-
PDF
- Split View
-
Views
-
Cite
Cite
Mattias Aronsson, Emma Svennberg, Mårten Rosenqvist, Johan Engdahl, Faris Al-Khalili, Leif Friberg, Viveka Frykman-Kull, Lars-Åke Levin, Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording, EP Europace, Volume 17, Issue 7, July 2015, Pages 1023–1029, https://doi.org/10.1093/europace/euv083
Close - Share Icon Share
Abstract
The aim of this study was to estimate the cost-effectiveness of 2 weeks of intermittent screening for asymptomatic atrial fibrillation (AF) in 75/76-year-old individuals.
The cost-effectiveness analysis of screening in 75-year-old individuals was based on a lifelong decision analytic Markov model. In this model, 1000 hypothetical individuals, who matched the population of the STROKESTOP study, were simulated. The population was analysed for different parameters such as prevalence, AF status, treatment with oral anticoagulation, stroke risk, utility, and costs. In the base-case scenario, screening of 1000 individuals resulted in 263 fewer patient-years with undetected AF. This implies eight fewer strokes, 11 more life-years, and 12 more quality-adjusted life years (QALYs) per 1000 screened individuals. The screening implies an incremental cost of €50 012, resulting in a cost of €4313 per gained QALY and €6583 per avoided stroke.
With the use of a decision analytic simulation model, it has been shown that screening for asymptomatic AF in 75/76-year-old individuals is cost-effective.
Introduction
In Sweden, ∼210 000 individuals, representing 3% of the adult (≥20 years) population, have a hospital diagnosis of atrial fibrillation (AF).1 The prevalence of diagnosed AF increases with age and has been reported to be 9% among individuals of 75–79 years, 13.5 and 17.8% among the age groups of 80–84 years and ≥85 years, respectively.2 It has already been predicted that the future prevalence of AF will increase further due to an ageing population.3
Atrial fibrillation does not always affect quality of life but is associated with an increased risk of thrombo-embolic events,4 especially ischaemic stroke, which is associated with high costs,5 decreased quality of life,6,7 and increased mortality.8,9 The risk of stroke can successfully be reduced in ∼70% of patients with AF using oral anticoagulants (OACs).10 Oral anticoagulant treatment, according to the guidelines of European Society of Cardiology,11 is recommended in patients with AF and CHA2DS2-VASc score ≥2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category; scores range from 0 to 9).11,12 Prior studies have shown that one-third of individuals with AF might be asymptomatic, or have symptoms that are not well recognized as caused by AF.13–15 As asymptomatic individuals are less likely to seek healthcare, they might not receive appropriate treatment; hence, they are at a higher risk of sustaining an ischaemic stroke.
Importance of screening for identifying asymptomatic AF has already been realized by medical fraternity;14,16,17 opportunistic screening for AF is also recommended by international guidelines.11 Screening may help in decreasing the risk of stroke, providing positive effects such as reduced mortality and morbidity. However, screening of a large number of individuals also entails high costs.18,19 Whether the cost can be considered reasonable for the clinical gain is still a matter of debate.
The STROKESTOP study, an ongoing randomized trial, already conducted on 28 757 Swedish individuals aged between 75 and 76 years in a multicentre set-up, with the object to study whether AF screening helps in reducing the risk of stroke in the screened population.20,21 Here, we report a simulation study, mainly based on STROKESTOP data, to estimate the cost-effectiveness of 2 weeks of intermittent screening for asymptomatic AF in 75/76-year-old individuals.
Methods
Analytic approach
The cost-effectiveness analysis of screening in 75/76-year-old individuals for AF was based on a decision analytic Markov model. Such a model consists of a number of health states in which the simulated individual can be in and move between. The statistical definition of the model is a discrete-time stochastic process with Markov property. This implies that the future behaviour of the model depends only on the current health state in which the simulated individual is in and not previous events. Hence, tunnel-states were used to model memory and time-dependency in our model. Atrial fibrillation, stroke, and OAC treatment are expected to affect individuals for the rest of their lives, seldom captured in clinical trials; therefore, a lifelong simulation model is required for such a study. Using a simulation model, we analysed 1000 hypothetical individuals who matched the population of the STROKESTOP study (according to sex, age, and CHA2DS2-VASc score). The simulation of the natural disease progression and the effect of screening in men and women require data including prevalence, incidence, risk of events, morbidity, and mortality. The data, mainly from a Swedish setting, were obtained from the STROKESTOP study, also additionally supplemented with data retrieved from the published literature and registers. Both deterministic and probabilistic sensitivity analyses were performed to study uncertainty of parameters and assumptions. Figure 1 depicts the core model.

