-
PDF
- Split View
-
Views
-
Cite
Cite
Marian Christoph, Carsten Wunderlich, Stefanie Moebius, Mathias Forkmann, Judith Sitzy, Jozef Salmas, Julia Mayer, Yan Huo, Christopher Piorkowski, Thomas Gaspar, Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias, EP Europace, Volume 17, Issue 6, June 2015, Pages 928–937, https://doi.org/10.1093/europace/euu334
Close - Share Icon Share
Abstract
Despite the use of established 3D-mapping systems, invasive electrophysiological studies and catheter ablation require high radiation exposure of patients and medical staff. This study investigated whether electroanatomic catheter tracking in prerecorded X-ray images on top of an existing 3D-mapping system has any impact on radiation exposure.
Two hundred and ninety-five consecutive patients were either ablated with the guidance of the traditional CARTO-3 system (c3) or with help of the CARTO-UNIVU system (cU): [typical atrial flutter (AFL) n = 58, drug refractory atrial fibrillation (AF) n = 81, ectopic atrial tachycardia (EAT) n = 37, accessory pathways (APs) n = 22, symptomatic, idiopathic premature ventricular complexes (PVCs) n = 56, ventricular tachycardias (VTs) n = 41]. The CARTO-UNIVU allowed a reduction in radiation exposure: fluoroscopy time: AFL c3: 8.6 ± 0.8 min vs. cU: 2.9 ± 0.3 min, P < 0.001; AF c3: 16.0 ± 1.3 min vs. cU: 6.4 ± 0.9 min, P < 0.001; EAT c3: 23.4 ± 3.1 min vs. cU: 9.7 ± 1.7 min, P < 0.001; AP c3: 7.1 ± 1.2min vs. cU: 6.0 ± 1.5 min, P = 0.59; PVCs c3: 17.6 ± 2.3 min vs. cU: 15.2 ± 2.8 min, P = 0.52; VT c3: 31.4 ± 3.4 min vs. cU: 17.5 ± 2.4 min, P = 0.003. Corresponding to the fluoroscopy time the fluoroscopy dose was also reduced significantly. These advantages were not at the cost of increased procedure times, periprocedural complications, or decreased acute ablation success rates.
In a wide spectrum of cardiac arrhythmias, and especially in AF and VT ablation, fluoroscopy integrated 3D mapping contributed to a dramatic reduction in radiation exposure without prolonging procedure times and compromising patient's safety. That effect, however, could not be maintained in patients with APs and PVCs.
We report about our single centre experience of the new 3D-mapping system CARTO-UNIVU, which provides electroanatomic catheter tracking in prerecorded X-ray images and angiograms.
The option of angiogram overlay improves intra-cardiac due to permanent visualization of the endocardial chamber surface within a rough generic fluoroscopic cardiac anatomy.
This study demonstrated that the use of CARTO-UNIVU contributed to a dramatic reduction in radiation exposure during ablation of a wide spectrum of cardiac arrhythmias without prolonging procedure times and compromising patient's safety.
Introduction
Although the use of 3D-mapping systems has increased, the majority of invasive electrophysiological procedures are fundamentally guided by fluoroscopy-based catheter visualization. With a better understanding of the different mechanisms and substrates of various arrhythmias radiofrequency ablation has emerged as an important treatment modality. This holds true also for more complex arrhythmias like atrial fibrillation (AF) and ventricular tachycardia (VT). Because improving ablation results and the increasing incidences of complex arrhythmias, the issue of radiation exposure to patients and involved medical staff has become increasingly important. Owing to the fact of even low-dose radiation inducing chromosomal abnormalities with possibly serious consequences, new technologies for non-fluoroscopic visualization of diagnostic and ablation catheter are desirable.1,2 Recently, a new 3D non-fluoroscopic navigation system software (CARTO-UNIVU™ Module of CARTO® 3 system, Biosense Webster) was developed that allows electroanatomical localization of diagnostic and ablation catheters in pre-recorded X-ray images or non-gated X-ray videos. In this study, we analysed the impact of such ‘fluoroscopy integrated 3D mapping’ using the CARTO-UNIVU module on fluoroscopy time, dose area product, procedural duration, and procedural safety in a wide spectrum of cardiac arrhythmias as compared with an established standalone 3D-mapping system CARTO-3® (Biosense Webster).
Methods
Study design
This study was a retrospective, single-centre study performed in compliance with the guidelines for good clinical practice and the Declaration of Helsinki. The study was approved by the institutional ethical review board. All data were collected, managed, and analysed at the Heart Centre, University of Dresden (ethics votum University of Dresden: EK 28409202).
The primary endpoints of this study was the change of fluoroscopy time and fluoroscopy dose during different ablation procedures using fluoroscopy integrated 3D mapping (CARTO-UNIVU) compared with the traditional 3D-mapping system (CARTO-3).
The secondary endpoints were the change of procedure time, radiofrequency application time, radiofrequency application dose as well as the procedure-step-specific fluoroscopy time and dose using CARTO-UNIVU compared with CARTO-3 only. Additionally, procedural success and procedure-related adverse clinical events were collected.
Study population and protocol
Eligible subjects were consecutive male or females >18 years of age suffering either from symptomatic drug-resistant supra-ventricular tachycardia [typical atrial flutter (AFL), AF, accessory pathway (AP), ectopic atrial tachycardia (EAT)], symptomatic premature ventricular complexes (PVCs) or from VT requiring catheter ablation between February and December 2013. Within this period in the first 5 months, only the CARTO-3 group was enrolled because the CARTO-UNIVU system was not available. In the following 6 months, only the CARTO-UNIVU system was used. Other inclusion or treatment group selection criteria did not exist. The only exclusion criteria were ablation procedures performed with a different 3D-mapping system other than CARTO or without 3D mapping at all. If a patient fulfilled all inclusion criteria and none of the exclusion criteria, the clinical data, intra-procedural data, and complications were analysed retrospectively. Five different experienced operators (more than 5 years of experience in electrophysiological studies with approximately 300 procedures per year) performed all ablation procedures.
The control group consisted of all consecutive patients, which were ablated with the CARTO-3 system (c3). In the treatment group included all consecutive patients, ablated with the CARTO-UNIVU (cU) system. A statistical-based matching of both groups was not performed.
Technology description
The CARTO-3® system (Biosense Webster) combines electromagnetic navigation with current-based catheter localization. A locator sensor, localized in the proximal end of a multi polar spiral mapping catheter (Lasso®eco NAV catheter, Biosense Webster) or in the distal end of the ablation catheter (Thermocool® SF catheter, Biosense Webster) interacts with three electromagnetic fields, generated by a three coil location pad positioned underneath the patient table, providing real-time 3D electromagnetic navigation. Electric fields between six surface electrode patches on the patient's front and back provide additional information for catheter electrode localization (electrodes without locator sensor) via current ratio calculations.3 With the help of this technology electroanatomic maps (EAMs) of the different heart chambers can be created either only with the magnetic sensor equipped tip of the ablation catheter or with all electrodes in case of the use of an MEM enabled catheter (multi-electrode mapping, for example Lasso®eco NAV catheter) for further non-fluoroscopic visualization during the further electrophysiology (EP) procedure.
