 ± 5.1 | 0.446 |
| Coronary heart disease, n (%) | 0 (0) | 2 (18.2) | 0.231 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | 0.836 |
| Hypertension, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Diabetes mellitus, n (%) | 1 (14.3) | 2 (18.2) | 0.829 |
| COPD, n (%) | 1 (14.3) | 0 (0) | 0.197 |
| LVEF (%) | 50 ± 13 | 56 ± 13 | 0.375 |
| LA diameter (mm) | 44 ± 6 | 46 ± 6 | 0.479 |
| Mitral insufficiency | 4 (57.1) | 9 (81.8) | 0.255 |
| Beta-blocker, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Digitalis, n (%) | 1 (14.3) | 3 (27.3) | 0.518 |
| ACEI or ARB, n (%) | 5 (71.4) | 5 (45.5) | 0.280 |
| AF Ablation within 30 days, n (%) | 7 (100) | 7 (63.6) | 0.070 |
| Potassium (mmol/L) | 4.2 ± 0.6 | 4.3 ± 0.3 | 0.447 |
| Vernakalant, n (%) | 6 (66.7) | 3 (33.3) | 0.016 |
| Amiodarone, n (%) | 1 (11.1) | 8 (88.9) |
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 7 | 11 | |
| Female, n (%) | 2 (28.6) | 2 (18.2) | 0.605 |
| Age (years) | 64.6 ± 8.4 | 62.3 ± 10.0 | 0.632 |
| Weight (kg) | 93 ± 18 | 85 ± 20 | 0.408 |
| Height (cm) | 175 ± 11 | 174 ± 8 | 0.827 |
| BMI (kg/m2) | 29.6 ± 4.6 | 28.0 ± 5.1 | 0.446 |
| Coronary heart disease, n (%) | 0 (0) | 2 (18.2) | 0.231 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | 0.836 |
| Hypertension, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Diabetes mellitus, n (%) | 1 (14.3) | 2 (18.2) | 0.829 |
| COPD, n (%) | 1 (14.3) | 0 (0) | 0.197 |
| LVEF (%) | 50 ± 13 | 56 ± 13 | 0.375 |
| LA diameter (mm) | 44 ± 6 | 46 ± 6 | 0.479 |
| Mitral insufficiency | 4 (57.1) | 9 (81.8) | 0.255 |
| Beta-blocker, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Digitalis, n (%) | 1 (14.3) | 3 (27.3) | 0.518 |
| ACEI or ARB, n (%) | 5 (71.4) | 5 (45.5) | 0.280 |
| AF Ablation within 30 days, n (%) | 7 (100) | 7 (63.6) | 0.070 |
| Potassium (mmol/L) | 4.2 ± 0.6 | 4.3 ± 0.3 | 0.447 |
| Vernakalant, n (%) | 6 (66.7) | 3 (33.3) | 0.016 |
| Amiodarone, n (%) | 1 (11.1) | 8 (88.9) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Baseline parameters of patients with previous AF ablation after drug-facilitated electrical cardioversion
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 7 | 11 | |
| Female, n (%) | 2 (28.6) | 2 (18.2) | 0.605 |
| Age (years) | 64.6 ± 8.4 | 62.3 ± 10.0 | 0.632 |
| Weight (kg) | 93 ± 18 | 85 ± 20 | 0.408 |
| Height (cm) | 175 ± 11 | 174 ± 8 | 0.827 |
| BMI (kg/m2) | 29.6 ± 4.6 | 28.0 ± 5.1 | 0.446 |
| Coronary heart disease, n (%) | 0 (0) | 2 (18.2) | 0.231 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | 0.836 |
| Hypertension, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Diabetes mellitus, n (%) | 1 (14.3) | 2 (18.2) | 0.829 |
| COPD, n (%) | 1 (14.3) | 0 (0) | 0.197 |
| LVEF (%) | 50 ± 13 | 56 ± 13 | 0.375 |
| LA diameter (mm) | 44 ± 6 | 46 ± 6 | 0.479 |
| Mitral insufficiency | 4 (57.1) | 9 (81.8) | 0.255 |
| Beta-blocker, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Digitalis, n (%) | 1 (14.3) | 3 (27.3) | 0.518 |
| ACEI or ARB, n (%) | 5 (71.4) | 5 (45.5) | 0.280 |
| AF Ablation within 30 days, n (%) | 7 (100) | 7 (63.6) | 0.070 |
| Potassium (mmol/L) | 4.2 ± 0.6 | 4.3 ± 0.3 | 0.447 |
| Vernakalant, n (%) | 6 (66.7) | 3 (33.3) | 0.016 |
| Amiodarone, n (%) | 1 (11.1) | 8 (88.9) |
| . | SR . | IRAF or FECV . | P-value . |
|---|---|---|---|
| Number of patients, n | 7 | 11 | |
| Female, n (%) | 2 (28.6) | 2 (18.2) | 0.605 |
| Age (years) | 64.6 ± 8.4 | 62.3 ± 10.0 | 0.632 |
| Weight (kg) | 93 ± 18 | 85 ± 20 | 0.408 |
| Height (cm) | 175 ± 11 | 174 ± 8 | 0.827 |
| BMI (kg/m2) | 29.6 ± 4.6 | 28.0 ± 5.1 | 0.446 |
| Coronary heart disease, n (%) | 0 (0) | 2 (18.2) | 0.231 |
| Dilative cardiomyopathy, n (%) | 0 (0) | 0 (0) | 0.836 |
| Hypertension, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Diabetes mellitus, n (%) | 1 (14.3) | 2 (18.2) | 0.829 |
| COPD, n (%) | 1 (14.3) | 0 (0) | 0.197 |
| LVEF (%) | 50 ± 13 | 56 ± 13 | 0.375 |
| LA diameter (mm) | 44 ± 6 | 46 ± 6 | 0.479 |
| Mitral insufficiency | 4 (57.1) | 9 (81.8) | 0.255 |
| Beta-blocker, n (%) | 7 (100.0) | 9 (81.8) | 0.231 |
| Digitalis, n (%) | 1 (14.3) | 3 (27.3) | 0.518 |
| ACEI or ARB, n (%) | 5 (71.4) | 5 (45.5) | 0.280 |
| AF Ablation within 30 days, n (%) | 7 (100) | 7 (63.6) | 0.070 |
| Potassium (mmol/L) | 4.2 ± 0.6 | 4.3 ± 0.3 | 0.447 |
| Vernakalant, n (%) | 6 (66.7) | 3 (33.3) | 0.016 |
| Amiodarone, n (%) | 1 (11.1) | 8 (88.9) |
Values are mean ± SD or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left-ventricular ejection fraction; LA, left atrium; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; IRAF, immediate recurrence of AF; FECV, failed electrical cardioversion.