A basic description of the structure in the decision analytic Markov model. The decision problem and screening procedure is described in Part 1 of the model, while the Part 2 shows how the risk of thrombo-embolic events and bleedings depends on AF-status and CHA2DS2-VASC score. All individuals may also suffer death from non-cardiac reasons. Part 2 was repeated every month for the rest of the life of the hypothetical individuals. AF, atrial fibrillation.
The STROKESTOP study and hand-held ECG
The STROKESTOP study is described elsewhere.20,21 In short, 75/76-year-old individuals were equipped with a hand-held ECG recorder (Zenicor-EKG; Zenicor Medical Systems AB, Stockholm, Sweden); hand-held ECG has been shown to be an efficient technique to detect AF.14,22 The device transmitted information regarding the heart rhythm through a built-in cell phone to a database. The participants in the STROKESTOP study were instructed to perform 30 s recordings twice daily, or when symptoms of palpitations for 2 weeks.20,21
Prevalence of atrial fibrillation
In this analysis, we used the data from the STROKESTOP study for determining the number of individuals with AF detected from the screening procedure. Previously undiagnosed AF was found in ∼3% of the participants. The total prevalence including newly detected (3%) and previously known AF (9%) in the screened population was 12%.21
The nature of asymptomatic AF is yet to be known. We, therefore, made assumptions regarding the proportion of the asymptomatic AF that would be detected by coincidence every year (for instance, from primary care or hospital visits). In the base-case scenario, we assumed that 5% of all undetected AF would be detected every year without screening based on estimates from cardiologists. This percentage was tested in the sensitivity analyses.
Treatment of atrial fibrillation
For a screening programme to be effective, there should be an accepted treatment for patients with recognized disease that affect mortality or morbidity.23 In the case of identifying AF patients, there is strong evidence supporting that OAC treatment reduces the risk of stroke.10
According to the international guidelines, patients with a CHA2DS2-VASc score of ≥2 should be treated with OAC. All individuals with AF in the STROKESTOP study had a CHA2DS2-VASc ≥ 2, OAC should, therefore, be considered if AF was found. Considering the contraindications and concordance for OAC treatment, based on the STROKESTOP study, 93% with detected AF and 1.5% with undetected AF were expected to be treated with OAC. The discontinuation rate was in the base-case scenario modelled according to the ARISTOTLE trial.24
The new OAC (NOAC) treatments, including apixaban, rivaroxaban, and dabigatran, have been increasingly used in most industrialized countries. We expected this trend to continue; for example, Swedish authorities (The National Board of Health and Welfare) recommend that NOAC or warfarin should be considered in all new treatment initiations.25 In the base-case scenario, we assumed that apixaban (Eliquis, Pfizer) was used as the OAC treatment, because the available data from the ARISTOTLE trial24 fitted our model better than data of dabigatran and rivaroxaban.26,27 This assumption was not considered critical, because the cost of the three NOACs is similar,28 and no direct or indirect comparisons, to the best of our knowledge, have found any significant difference between the drugs in terms of effect on ischaemic stroke. However, warfarin is currently the most commonly used OAC in Sweden; hence, we included the results for warfarin in the sensitivity analysis.
Risk of events
The primary objective of the screening programme was to limit the number of ischaemic strokes. Atrial fibrillation, as an independent risk factor, causes a four- to five-fold increase in the risk of stroke.29 Apart from AF, congestive heart failure, hypertension, age, diabetes, prior stroke/TIA, vascular disease, and female gender are also expected to affect the risk of stroke. We used CHA2DS2-VASc score in the model, the widely used scoring system using those variables (mentioned above) to predict the risk of stroke. In the base-case scenario, we assumed that asymptomatic, often paroxysmal episodes of AF implied the same risk of stroke as longer symptomatic episodes.30,31 This assumption was tested in the sensitivity analyses.