The CARTO-UNIVU module (CARTO-UNIVU™ Module of CARTO® 3 system, Biosense Webster, California, USA) is an additional tool of CARTO-3, which consists of a registration plate fixed on the three-coil location pad and a software module. Thus, CARTO-UNIVU is a ‘conventional’ CARTO-3 system with image (and non-gated video) integration options. The aim of the CARTO-UNIVU module is electroanatomic catheter visualization in prerecorded X-ray images or prerecorded non-gated fluoroscopy video loops and angiographies. Noteworthy, the catheter visualization on the prerecorded images or cine-loops is neither electrocardiogram nor respiratory movement compensated. This is the most important limitation of this technology. As a result, the operator is able to manipulate the catheters, simulating the use of fluoroscopy (optional in two different fluoroscopic views simultaneously) but without the use of further irradiation exposure. Alignment of CARTO-UNIVU™ with the X-ray machine requires only one registration X-ray image of the registration plate. In all procedures during the study a map shift of the pre-recorded images was never recognized. But in case a map shift would have been detected, a new registration step and the recording of new X-ray images are recommended. The set-up of CARTO-UNIVU is illustrated in Figure 1.
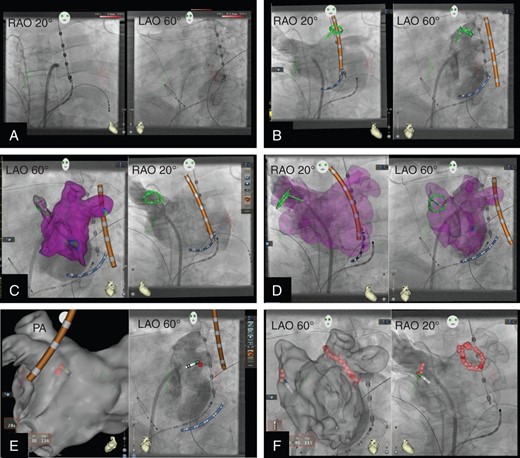
Representative periprocedural CARTO-UNIVU (cU) images during AF ablation. (A) cU display of pseudo biplane X-ray images in RAO 20° and LAO 60° recorded with a monoplane fluoroscopy system; diagnostic catheters in right ventricular apex and coronary sinus, trans-septal sheath and oesophageal temperature probe are shown. (B) cU display of pseudo biplane angiography of the left atrium (LA) including the pulmonary veins; active cU tracking of the CS catheter (blue), the temperature probe (orange) and the spiral mapping chatheter (green) which is positioned in the left superior pulmonary vein. (C) Left panel active cU tracking of catheters within the angiography of the LA and overlay of a part of the corresponding EAM including substrate mapping (purple colour encodes healthy myocardium); right panel display simultaneously to the left panel the pseudo biplane angiography in LAO view with active cU catheter tracking; the spiral catheter is positioned in the right superior pulmonary vein. (D) Angiography of the LA and overlay of the EAM including substrate mapping in pseudo-biplane view. (E) Start of the PVI, the left panel shows the EAM without substrate mapping in PA view; the temperature probe (orange) and the tip ablation catheter (red tip) are displayed; the first ablation point which was acquired with automated point acquisition tool (VISITAG) is displayed, the left plane shows the corresponding angiography in LAO view with active cU catheter tracking and the first ablation point. (F) Pseudo-biplane view of the LA angiography with the EAM as overlay (high transparency) and active cU tracking of the ablation catheter, the isolation of the left pulmonary veins is finished and the isolation of the right veins is started (all ablation points were taken with VISITAG).
In all patients the VisiTag Module (VisiTag™ Module, Biosense Webster) of CARTO-3 was used. This tool automatically acquires ablation points in the EAM, if predefined conditions are fulfilled. Additionally it displays parameter of lesion formation (ablation time, impedance, radiofrequency energy) to objectify the lesions. In the current trial the predefined ablation point acquisition conditions were defined as follows: (catheter stability for at least 6 s range of catheter movement during ablation maximum 3 mm).
Ablation procedures
The details of the specific ablation procedures are described in the supplements. In short, in all procedures one quadripolar diagnostic catheter (Biosense Webster) was advanced into right ventricular apex and one decapolar catheter (Webster® CS uni-directional catheter, Biosense Webster) into the coronary sinus under fluoroscopic guidance. If required, a single trans-septal puncture was performed with a steerable intra-cardiac sheath (Agilis™ NxT, St. Jude Medical, Inc., Minnesota, USA) under fluoroscopic guidance. Subsequently, in the CARTO-3 group EAMs including activation and substrate mapping of the tachycardia involved cardiac chambers were acquired either with the ablation catheter or with the spiral mapping-catheter under active fluoroscopic guidance if necessary. In case of an AF ablation the EAM of the left atrium and the pulmonary veins were merged with a reconstructed computed tomography (CT) model of the left atrium and pulmonary veins. Finally the ablation as well as the conformation of the ablation lines was performed with the help of the EAM and active fluoroscopy. In the CARTO-UNIVU group after positioning of the diagnostic catheter and optional trans-septal puncture the UNIVU module was registered and cine loops or angiographies were recorded in right anterior oblique (RAO) (20–30°) and left anterior oblique (LAO) (50–60°) views. Then the EAMs were created under active catheter tracking in the pre-recorded cine loops or angiographies and only if necessary under further active fluoroscopic guidance. Finally, the ablation and conformation of the ablation lines were guided with the EAMs, the pre-recorded cine loops and only if necessary with active fluoroscopy. In both groups the same fluoroscopy system and the same fluoroscopy settings were used (low radiation dose during fluoroscopy, 7.5 pictures/s with low radiation dose during film acquisition) in all procedures. In all procedures the image section was actively adapted to the area of interest by the operator for optimal radiation dose reduction.
Statistical analysis
Data were tested for normal distribution. Results of continuous variables are expressed as mean ± standard deviation. Statistical analyses were done using the 2 tailed, unpaired Student's t-test. Level of significance was set to P < 0.05. Categorical variables are expressed as number and percentage of patients. Statistical analyses of categorical variables were done using χ2 statistics and Fisher's exact test. Significance level was set at P < 0.05. The subgroup analyses of the AF patients were also performed with the 2 tailed, unpaired Student's t-test.