Adverse effects
There were no major adverse events. Three patients (9.1%) in the vernakalant group described transient tingling paraesthesia in their upper body during drug infusion (vs. 0% in the amiodarone group. QT prolongation over 500 ms or QRS widening >50% has not been observed. None of the patients experienced atrial flutter.
Discussion
The efficacy of vernakalant to convert recent onset AF to sinus rhythm has been shown by previous studies.7–10 This study is the first to show that vernakalant, a novel antiarrhythmic drug for acute termination of AF, may also be useful for facilitated electrical cardioversion in cardioversion-resistant AF. In this non-randomized, observational trial, vernakalant was similarly effective as amiodarone that is frequently used in this clinical setting.
Electrical CV of AF may be unsuccessful either because of a complete inability to convert the AF to sinus rhythm, or because of IRAF. The most common cause of the failure of electrical CV to result in sinus rhythm is IRAF, which may occur in up to 26% of patients undergoing electrical CV.1–4 Failed electrical cardioversions with persistence of AF lead to the term permanent AF. The failure of complete capture by the electrical shock and persistence of electrical activity in the LA (left atrium) is considered as the pathophysiological mechanism of FECV.
The failure of transthoracic shocks to restore sinus rhythm has been largely obviated by treatment with antiarrhythmic drugs11 and by the use of biphasic shocks.12
Several drugs with antiarrhythmic properties have been tested to facilitate unsuccessful electrical CV caused by IRAF and FECV. Both, amiodarone and verapamil were effective in preventing IRAF in 50%.13 Ibutilide that is not available in many European countries was 95% effective in 22 amiodarone- or sotalol-pretreated patients14—a different cohort than our antiarrhythmic-naïve patients. However, ibutilide was frequently associated with sustained polymorphic ventricular tachycardia. More recently, ranolazine has been reported to be effective to support restoration of sinus rhythm after unsuccessful ECV in 19 of 25 (76%) patients.15
The findings of our study attest vernakalant a possible new indication in the case of cardioversion-resistant AF as the rate of sinus rhythm restoration was quite high and the drug was well tolerated. Our results imply a significantly higher success rate for treatment with vernakalant compared with amiodarone in patients with AF recurrence after previous AF ablation. Larger studies will have to confirm this finding. Despite additional costs for medication, drug-facilitated cardioversion can lead to shorter hospitalization and might therefore be cost-effective.16
Mechanisms that may lead to the suppression of IRAF and reduced defibrillation threshold include the suppression of premature atrial contractions and atrial or pulmonary vein tachycardia, as well as a reduction of atrial refractoriness heterogeneity.
Based on current concepts, AF results from the effect of triggering factors such as PAC (premature atrial contraction) and atrial tachycardia on a susceptible left atrial substrate.17 Altered electrical properties of the left atrial substrate are due to electrical and structural remodelling. Rapid atrial frequencies have been shown to result in a reversible shortening of the atrial ERP and an increased likelihood of AF recurrence. This finding is known as atrial electrical remodelling.18 Based on this concept, prolongation of the atrial effective refractory period diminishes the probability that AF will occur.18 Therefore, it has been hypothesized that a single dose of antiarrhythmic drug exerts antiarrhythmic effect beyond its pharmacological effect on cellular ion channels by stabilising sinus rhythm during the ‘super-vulnerable period’ immediately after electrical CV.
Study limitations
This observational trial provides the first data for improved cardioversion rhythm outcome using vernakalant, thus only a limited number of patients was included.
As one important limitation, we acknowledge that patient selection was not randomized. Therefore, we cannot exclude selection bias. However, both groups were similarly distributed.
Another limitation of this study is that only single doses of vernakalant and amiodarone were compared. It is also possible that higher dosages and/or longer exposure would have led to a different result after electrical CV or to conversions into sinus rhythm without the need of electrical cardioversion. This holds true especially for the efficacy of amiodarone, which seems to have a better antiarrhythmic effect after prolonged administration. A significant number of patients underwent ablation for AF before inclusion into this study. Because they were equally distributed in both groups, it is not considered as a potential confounder. Lastly, it is unclear whether the administration of the respective agent exerted its effect by prevention of the shortening of the atrial ERP or by the suppression of ectopic beats or bursts of atrial tachycardia.
Conclusion
Vernakalant was more efficient than amiodarone for drug-facilitated electrical cardioversion in patients with previous AF ablation. In patients without previous AF ablation, vernakalant was similarly effective as amiodarone. Vernakalant may therefore be considered as a useful agent for facilitated electrical cardioversion in cardioversion-resistant AF.
Conflict of interest: none declared.