The risk of major bleeding and the risk reduction for stroke of apixaban compared with warfarin were obtained from the ARISTOTLE trial24,32,33 for patients with AF treated with NOAC. The risk reductions for stroke from warfarin compared with no treatment were obtained from a meta-analysis of Hart et al.10 The risk of stroke and bleedings for individuals with undetected and untreated AF was obtained from a Swedish registry study.34 Standard mortality based on age for Sweden in 2013 was used in the simulation model.35
Resource usage and unit costs
Resources used in the screening procedure included invitation to screening, staff costs, materials, equipment, and additional examinations due to difficulties in diagnosing AF. After the screening procedure, resource usage related to thrombo-embolic events, bleedings, and OAC treatment was included. No production losses were included as few individuals in the study were expected to be in the work force due to their high age (≥75 years). A societal perspective was used otherwise.
Cost data were mainly obtained from the Southeast Healthcare region of Sweden. The mean cost of a stroke was obtained from a calculation of stroke costs in Sweden.36 The monthly drug costs were gathered from Pharmaceutical Specialties in Sweden.28 Other unit costs were obtained from published literature, and Table 1 lists all of them. Three per cent was used as the discount rate for both costs and effects in the base-case scenario. All unit costs were adjusted to the year 2014 and converted to Euro using the exchange rate on 10 January 2014 (€1 = 9 SEK).
| Parameter . | Mean . | Reference . |
|---|---|---|
| Baseline characteristics | ||
| Age | 75 | a |
| Female gender | 55.9% | a |
| CHA2DS2-VASc score | 3.45 | a |
| Prevalence unknown AF | 3.0% | 21 |
| Persistent AF | 0.5% | a |
| Paroxysmal AF | 2.5% | a |
| ECG recordings per subject | 26 | Assumption |
| Probabilities | ||
| Mortality stroke (CHADS2 = 2) | 0.269 | 8 |
| Yearly spontaneous detection of asymptomatic AF (base case) | 5% | Assumption |
| AF detected after stroke | 88.2% | 14 |
| Intracranial bleedings | 0.6 | 34 |
| Major bleedings warfarin | 5.2 | 24 |
| Major bleedings no OAC | 2.3 | 34 |
| Yearly stroke risk in AF | ||
| With apixaban | ||
| CHA2DS2-VASc 3 | 0.012 | 10,24,34 |
| CHA2DS2-VASc 4 | 0.018 | 10,24,34 |
| CHA2DS2-VASc 5 | 0.027 | 10,24,34 |
| CHA2DS2-VASc 6 | 0.037 | 10,24,34 |
| With no OAC | ||
| CHA2DS2-VASc 3 | 0.036 | 34 |
| CHA2DS2-VASc 4 | 0.054 | 34 |
| CHA2DS2-VASc 5 | 0.083 | 34 |
| CHA2DS2-VASc 6 | 0.113 | 34 |
| Proportion treated with (AF patients) | ||
| Warfarin | 0% | Base case |
| Apixaban | 93% | Base case |
| Aspirin | 0% | Base case |
| Costs (€) | ||
| Screening hand-held ECG | 106 | 37 |
| Invitation screening | 2 | 37 |
| 24-h ECG | 266 | b |
| Apixaban (yearly) | 844 | 28 |
| Stroke ≤1 year | 18 172 | 36 |
| Stroke >1 year | 4336 | 36 |
| Severe bleeding | 2927 | 38 |
| Minor bleeding | 40 | Assumption |
| Quality of life | ||
| Age 70–79 | 0.794 | 39 |
| Age 80–88 | 0.733 | 39 |
| QALY-loss ischaemic stroke (yearly) | 0.15 | 6 |
| QALY-loss bleeding stroke (yearly) | 0.30 | 6 |
| Parameter . | Mean . | Reference . |