Results
Study population, efficacy, and safety endpoints
Between February and December 2013 in total 295 consecutive patients were included in this retrospective analyses. Both treatment groups CARTO-3 and CARTO-UNIVU were well balanced with regard to the demographics and clinical baseline characteristics for every kind of arrhythmia (Table 1). There were no relevant differences in age, gender, co-morbidities, and concomitant medications.
| . | AFL . | AF . | EAT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 26 | 32 | 37 | 44 | 16 | 21 | |||
| Gender, male % | 21 (80.8) | 25 (78.1) | 1.00 | 25 (67.6) | 27 (61.4) | 0.65 | 2 (12.5) | 3 (14.3) | 1.00 |
| Age, years (SD) | 67 (10) | 67 (11) | 0.91 | 66 (10) | 62 (12) | 0.14 | 52 (17) | 59 (15) | 0.21 |
| Body hight, meter (SD) | 1.76 (0.10) | 1.75 (0.08) | 0.77 | 1.75 (0.11) | 1.75 (0.09) | 0.95 | 1.69 (0.07) | 1.66 (0.08) | 0.29 |
| Body weight, kg (SD) | 86.6 (18.3) | 84.6 (16.2) | 0.68 | 88.3 (15.0) | 89.2 (17.9) | 0.81 | 76.5 (15.3) | 71.7 (16.8) | 0.40 |
| BMI (SD) | 28 (6) | 27.5 (4.1) | 0.70 | 28.9 (4.1) | 29.1 (5.6) | 0.83 | 26.7 (3.8) | 25.9 (5.1) | 0.63 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 56 (9.5) | 53 (11.0) | 0.39 | 52 (11) | 55 (11) | 0.22 | 56.3 (10.6) | 57.7 (9.4) | 0.66 |
| Left atrium size, mm (SD) | 44.6 (4.9) | 43.6 (6.6) | 0.77 | 45.9 (6.0) | 43.5 (5.6) | 0.15 | 34.0 (4.8) | 36.5 (6.5) | 0.40 |
| CHA2DS2-VASc-score, mean (SD) | 2.6 (1.4) | 2.4 (1.6) | 0.5 | ||||||
| Hypertension, N (%) | 14 (53.8) | 23 (71.9) | 0.18 | 29 (78.4) | 30 (68.2) | 0.33 | 6 (37.5) | 12 (57.1) | 0.33 |
| Hyperlipidemia, N (%) | 14 (53.8) | 19 (59.4) | 0.79 | 19 (51.4) | 22 (50.0) | 1.0 | 4 (25.0) | 5 (23.8) | 1.00 |
| Diabetes, N (%) | 4 (15.4) | 12 (37.5) | 0.08 | 10 (27.0) | 10 (22.7) | 0.8 | 2 (12.5) | 2 (9.5) | 1.00 |
| Coronary heart disease, N (%) | 8 (30.8) | 11 (34.4) | 1.00 | 8 (21.6) | 10 (22.7) | 1.0 | 2 (12.5) | 1 (4.8) | 0.57 |
| Device (ICD or pacemaker), N (%) | 1 (3.8) | 2 (6.25) | 1.00 | 8 (21.6) | 6 (13.6) | 0.39 | 1 (6.3) | 2 (9.5) | 1.00 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 17 (65.4) | 22 (68.8) | 1.00 | 25 (67.6) | 34 (77.3) | 0.62 | 6 (37.5) | 12 (57.1) | 0.33 |
| Beta-blocker, N (%) | 23 (88.5) | 29 (90.6) | 1.00 | 36 (97.3) | 34 (77.3) | 0.01 | 9 (56.3) | 17 (81.0) | 0.15 |
| Antiarrhythmic, N (%) | 5 (19.2) | 2 (6.25) | 0.23 | 11 (29.7) | 13 (29.5) | 1.0 | 3 (18.8) | 1 (4.8) | 0.30 |
| Statin, N (%) | 10 (38.5) | 15 (46.9) | 0.60 | 14 (37.8) | 18 (40.9) | 0.82 | 2 (12.5) | 4 (19.0) | 0.68 |
| Anticoagulation, N (%) | 17 (65.4) | 27 (84.4) | 0.13 | 36 (97.3) | 44 (100) | 1.0 | 5 (31.3) | 4 (19.0) | 0.46 |
| . | AP . | PVCs . | VT . | ||||||
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 11 | 11 | 26 | 30 | 22 | 19 | |||
| Gender, male % | 8 (72.7) | 4 (36.4) | 0.20 | 17 (65.4) | 23 (76.7) | 0.39 | 16 (72.7) | 17 (89.5) | 0.25 |
| Age, years (SD) | 46 (19) | 47 (21) | 0.87 | 63 (13) | 66 (13) | 0.45 | 72 (10) | 64 (10) | 0.03 |
| Body hight, meter (SD) | 1.76 (0.07) | 1.70 (1.10) | 0.19 | 1.73 (0.09) | 1.74 (0.09) | 0.63 | 1.69 (0.09) | 1.74 (0.07) | 0.08 |
| Body weight, kg (SD) | 78.9 (16.8) | 81.6 (23.0) | 0.78 | 81.6 (15.2) | 87.2 (20.0) | 0.25 | 79.6 (21) | 84 (15) | 0.5 |
| BMI (SD) | 25.4 (4.2) | 27.8 (4.9) | 0.29 | 27.3 (4.7) | 28.7 (5.4) | 0.32 | 27.7 (4.6) | 27.8 (4.6) | 0.95 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 57.1 (6.9) | 60.0 (0) | 0.18 | 52.3 (13.2) | 49.1 (14.0) | 0.38 | 31 (12) | 29 (9) | 0.57 |
| Left atrium size, mm, (SD) | 36.8 (5.6) | 33.5 (2.1) | 0.48 | 42.9 (5.5) | 44.0 (8.7) | 0.63 | 47.4 (3.7) | 47.7 (4.8) | 0.84 |
| CHA2DS2-VASc-score, mean (SD) | |||||||||
| Hypertension, N (%) | 4 (36.4) | 3 (27.3) | 1.00 | 16 (61.5) | 25 (83.3) | 0.08 | 20 (90.9) | 13 (68.4) | 0.11 |
| Hyperlipidemia, N (%) | 3 (27.3) | 5 (45.5) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 18 (94.7) | 0.61 |
| Diabetes, N (%) | 3 (27.3) | 2 (18.2) | 1.00 | 7 (26.9) | 8 (26.7) | 1.00 | 9 (40.9) | 5 (26.3) | 0.51 |
| Coronary heart disease, N (%) | 1 (9.1) | 1 (9.1) | 1.00 | 11 (42.3) | 17 (56.7) | 0.42 | 18 (81.8) | 14 (73.7) | 0.71 |
| Device (ICD or pacemaker), N (%) | 1 (9.1) | 0 | 1.00 | 4 (15.4) | 8 (26.7) | 0.35 | 19 (86.4) | 16 (84.2) | 1.0 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 5 (45.5) | 3 (27.3) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 17 (89.5) | 1.0 |
| Beta-blocker, N (%) | 6 (54.5) | 5 (45.5) | 1.00 | 19 (73.1) | 24 (80.0) | 0.75 | 22 (100) | 19 (100) | 1.0 |
| Antiarrhythmic, N (%) | 0 | 0 | 1.00 | 1 (3.9) | 0 | 0.46 | 8 (36.4) | 6 (31.6) | 1.0 |
| Statin, N (%) | 4 (36.4) | 2 (18.2) | 0.64 | 13 (50.0) | 18 (60.0) | 0.59 | 19 (86.4) | 16 (84.2) | 1.0 |