|---|---|---|
| Baseline characteristics | ||
| Age | 75 | a |
| Female gender | 55.9% | a |
| CHA2DS2-VASc score | 3.45 | a |
| Prevalence unknown AF | 3.0% | 21 |
| Persistent AF | 0.5% | a |
| Paroxysmal AF | 2.5% | a |
| ECG recordings per subject | 26 | Assumption |
| Probabilities | ||
| Mortality stroke (CHADS2 = 2) | 0.269 | 8 |
| Yearly spontaneous detection of asymptomatic AF (base case) | 5% | Assumption |
| AF detected after stroke | 88.2% | 14 |
| Intracranial bleedings | 0.6 | 34 |
| Major bleedings warfarin | 5.2 | 24 |
| Major bleedings no OAC | 2.3 | 34 |
| Yearly stroke risk in AF | ||
| With apixaban | ||
| CHA2DS2-VASc 3 | 0.012 | 10,24,34 |
| CHA2DS2-VASc 4 | 0.018 | 10,24,34 |
| CHA2DS2-VASc 5 | 0.027 | 10,24,34 |
| CHA2DS2-VASc 6 | 0.037 | 10,24,34 |
| With no OAC | ||
| CHA2DS2-VASc 3 | 0.036 | 34 |
| CHA2DS2-VASc 4 | 0.054 | 34 |
| CHA2DS2-VASc 5 | 0.083 | 34 |
| CHA2DS2-VASc 6 | 0.113 | 34 |
| Proportion treated with (AF patients) | ||
| Warfarin | 0% | Base case |
| Apixaban | 93% | Base case |
| Aspirin | 0% | Base case |
| Costs (€) | ||
| Screening hand-held ECG | 106 | 37 |
| Invitation screening | 2 | 37 |
| 24-h ECG | 266 | b |
| Apixaban (yearly) | 844 | 28 |
| Stroke ≤1 year | 18 172 | 36 |
| Stroke >1 year | 4336 | 36 |
| Severe bleeding | 2927 | 38 |
| Minor bleeding | 40 | Assumption |
| Quality of life | ||
| Age 70–79 | 0.794 | 39 |
| Age 80–88 | 0.733 | 39 |
| QALY-loss ischaemic stroke (yearly) | 0.15 | 6 |
| QALY-loss bleeding stroke (yearly) | 0.30 | 6 |
OAC, oral anticoagulant; AF, Atrial fibrillation; ECG, Electrocardiography; QALY, Quality-adjusted life-year.
aUnpublished data from the STROKESTOP study.
bCost at Department of Cardiology, Linköping University Hospital.
| Parameter . | Mean . | Reference . |
|---|---|---|
| Baseline characteristics | ||
| Age | 75 | a |
| Female gender | 55.9% | a |
| CHA2DS2-VASc score | 3.45 | a |
| Prevalence unknown AF | 3.0% | 21 |
| Persistent AF | 0.5% | a |
| Paroxysmal AF | 2.5% | a |
| ECG recordings per subject | 26 | Assumption |
| Probabilities | ||
| Mortality stroke (CHADS2 = 2) | 0.269 | 8 |
| Yearly spontaneous detection of asymptomatic AF (base case) | 5% | Assumption |
| AF detected after stroke | 88.2% | 14 |
| Intracranial bleedings | 0.6 | 34 |
| Major bleedings warfarin | 5.2 | 24 |
| Major bleedings no OAC | 2.3 | 34 |
| Yearly stroke risk in AF | ||
| With apixaban | ||
| CHA2DS2-VASc 3 | 0.012 | 10,24,34 |
| CHA2DS2-VASc 4 | 0.018 | 10,24,34 |
| CHA2DS2-VASc 5 | 0.027 | 10,24,34 |
| CHA2DS2-VASc 6 | 0.037 | 10,24,34 |
| With no OAC | ||
| CHA2DS2-VASc 3 | 0.036 | 34 |
| CHA2DS2-VASc 4 | 0.054 | 34 |
| CHA2DS2-VASc 5 | 0.083 | 34 |
| CHA2DS2-VASc 6 | 0.113 | 34 |
| Proportion treated with (AF patients) | ||
| Warfarin | 0% | Base case |
| Apixaban | 93% | Base case |
| Aspirin | 0% | Base case |
| Costs (€) | ||
| Screening hand-held ECG | 106 | 37 |
| Invitation screening | 2 | 37 |
| 24-h ECG | 266 | b |
| Apixaban (yearly) | 844 | 28 |
| Stroke ≤1 year | 18 172 | 36 |
| Stroke >1 year | 4336 | 36 |
| Severe bleeding | 2927 | 38 |
| Minor bleeding | 40 | Assumption |
| Quality of life | ||
| Age 70–79 | 0.794 | 39 |
| Age 80–88 | 0.733 | 39 |
| QALY-loss ischaemic stroke (yearly) | 0.15 | 6 |
| QALY-loss bleeding stroke (yearly) | 0.30 | 6 |
| Parameter . | Mean . | Reference . |
|---|---|---|
| Baseline characteristics | ||
| Age | 75 | a |
| Female gender | 55.9% | a |
| CHA2DS2-VASc score | 3.45 | a |
| Prevalence unknown AF | 3.0% | 21 |
| Persistent AF | 0.5% | a |
| Paroxysmal AF | 2.5% | a |
| ECG recordings per subject | 26 | Assumption |
| Probabilities | ||
| Mortality stroke (CHADS2 = 2) | 0.269 | 8 |
| Yearly spontaneous detection of asymptomatic AF (base case) | 5% | Assumption |
| AF detected after stroke | 88.2% | 14 |
| Intracranial bleedings | 0.6 | 34 |
| Major bleedings warfarin | 5.2 | 24 |
| Major bleedings no OAC | 2.3 | 34 |
| Yearly stroke risk in AF | ||
| With apixaban | ||
| CHA2DS2-VASc 3 | 0.012 | 10,24,34 |
| CHA2DS2-VASc 4 | 0.018 | 10,24,34 |
| CHA2DS2-VASc 5 | 0.027 | 10,24,34 |
| CHA2DS2-VASc 6 | 0.037 | 10,24,34 |
| With no OAC | ||
| CHA2DS2-VASc 3 | 0.036 | 34 |
| CHA2DS2-VASc 4 | 0.054 | 34 |
| CHA2DS2-VASc 5 | 0.083 | 34 |
| CHA2DS2-VASc 6 | 0.113 | 34 |
| Proportion treated with (AF patients) | ||
| Warfarin | 0% | Base case |
| Apixaban | 93% | Base case |
| Aspirin | 0% | Base case |
| Costs (€) | ||
| Screening hand-held ECG | 106 | 37 |
| Invitation screening | 2 | 37 |
| 24-h ECG | 266 | b |
| Apixaban (yearly) | 844 | 28 |
| Stroke ≤1 year | 18 172 | 36 |
| Stroke >1 year | 4336 | 36 |
| Severe bleeding | 2927 | 38 |
| Minor bleeding | 40 | Assumption |
| Quality of life | ||
| Age 70–79 | 0.794 | 39 |
| Age 80–88 | 0.733 | 39 |
| QALY-loss ischaemic stroke (yearly) | 0.15 | 6 |
| QALY-loss bleeding stroke (yearly) | 0.30 | 6 |
OAC, oral anticoagulant; AF, Atrial fibrillation; ECG, Electrocardiography; QALY, Quality-adjusted life-year.
aUnpublished data from the STROKESTOP study.
bCost at Department of Cardiology, Linköping University Hospital.
Utility weights
The quality-adjusted life year (QALY) weights used in the model were attributed to the participants' age based on the utility in the overall population of Sweden (collected with EQ-5D).39 Ischaemic and haemorrhagic stroke were expected to decrease the quality of life for the individuals.6Table 1 presents the quality of life and utility decrements used in the model together with the other parameters.
Results
Base-case scenario
Figure 2 shows the number of individuals diagnosed with AF among 1000 screened hypothetical individuals. Our lifelong model simulation showed that mass screening results in earlier and higher detection of AF. The purple area, in Figure 2, shows the number of patients with detected AF without screening, while the red line shows the number of patients with detected AF when screening was implemented. The yellow area between the red and the purple line is the incremental number of patient-years with detected AF resulting from the screening. This area could be considered as patient-years in a lower risk of stroke due to treatment with OAC.

The number of patients experience AF when 1000 individuals are simulated in the model. The red and purple lines show the number of individuals with detected AF. AF, atrial fibrillation.