| Anticoagulation, N (%) | 2 (18.2) | 0 | 0.48 | 7 (26.9) | 13 (43.3) | 0.27 | 11 (50.0) | 11 (57.9) | 0.76 |
| . | AFL . | AF . | EAT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 26 | 32 | 37 | 44 | 16 | 21 | |||
| Gender, male % | 21 (80.8) | 25 (78.1) | 1.00 | 25 (67.6) | 27 (61.4) | 0.65 | 2 (12.5) | 3 (14.3) | 1.00 |
| Age, years (SD) | 67 (10) | 67 (11) | 0.91 | 66 (10) | 62 (12) | 0.14 | 52 (17) | 59 (15) | 0.21 |
| Body hight, meter (SD) | 1.76 (0.10) | 1.75 (0.08) | 0.77 | 1.75 (0.11) | 1.75 (0.09) | 0.95 | 1.69 (0.07) | 1.66 (0.08) | 0.29 |
| Body weight, kg (SD) | 86.6 (18.3) | 84.6 (16.2) | 0.68 | 88.3 (15.0) | 89.2 (17.9) | 0.81 | 76.5 (15.3) | 71.7 (16.8) | 0.40 |
| BMI (SD) | 28 (6) | 27.5 (4.1) | 0.70 | 28.9 (4.1) | 29.1 (5.6) | 0.83 | 26.7 (3.8) | 25.9 (5.1) | 0.63 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 56 (9.5) | 53 (11.0) | 0.39 | 52 (11) | 55 (11) | 0.22 | 56.3 (10.6) | 57.7 (9.4) | 0.66 |
| Left atrium size, mm (SD) | 44.6 (4.9) | 43.6 (6.6) | 0.77 | 45.9 (6.0) | 43.5 (5.6) | 0.15 | 34.0 (4.8) | 36.5 (6.5) | 0.40 |
| CHA2DS2-VASc-score, mean (SD) | 2.6 (1.4) | 2.4 (1.6) | 0.5 | ||||||
| Hypertension, N (%) | 14 (53.8) | 23 (71.9) | 0.18 | 29 (78.4) | 30 (68.2) | 0.33 | 6 (37.5) | 12 (57.1) | 0.33 |
| Hyperlipidemia, N (%) | 14 (53.8) | 19 (59.4) | 0.79 | 19 (51.4) | 22 (50.0) | 1.0 | 4 (25.0) | 5 (23.8) | 1.00 |
| Diabetes, N (%) | 4 (15.4) | 12 (37.5) | 0.08 | 10 (27.0) | 10 (22.7) | 0.8 | 2 (12.5) | 2 (9.5) | 1.00 |
| Coronary heart disease, N (%) | 8 (30.8) | 11 (34.4) | 1.00 | 8 (21.6) | 10 (22.7) | 1.0 | 2 (12.5) | 1 (4.8) | 0.57 |
| Device (ICD or pacemaker), N (%) | 1 (3.8) | 2 (6.25) | 1.00 | 8 (21.6) | 6 (13.6) | 0.39 | 1 (6.3) | 2 (9.5) | 1.00 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 17 (65.4) | 22 (68.8) | 1.00 | 25 (67.6) | 34 (77.3) | 0.62 | 6 (37.5) | 12 (57.1) | 0.33 |
| Beta-blocker, N (%) | 23 (88.5) | 29 (90.6) | 1.00 | 36 (97.3) | 34 (77.3) | 0.01 | 9 (56.3) | 17 (81.0) | 0.15 |
| Antiarrhythmic, N (%) | 5 (19.2) | 2 (6.25) | 0.23 | 11 (29.7) | 13 (29.5) | 1.0 | 3 (18.8) | 1 (4.8) | 0.30 |
| Statin, N (%) | 10 (38.5) | 15 (46.9) | 0.60 | 14 (37.8) | 18 (40.9) | 0.82 | 2 (12.5) | 4 (19.0) | 0.68 |
| Anticoagulation, N (%) | 17 (65.4) | 27 (84.4) | 0.13 | 36 (97.3) | 44 (100) | 1.0 | 5 (31.3) | 4 (19.0) | 0.46 |
| . | AP . | PVCs . | VT . | ||||||
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 11 | 11 | 26 | 30 | 22 | 19 | |||
| Gender, male % | 8 (72.7) | 4 (36.4) | 0.20 | 17 (65.4) | 23 (76.7) | 0.39 | 16 (72.7) | 17 (89.5) | 0.25 |
| Age, years (SD) | 46 (19) | 47 (21) | 0.87 | 63 (13) | 66 (13) | 0.45 | 72 (10) | 64 (10) | 0.03 |
| Body hight, meter (SD) | 1.76 (0.07) | 1.70 (1.10) | 0.19 | 1.73 (0.09) | 1.74 (0.09) | 0.63 | 1.69 (0.09) | 1.74 (0.07) | 0.08 |
| Body weight, kg (SD) | 78.9 (16.8) | 81.6 (23.0) | 0.78 | 81.6 (15.2) | 87.2 (20.0) | 0.25 | 79.6 (21) | 84 (15) | 0.5 |
| BMI (SD) | 25.4 (4.2) | 27.8 (4.9) | 0.29 | 27.3 (4.7) | 28.7 (5.4) | 0.32 | 27.7 (4.6) | 27.8 (4.6) | 0.95 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 57.1 (6.9) | 60.0 (0) | 0.18 | 52.3 (13.2) | 49.1 (14.0) | 0.38 | 31 (12) | 29 (9) | 0.57 |
| Left atrium size, mm, (SD) | 36.8 (5.6) | 33.5 (2.1) | 0.48 | 42.9 (5.5) | 44.0 (8.7) | 0.63 | 47.4 (3.7) | 47.7 (4.8) | 0.84 |
| CHA2DS2-VASc-score, mean (SD) | |||||||||
| Hypertension, N (%) | 4 (36.4) | 3 (27.3) | 1.00 | 16 (61.5) | 25 (83.3) | 0.08 | 20 (90.9) | 13 (68.4) | 0.11 |
| Hyperlipidemia, N (%) | 3 (27.3) | 5 (45.5) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 18 (94.7) | 0.61 |
| Diabetes, N (%) | 3 (27.3) | 2 (18.2) | 1.00 | 7 (26.9) | 8 (26.7) | 1.00 | 9 (40.9) | 5 (26.3) | 0.51 |
| Coronary heart disease, N (%) | 1 (9.1) | 1 (9.1) | 1.00 | 11 (42.3) | 17 (56.7) | 0.42 | 18 (81.8) | 14 (73.7) | 0.71 |
| Device (ICD or pacemaker), N (%) | 1 (9.1) | 0 | 1.00 | 4 (15.4) | 8 (26.7) | 0.35 | 19 (86.4) | 16 (84.2) | 1.0 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 5 (45.5) | 3 (27.3) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 17 (89.5) | 1.0 |
| Beta-blocker, N (%) | 6 (54.5) | 5 (45.5) | 1.00 | 19 (73.1) | 24 (80.0) | 0.75 | 22 (100) | 19 (100) | 1.0 |
| Antiarrhythmic, N (%) | 0 | 0 | 1.00 | 1 (3.9) | 0 | 0.46 | 8 (36.4) | 6 (31.6) | 1.0 |
| Statin, N (%) | 4 (36.4) | 2 (18.2) | 0.64 | 13 (50.0) | 18 (60.0) | 0.59 | 19 (86.4) | 16 (84.2) | 1.0 |
| Anticoagulation, N (%) | 2 (18.2) | 0 | 0.48 | 7 (26.9) | 13 (43.3) | 0.27 | 11 (50.0) | 11 (57.9) | 0.76 |
AFL, typical atrial flutter; AF, atrial fibrillation; EAT, ectopic atrial tachycardia; BMI, body-mass index (weight in kilograms divided by the square of the height in meters); AP, accessory pathway; PVCs, premature ventricular complexes; VT, ventricular tachycardia.