In the base-case scenario presented in Table 2, screening of 1000 individuals resulted in 263 more patient-years with detected AF (dotted area). This implied 3 more major bleedings but 8 fewer strokes, 11 more life-years, and 12 gained QALYs per 1000 screened individuals. The screening implied an incremental cost of €50 012, resulting in a cost of €4313 per gained QALY and €6583 per avoided stroke.
| . | Lifetime costs . | Strokes . | Life years . | QALY . | Cost per gained life year . | Cost per gained QALY . |
|---|---|---|---|---|---|---|
| No screening | €3 885 879 | 92 | 9835 | 6646 | ||
| Screening | €3 935 891 | 85 | 9847 | 6657 | €4365 | €4313 |
| . | Lifetime costs . | Strokes . | Life years . | QALY . | Cost per gained life year . | Cost per gained QALY . |
|---|---|---|---|---|---|---|
| No screening | €3 885 879 | 92 | 9835 | 6646 | ||
| Screening | €3 935 891 | 85 | 9847 | 6657 | €4365 | €4313 |
QALY, quality-adjusted life year.
| . | Lifetime costs . | Strokes . | Life years . | QALY . | Cost per gained life year . | Cost per gained QALY . |
|---|---|---|---|---|---|---|
| No screening | €3 885 879 | 92 | 9835 | 6646 | ||
| Screening | €3 935 891 | 85 | 9847 | 6657 | €4365 | €4313 |
| . | Lifetime costs . | Strokes . | Life years . | QALY . | Cost per gained life year . | Cost per gained QALY . |
|---|---|---|---|---|---|---|
| No screening | €3 885 879 | 92 | 9835 | 6646 | ||
| Screening | €3 935 891 | 85 | 9847 | 6657 | €4365 | €4313 |
QALY, quality-adjusted life year.
Sensitivity analyses
In order to study the uncertainty in our results, the statistical uncertainty was tested probabilistically 10 000 times. The result of the probabilistic analysis, presented as acceptability curves in Figure 3, shows that if the willingness to pay for a QALY is higher than €5000, screening with hand-held ECG is probably cost-effective.
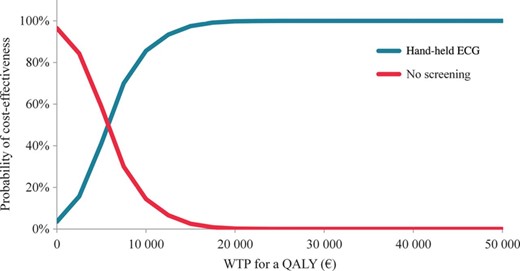
Acceptability curves. The purple curve shows the probability of screening to be cost-effective, while the red line corresponds to the equivalent of no screening.
Table 3 presents the deterministic sensitivity analyses. The results of the simulation model are sensitive to changes in the time horizon, the prevalence of undetected AF, and the stroke risk in asymptomatic AF.
| Scenario . | Value . | Cost per gained QALY (€) . |
|---|---|---|
| Time horizon | 5 years | 78 049 |
| 10 years | 19 092 | |
| 15 years | 7855 | |
| Discount rate | 0% | 34 |
| 5% | 7874 | |
| Prevalence of unknown AF (in 75-years-old individuals) | 1% | 26 892 |
| 5% | 373 | |
| Yearly spontaneous detection of AF | 0% | 2590 |
| 10% | 6232 | |
| OAC adherence | 50% | 12 423 |
| 100% | 3661 | |
| CHA2DS2-VASc (mean) | 2.5 | 16 176 |
| 4.5 | –628 | |
| Asymptomatic AF stroke risk (in relation to symptomatic AF) | +25% | −1467 |
| −25% | 15 090 | |
| −50% | 40 422 | |
| Warfarin (used as OAC) | 4040 | |
| Discontinuation rate OAC | 20% year 1 (decreases yearly with 30%) | 10 175 |
| Scenario . | Value . | Cost per gained QALY (€) . |
|---|---|---|
| Time horizon | 5 years | 78 049 |
| 10 years | 19 092 | |
| 15 years | 7855 | |
| Discount rate | 0% | 34 |
| 5% | 7874 | |
| Prevalence of unknown AF (in 75-years-old individuals) | 1% | 26 892 |
| 5% | 373 | |
| Yearly spontaneous detection of AF | 0% | 2590 |
| 10% | 6232 | |
| OAC adherence | 50% | 12 423 |
| 100% | 3661 | |
| CHA2DS2-VASc (mean) | 2.5 | 16 176 |
| 4.5 | –628 | |
| Asymptomatic AF stroke risk (in relation to symptomatic AF) | +25% | −1467 |
| −25% | 15 090 | |
| −50% | 40 422 | |
| Warfarin (used as OAC) | 4040 | |
| Discontinuation rate OAC | 20% year 1 (decreases yearly with 30%) | 10 175 |
| Scenario . | Value . | Cost per gained QALY (€) . |
|---|---|---|
| Time horizon | 5 years | 78 049 |
| 10 years | 19 092 | |
| 15 years | 7855 | |
| Discount rate | 0% | 34 |
| 5% | 7874 | |
| Prevalence of unknown AF (in 75-years-old individuals) | 1% | 26 892 |
| 5% | 373 | |
| Yearly spontaneous detection of AF | 0% | 2590 |
| 10% | 6232 | |
| OAC adherence | 50% | 12 423 |
| 100% | 3661 | |
| CHA2DS2-VASc (mean) | 2.5 | 16 176 |
| 4.5 | –628 | |
| Asymptomatic AF stroke risk (in relation to symptomatic AF) | +25% | −1467 |
| −25% | 15 090 | |