| . | AFL . | AF . | EAT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 26 | 32 | 37 | 44 | 16 | 21 | |||
| Gender, male % | 21 (80.8) | 25 (78.1) | 1.00 | 25 (67.6) | 27 (61.4) | 0.65 | 2 (12.5) | 3 (14.3) | 1.00 |
| Age, years (SD) | 67 (10) | 67 (11) | 0.91 | 66 (10) | 62 (12) | 0.14 | 52 (17) | 59 (15) | 0.21 |
| Body hight, meter (SD) | 1.76 (0.10) | 1.75 (0.08) | 0.77 | 1.75 (0.11) | 1.75 (0.09) | 0.95 | 1.69 (0.07) | 1.66 (0.08) | 0.29 |
| Body weight, kg (SD) | 86.6 (18.3) | 84.6 (16.2) | 0.68 | 88.3 (15.0) | 89.2 (17.9) | 0.81 | 76.5 (15.3) | 71.7 (16.8) | 0.40 |
| BMI (SD) | 28 (6) | 27.5 (4.1) | 0.70 | 28.9 (4.1) | 29.1 (5.6) | 0.83 | 26.7 (3.8) | 25.9 (5.1) | 0.63 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 56 (9.5) | 53 (11.0) | 0.39 | 52 (11) | 55 (11) | 0.22 | 56.3 (10.6) | 57.7 (9.4) | 0.66 |
| Left atrium size, mm (SD) | 44.6 (4.9) | 43.6 (6.6) | 0.77 | 45.9 (6.0) | 43.5 (5.6) | 0.15 | 34.0 (4.8) | 36.5 (6.5) | 0.40 |
| CHA2DS2-VASc-score, mean (SD) | 2.6 (1.4) | 2.4 (1.6) | 0.5 | ||||||
| Hypertension, N (%) | 14 (53.8) | 23 (71.9) | 0.18 | 29 (78.4) | 30 (68.2) | 0.33 | 6 (37.5) | 12 (57.1) | 0.33 |
| Hyperlipidemia, N (%) | 14 (53.8) | 19 (59.4) | 0.79 | 19 (51.4) | 22 (50.0) | 1.0 | 4 (25.0) | 5 (23.8) | 1.00 |
| Diabetes, N (%) | 4 (15.4) | 12 (37.5) | 0.08 | 10 (27.0) | 10 (22.7) | 0.8 | 2 (12.5) | 2 (9.5) | 1.00 |
| Coronary heart disease, N (%) | 8 (30.8) | 11 (34.4) | 1.00 | 8 (21.6) | 10 (22.7) | 1.0 | 2 (12.5) | 1 (4.8) | 0.57 |
| Device (ICD or pacemaker), N (%) | 1 (3.8) | 2 (6.25) | 1.00 | 8 (21.6) | 6 (13.6) | 0.39 | 1 (6.3) | 2 (9.5) | 1.00 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 17 (65.4) | 22 (68.8) | 1.00 | 25 (67.6) | 34 (77.3) | 0.62 | 6 (37.5) | 12 (57.1) | 0.33 |
| Beta-blocker, N (%) | 23 (88.5) | 29 (90.6) | 1.00 | 36 (97.3) | 34 (77.3) | 0.01 | 9 (56.3) | 17 (81.0) | 0.15 |
| Antiarrhythmic, N (%) | 5 (19.2) | 2 (6.25) | 0.23 | 11 (29.7) | 13 (29.5) | 1.0 | 3 (18.8) | 1 (4.8) | 0.30 |
| Statin, N (%) | 10 (38.5) | 15 (46.9) | 0.60 | 14 (37.8) | 18 (40.9) | 0.82 | 2 (12.5) | 4 (19.0) | 0.68 |
| Anticoagulation, N (%) | 17 (65.4) | 27 (84.4) | 0.13 | 36 (97.3) | 44 (100) | 1.0 | 5 (31.3) | 4 (19.0) | 0.46 |
| . | AP . | PVCs . | VT . | ||||||
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 11 | 11 | 26 | 30 | 22 | 19 | |||
| Gender, male % | 8 (72.7) | 4 (36.4) | 0.20 | 17 (65.4) | 23 (76.7) | 0.39 | 16 (72.7) | 17 (89.5) | 0.25 |
| Age, years (SD) | 46 (19) | 47 (21) | 0.87 | 63 (13) | 66 (13) | 0.45 | 72 (10) | 64 (10) | 0.03 |
| Body hight, meter (SD) | 1.76 (0.07) | 1.70 (1.10) | 0.19 | 1.73 (0.09) | 1.74 (0.09) | 0.63 | 1.69 (0.09) | 1.74 (0.07) | 0.08 |
| Body weight, kg (SD) | 78.9 (16.8) | 81.6 (23.0) | 0.78 | 81.6 (15.2) | 87.2 (20.0) | 0.25 | 79.6 (21) | 84 (15) | 0.5 |
| BMI (SD) | 25.4 (4.2) | 27.8 (4.9) | 0.29 | 27.3 (4.7) | 28.7 (5.4) | 0.32 | 27.7 (4.6) | 27.8 (4.6) | 0.95 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 57.1 (6.9) | 60.0 (0) | 0.18 | 52.3 (13.2) | 49.1 (14.0) | 0.38 | 31 (12) | 29 (9) | 0.57 |
| Left atrium size, mm, (SD) | 36.8 (5.6) | 33.5 (2.1) | 0.48 | 42.9 (5.5) | 44.0 (8.7) | 0.63 | 47.4 (3.7) | 47.7 (4.8) | 0.84 |
| CHA2DS2-VASc-score, mean (SD) | |||||||||
| Hypertension, N (%) | 4 (36.4) | 3 (27.3) | 1.00 | 16 (61.5) | 25 (83.3) | 0.08 | 20 (90.9) | 13 (68.4) | 0.11 |
| Hyperlipidemia, N (%) | 3 (27.3) | 5 (45.5) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 18 (94.7) | 0.61 |
| Diabetes, N (%) | 3 (27.3) | 2 (18.2) | 1.00 | 7 (26.9) | 8 (26.7) | 1.00 | 9 (40.9) | 5 (26.3) | 0.51 |
| Coronary heart disease, N (%) | 1 (9.1) | 1 (9.1) | 1.00 | 11 (42.3) | 17 (56.7) | 0.42 | 18 (81.8) | 14 (73.7) | 0.71 |
| Device (ICD or pacemaker), N (%) | 1 (9.1) | 0 | 1.00 | 4 (15.4) | 8 (26.7) | 0.35 | 19 (86.4) | 16 (84.2) | 1.0 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 5 (45.5) | 3 (27.3) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 17 (89.5) | 1.0 |