| −50% | 40 422 | |
| Warfarin (used as OAC) | 4040 | |
| Discontinuation rate OAC | 20% year 1 (decreases yearly with 30%) | 10 175 |
| Scenario . | Value . | Cost per gained QALY (€) . |
|---|---|---|
| Time horizon | 5 years | 78 049 |
| 10 years | 19 092 | |
| 15 years | 7855 | |
| Discount rate | 0% | 34 |
| 5% | 7874 | |
| Prevalence of unknown AF (in 75-years-old individuals) | 1% | 26 892 |
| 5% | 373 | |
| Yearly spontaneous detection of AF | 0% | 2590 |
| 10% | 6232 | |
| OAC adherence | 50% | 12 423 |
| 100% | 3661 | |
| CHA2DS2-VASc (mean) | 2.5 | 16 176 |
| 4.5 | –628 | |
| Asymptomatic AF stroke risk (in relation to symptomatic AF) | +25% | −1467 |
| −25% | 15 090 | |
| −50% | 40 422 | |
| Warfarin (used as OAC) | 4040 | |
| Discontinuation rate OAC | 20% year 1 (decreases yearly with 30%) | 10 175 |
Discussion
Our analyses showed that the screening programme studied in the STROKESTOP study implied a cost of €4313 per QALY considering the lifelong perspective. This is a cost-effectiveness ratio, traditionally regarded as a low cost per QALY. If this screening programme is used in a country with 10 million residents (Sweden), ∼36 000 (0.4% of the total population) individuals aged 75 years would be suitable for screening every year (participation: 53.5%).40 Based on this analysis, the screening for asymptomatic AF would result in ∼270 fewer strokes, 410 more life-years, and 420 more QALYs to an incremental cost of €1 800 000.
To the best of our knowledge, only three other cost-effectiveness analyses of screening for AF exist.17,37,41 Levin et al.37 found that screening of individuals, who have previously experienced a stroke, implies lower costs and gained QALYs compared with no screening. In the same study, the authors showed that screening with hand-held ECG for 30 days was both less costly and more efficient in finding AF than the 24-h Holter ECG. Hand-held ECG could be applicable also for primary prevention as it is important for the screening technique to imply a low unit cost in case of numerous screenings. Lowres et al.41 presented a cost of €3142 per gained QALY when screening for AF in pharmacies using iPhone electrocardiogram. The results from that model-based analysis are similar to the results from the present study, despite being conducted in another setting (Australian) and in an older population (mean age was 79 years). Further, Hobbs et al.17 showed, in a piggyback study, that opportunistic screening of 65-year-old individuals in UK implies a cost of £363 per case detected. Additionally, two screening techniques that have been shown to be effective in identifying unknown AF, but have not been studied from a health economic perspective, are insertable cardiac monitors and 14-day ambulatory ECG adhesive patch monitors.42,43 Future studies need to investigate the relative effectiveness of these continuous monitoring devices compared with intermittent long-term screening techniques such as hand-held ECG and MyDiagnostick.44
A limitation with the use of Markov model simulations is that we have to rely on assumptions about the model structure and parameters when no reliable data is available. In the present screening model, we assumed that asymptomatic AF is associated with the same risks as symptomatic AF. This assumption was based on the findings of trials on the device-treated patients30 and type 2 diabetic patients.31 However, it is yet to be established whether this is a correct assumption. As shown by the sensitivity analyses (Table 3), this assumption could have an effect on the study results; therefore, it is important to conduct further studies on the risk of stroke in individuals with asymptomatic AF. However, even if this risk is 50% compared with the risk in symptomatic AF, the cost per QALY is reasonable. In the model, we also assumed that 93% of AF detected with screening will be treated with OAC based on the results from the STROKESTOP study. The importance of initiating OAC treatment when AF is found is shown in the sensitivity analyses (Table 3). We tried to minimize the uncertainty in the assumptions by using deterministic one-way sensitivity analyses and the statistical uncertainty by using Monte-Carlo simulations.