| Beta-blocker, N (%) | 6 (54.5) | 5 (45.5) | 1.00 | 19 (73.1) | 24 (80.0) | 0.75 | 22 (100) | 19 (100) | 1.0 |
| Antiarrhythmic, N (%) | 0 | 0 | 1.00 | 1 (3.9) | 0 | 0.46 | 8 (36.4) | 6 (31.6) | 1.0 |
| Statin, N (%) | 4 (36.4) | 2 (18.2) | 0.64 | 13 (50.0) | 18 (60.0) | 0.59 | 19 (86.4) | 16 (84.2) | 1.0 |
| Anticoagulation, N (%) | 2 (18.2) | 0 | 0.48 | 7 (26.9) | 13 (43.3) | 0.27 | 11 (50.0) | 11 (57.9) | 0.76 |
| . | AFL . | AF . | EAT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 26 | 32 | 37 | 44 | 16 | 21 | |||
| Gender, male % | 21 (80.8) | 25 (78.1) | 1.00 | 25 (67.6) | 27 (61.4) | 0.65 | 2 (12.5) | 3 (14.3) | 1.00 |
| Age, years (SD) | 67 (10) | 67 (11) | 0.91 | 66 (10) | 62 (12) | 0.14 | 52 (17) | 59 (15) | 0.21 |
| Body hight, meter (SD) | 1.76 (0.10) | 1.75 (0.08) | 0.77 | 1.75 (0.11) | 1.75 (0.09) | 0.95 | 1.69 (0.07) | 1.66 (0.08) | 0.29 |
| Body weight, kg (SD) | 86.6 (18.3) | 84.6 (16.2) | 0.68 | 88.3 (15.0) | 89.2 (17.9) | 0.81 | 76.5 (15.3) | 71.7 (16.8) | 0.40 |
| BMI (SD) | 28 (6) | 27.5 (4.1) | 0.70 | 28.9 (4.1) | 29.1 (5.6) | 0.83 | 26.7 (3.8) | 25.9 (5.1) | 0.63 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 56 (9.5) | 53 (11.0) | 0.39 | 52 (11) | 55 (11) | 0.22 | 56.3 (10.6) | 57.7 (9.4) | 0.66 |
| Left atrium size, mm (SD) | 44.6 (4.9) | 43.6 (6.6) | 0.77 | 45.9 (6.0) | 43.5 (5.6) | 0.15 | 34.0 (4.8) | 36.5 (6.5) | 0.40 |
| CHA2DS2-VASc-score, mean (SD) | 2.6 (1.4) | 2.4 (1.6) | 0.5 | ||||||
| Hypertension, N (%) | 14 (53.8) | 23 (71.9) | 0.18 | 29 (78.4) | 30 (68.2) | 0.33 | 6 (37.5) | 12 (57.1) | 0.33 |
| Hyperlipidemia, N (%) | 14 (53.8) | 19 (59.4) | 0.79 | 19 (51.4) | 22 (50.0) | 1.0 | 4 (25.0) | 5 (23.8) | 1.00 |
| Diabetes, N (%) | 4 (15.4) | 12 (37.5) | 0.08 | 10 (27.0) | 10 (22.7) | 0.8 | 2 (12.5) | 2 (9.5) | 1.00 |
| Coronary heart disease, N (%) | 8 (30.8) | 11 (34.4) | 1.00 | 8 (21.6) | 10 (22.7) | 1.0 | 2 (12.5) | 1 (4.8) | 0.57 |
| Device (ICD or pacemaker), N (%) | 1 (3.8) | 2 (6.25) | 1.00 | 8 (21.6) | 6 (13.6) | 0.39 | 1 (6.3) | 2 (9.5) | 1.00 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 17 (65.4) | 22 (68.8) | 1.00 | 25 (67.6) | 34 (77.3) | 0.62 | 6 (37.5) | 12 (57.1) | 0.33 |
| Beta-blocker, N (%) | 23 (88.5) | 29 (90.6) | 1.00 | 36 (97.3) | 34 (77.3) | 0.01 | 9 (56.3) | 17 (81.0) | 0.15 |
| Antiarrhythmic, N (%) | 5 (19.2) | 2 (6.25) | 0.23 | 11 (29.7) | 13 (29.5) | 1.0 | 3 (18.8) | 1 (4.8) | 0.30 |
| Statin, N (%) | 10 (38.5) | 15 (46.9) | 0.60 | 14 (37.8) | 18 (40.9) | 0.82 | 2 (12.5) | 4 (19.0) | 0.68 |
| Anticoagulation, N (%) | 17 (65.4) | 27 (84.4) | 0.13 | 36 (97.3) | 44 (100) | 1.0 | 5 (31.3) | 4 (19.0) | 0.46 |
| . | AP . | PVCs . | VT . | ||||||
| CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | CARTO-3 . | CARTO-UNIVU . | P-value . | |
| Patients (N) | 11 | 11 | 26 | 30 | 22 | 19 | |||
| Gender, male % | 8 (72.7) | 4 (36.4) | 0.20 | 17 (65.4) | 23 (76.7) | 0.39 | 16 (72.7) | 17 (89.5) | 0.25 |
| Age, years (SD) | 46 (19) | 47 (21) | 0.87 | 63 (13) | 66 (13) | 0.45 | 72 (10) | 64 (10) | 0.03 |
| Body hight, meter (SD) | 1.76 (0.07) | 1.70 (1.10) | 0.19 | 1.73 (0.09) | 1.74 (0.09) | 0.63 | 1.69 (0.09) | 1.74 (0.07) | 0.08 |
| Body weight, kg (SD) | 78.9 (16.8) | 81.6 (23.0) | 0.78 | 81.6 (15.2) | 87.2 (20.0) | 0.25 | 79.6 (21) | 84 (15) | 0.5 |
| BMI (SD) | 25.4 (4.2) | 27.8 (4.9) | 0.29 | 27.3 (4.7) | 28.7 (5.4) | 0.32 | 27.7 (4.6) | 27.8 (4.6) | 0.95 |
| Qualifying risk factors | |||||||||
| Ejection fraction, % (SD) | 57.1 (6.9) | 60.0 (0) | 0.18 | 52.3 (13.2) | 49.1 (14.0) | 0.38 | 31 (12) | 29 (9) | 0.57 |
| Left atrium size, mm, (SD) | 36.8 (5.6) | 33.5 (2.1) | 0.48 | 42.9 (5.5) | 44.0 (8.7) | 0.63 | 47.4 (3.7) | 47.7 (4.8) | 0.84 |
| CHA2DS2-VASc-score, mean (SD) | |||||||||
| Hypertension, N (%) | 4 (36.4) | 3 (27.3) | 1.00 | 16 (61.5) | 25 (83.3) | 0.08 | 20 (90.9) | 13 (68.4) | 0.11 |
| Hyperlipidemia, N (%) | 3 (27.3) | 5 (45.5) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 18 (94.7) | 0.61 |