The parameters used in this model simulation were mainly based on STROKESTOP study data from the Stockholm County. As shown in the STROKESTOP study, the participation and the proportion of previously undetected AF were lower in this area compared with the rural region of Halland.21 If Halland is more representative for the overall population of Sweden, then the cost per gained QALY and avoided stroke would be lower.
A pilot study prior to the STROKESTOP study also presented relatively large differences in participation between demographic areas.45 Additionally, this study found a correlation between areas with low participation, high proportion of immigrants, and a high CHA2DS2-VASc score. As shown in Table 3, a higher risk of stroke implies a lower cost-effectiveness ratio. This could motivate tailored efforts to increase the uptake in such areas. The participation rate is, in general, an important parameter from a public health perspective. Screening programmes that are shown to be clinical- and cost-effective should be designed to include as many participants as possible. Simplifying the screening procedure and other methods to increase the participation rate from the STROKESTOP should be discussed in further analyses.
As the cost per gained QALY is relatively low, the discussion should not be limited to whether a screening programme should be applied in the healthcare or not, but also in whom, when, and how it should be implemented. For instance, repeated intermittent screening could find previously undetected AF to a reasonable cost, and the age of 75 years may not be optimal in terms of clinical- and cost-effectiveness. The discussion of the appropriate age at which the screening should start has previously been highlighted.46 The screening age of 75 and 76 was chosen in the STROKESTOP study primarily because of three reasons: high prevalence of AF, a high risk for stroke according to the CHA2DS2-VASc score, and a strong recommendation for OAC treatment when AF is diagnosed. However, there are probably no major differences between 74- and 75-year-old individuals in terms of stroke risk and prevalence. There are no reasons for exposing 74-year-old individuals to an increased risk if it could be avoided at a reasonable cost. Additionally, studies have shown that intermittent screening can detect AF after the 14 days used in this model and the STROKESTOP study.14 Future analyses, therefore, have to investigate whether the screening should be conducted for more days, implemented in a younger population or be repeated.
Conclusions
With the use of a decision analytic simulation model, it has been shown that screening for asymptomatic AF in 75/76-year-old individuals is cost-effective.
Funding
This work was supported by grants from the Swedish Heart and Lung Foundation, the Board of Benevolence of the Swedish Order of Freemasons, and Tornspiran.
Conflict of interest: E.S. has received research grant from Boehringer-Ingelheim, and fees for lectures from MSD, Boehringer-Ingelheim, and Sanofi. M.R. has received research grants, been a consultant and given lectures for Bayer, Boehringer-Ingelheim, Pfizer, Medtronic, Zenicor, and St Jude Medical. J.E. has received consultant fees from Boehringen-Ingelheim, Pfizer, Bristol-Myers-Squibb, AstraZeneca, Medtronic, and Sanofi. F.A. has received fees for lectures from Bayer, Boehringer-Ingelheim, and Pfizer. L.F. has received research grants from Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, and Sanofi and fees for lectures from Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, Sanofi, and St Jude Medical. V.F.-K. received grants and performed study collaboration with Medtronic and study collaboration with St Jude Medical outside the submitted work. L.-Å.L reports economic support for lecturing, advisory boards and research from AstraZeneca, Bayer, Boehringer Ingelheim, Pfizer, and St Jude Medicals.