| Diabetes, N (%) | 3 (27.3) | 2 (18.2) | 1.00 | 7 (26.9) | 8 (26.7) | 1.00 | 9 (40.9) | 5 (26.3) | 0.51 |
| Coronary heart disease, N (%) | 1 (9.1) | 1 (9.1) | 1.00 | 11 (42.3) | 17 (56.7) | 0.42 | 18 (81.8) | 14 (73.7) | 0.71 |
| Device (ICD or pacemaker), N (%) | 1 (9.1) | 0 | 1.00 | 4 (15.4) | 8 (26.7) | 0.35 | 19 (86.4) | 16 (84.2) | 1.0 |
| Medications | |||||||||
| ACE inhibitor or ARB, N (%) | 5 (45.5) | 3 (27.3) | 0.66 | 17 (65.4) | 23 (76.7) | 0.39 | 19 (86.4) | 17 (89.5) | 1.0 |
| Beta-blocker, N (%) | 6 (54.5) | 5 (45.5) | 1.00 | 19 (73.1) | 24 (80.0) | 0.75 | 22 (100) | 19 (100) | 1.0 |
| Antiarrhythmic, N (%) | 0 | 0 | 1.00 | 1 (3.9) | 0 | 0.46 | 8 (36.4) | 6 (31.6) | 1.0 |
| Statin, N (%) | 4 (36.4) | 2 (18.2) | 0.64 | 13 (50.0) | 18 (60.0) | 0.59 | 19 (86.4) | 16 (84.2) | 1.0 |
| Anticoagulation, N (%) | 2 (18.2) | 0 | 0.48 | 7 (26.9) | 13 (43.3) | 0.27 | 11 (50.0) | 11 (57.9) | 0.76 |
AFL, typical atrial flutter; AF, atrial fibrillation; EAT, ectopic atrial tachycardia; BMI, body-mass index (weight in kilograms divided by the square of the height in meters); AP, accessory pathway; PVCs, premature ventricular complexes; VT, ventricular tachycardia.
The following procedural outcome was reached: In all (n) patients with typical AFL the cavo-tricuspid isthmus could be bi-directionally blocked. In all 81 patients treated for symptomatic AF complete PVI was acutely achieved in both groups. Furthermore, all 37 patients with EAT and 22 patients with AP were successfully ablated in both groups. For patients suffering from idiopathic PVCs in 22 of 26 patients (85%) in the c3 group and 27 of 30 patients (90%) in the cU group, the ectopy was completely eliminated (P = 0.88). The predominant origins were the right and left ventricular outflow tract, but also PVCs from the aortic bulb, mitral annulus, papillary muscles, or within the coronary sinus. Because of the relative small numbers of patients, a subgroup analysis of the different origin was not performed. For patients undergoing VT ablation, the clinical VT was not inducible after ablation in all patients in both treatment groups. However, 2 of 22 patients (9%) in the c3 group and 2 of 19 patients (11%) in the cU group were still inducible with a non-clinical VT after ablation (P = 0.7).
The incidences of adverse events were similar among both groups. There was one pericardial effusion in the CARTO-3 group during a PVC ablation, which could be treated conservatively without pericardiocentesis. Additionally five pseudoaneurysms were registered: two in the CARTO-3 group and three in the CARTO-UNIVU group.
Changes of fluoroscopy time and dose (primary endpoints)
The results of fluoroscopy time and fluoroscopic dose are illustrated in Figure 2. In contrast to the CARTO-3 group, the CARTO-UNIVU group revealed a significant 66% relative reduction of the fluoroscopy time during ablation of AFL (c3: 8.6 ± 0.8 min vs. cU: 2.9 ± 0.3 min, P < 0.001), a 60% relative reduction in AF patients (c3: 16.0 ± 1.3 min vs. cU: 6.4 ± 0.9 min, P < 0.001), a 59% reduction during ablation of EATs (c3: 23.4 ± 3.1 min vs. cU: 9.7 ± 1.7 min, P < 0.001) and a significant 44% reduction during VT ablation (c3: 31.4 ± 3.4 min vs. cU: 17.5 ± 2.4 min, P = 0.003). During ablation of APs and PVCs, no relevant reduction of the fluoroscopy time could be detected (AP: c3: 7.1 ± 1.2 min vs. cU: 6.0 ± 1.5 min, P = 0.59; PVCs: c3: 17.6 ± 2.3 min vs. cU: 15.2 ± 2.8 min, P = 0.52).
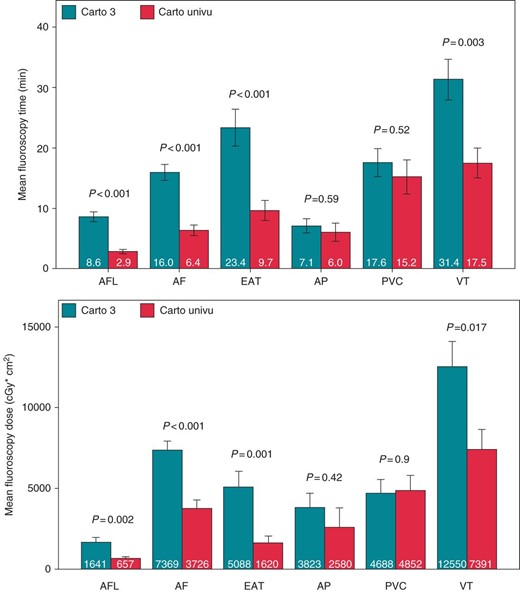
Fluoroscopy time and fluoroscopy dose CARTO-3 vs. CARTO-UNIVU fluoroscopy time in minutes and dose used for the different ablation procedures CARTO-3 vs. CARTO-UNIVU, AFL, ablation of typical flutter; AF, atrial fibrillation; EAT, ectopic atrial tachycardia; AP, accessory pathway; PVC, premature ventricular contractions; VT, ventricular tachycardia. Data presented as mean ± SEM, P < 0.05 was defined as significant.
Corresponding to the fluoroscopy time, the fluoroscopy dose was also reduced significantly in AFL ablation: relative reduction of 60% (c3: 1641 ± 315 cGy*cm2 vs. cU: 657 ± 102 cGy*cm2, P = 0.002), in AF ablation: relative reduction of 49% (c3: 7369 ± 558 cGy*cm2 vs. cU: 3726 ± 555 cGy*cm2, P < 0.001), in EAT ablation: relative reduction of 68% (c3: 5088 ± 969 cGy*cm2 vs. cU: 1620 ± 431 cGy*cm2, P = 0.001), and in VT ablation: relative reduction of 41% (c3: 12 550 ± 1557 cGy*cm2 vs. cU: 7391 ± 1248 cGy*cm2, P = 0.017). There was no change in the fluoroscopy dose between both groups in the AP and PVC cohorts (AP: c3: 3823 ± 868cGy*cm2 vs. cU: 2580 ± 1215 cGy*cm2, P = 0.42; PVC: c3: 4688 ± 838 cGy*cm2 vs. cU: 4852 ± 982 cGy*cm2, P = 0.9).
Subgroup-analyses of the AF patients showed a significant radiation reduction in the AF ablation procedures with only PVI (c3: 16 patients, cU 25 patients) as well as in the subgroup with PVI and additional left atrial lines, which were applied as described previously4 (c3: 21 patients, cU 19 patients) (data shown in Supplementary material online, Figure 1).
Procedure time, radiofrequency application time and dose (secondary endpoint)
The comparison of the procedure times (puncture of the femoral veins until removal of the sheaths) between both groups is illustrated in Figure 3. In the whole spectrum of procedures, there were no relevant differences in the procedure times between the CARTO-3 and CARTO-UNIVU group. Additionally, no differences in the radiofrequency application time and dose could be detected between both treatment groups (details are illustrated in Supplementary material online, Figure 2).
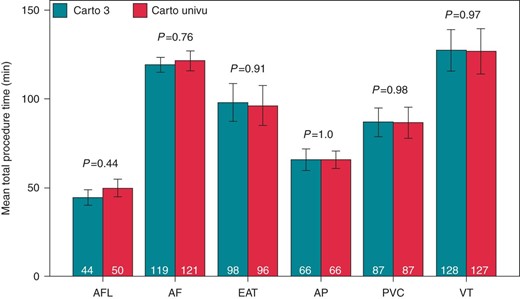
Total procedure time CARTO-3 vs. CARTO-UNIVU total procedure time in minutes (puncture of the femoral veins—remove of the sheaths) of the different ablation procedures CARTO-3 vs. CARTO-UNIVU. AFL, ablation of typical flutter; AF, atrial fibrillation; EAT, ectopic atrial tachycardia; AP, accessory pathway; PVC, premature ventricular contractions; VT, ventricular tachycardia. Data presented as mean ± SEM, P < 0.05 was defined as significant.
Procedure-step-specific fluoroscopy time and dose during atrial flutter ablation
To further analyse the specific reasons for the substantial changes of the fluoroscopy exposure, we choose to look in detail on the different steps during five representative cases of AF ablation and choose to separately register fluoroscopy time and dose for each procedural step: (1) positioning of the diagnostic catheters in the coronary sinus and right ventricular apex, (2) trans-septal puncture, (3) angiography of the left atrium and pulmonary veins and creation of the EAM, (4) ablation of the pulmonary veins, and (5) conformation of PVI with the diagnostic spiral catheter for entrance and exit block. The respective results are shown in Figure 4. During positioning of the diagnostic catheters and the trans-septal puncture no relevant reduction of the fluoroscopy time and dose could be detected. Noteworthy, only a small part of the total fluoroscopy time and dose was spent in these two procedural steps. The most radiation was used during LA angiography with EAM acquisition (step 3) and ablation (step 4). In these two steps, the fluoroscopy time and dose were significantly reduced by 74%/93% and 72%/76%, respectively.
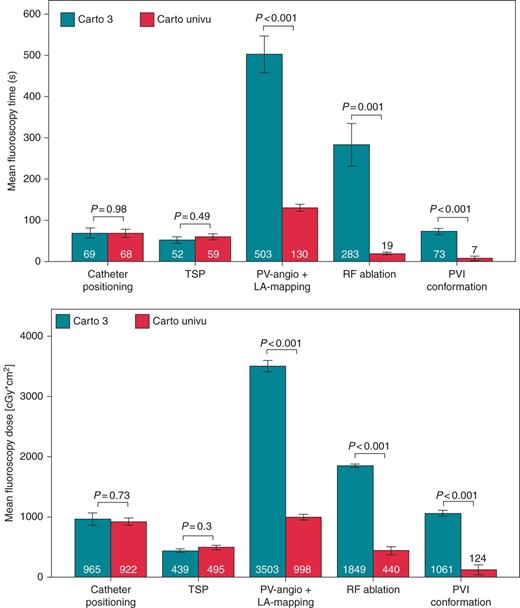
Procedure-step-specific fluoroscopy time and dose during AF ablation fluoroscopy time in seconds and dose used for the different procedure steps during AF ablation CARTO-3 vs. CARTO-UNIVU, catheter positioning: positioning of the diagnostic catheters in the coronary sinus and right ventricular apex, TSP, trans-septal puncture, PV-angio + LA mapping: angiography of the left atrium and pulmonary veins and creation of the EAM, radiofrequency ablation: ablation of the pulmonary veins, PVI conformation: conformation of PVI with the diagnostic spiral catheter for entrance and exit block. Data presented as mean ± SEM, P < 0.05 was defined as significant.
Discussion
The salient finding of this study is the observation, that fluoroscopy integrated 3D mapping enables a pronounced reduction in radiation exposure during ablation of a wide spectrum of cardiac arrhythmias that typically require substantial use of fluorosopy (e.g. AF and VT). This decline in fluoroscopy time and dose was not at the cost of increased procedure times, radiofrequency times and patient safety. On the other hand, during 3D mapping/ablation of arrhythmias with an already inherently low fluoroscopy exposure or 3D mapping/ablation of arrhythmias with difficult anatomical access/delicate neighbouring structures (APs, PVCs), the usage of this novel technology did not result in a further significant decrease of radiation usage.
Radiofrequency ablation has evolved to an effective and safe technique for the treatment of many symptomatic supraventricular and ventricular arrhythmias. Currently, the fundamental imaging modality during electrophysiological interventions is still fluoroscopy-based visualization of diagnostic and ablation catheter. Depending on the radiation dose, the patient may develop acute skin injury and/or may have an enhanced lifetime risk of developing a malignancy due to damage to the human DNA.1,2 Furthermore, also the involved medical staff is exposed to increasing radiation hazards like cataracts, neoplasms, and possibly an excess risk of left-sided brain tumours.5,6 In the past, several tools were developed to reduce patient and operator radiation exposure. In all labs performing fluoroscopy-based cardiac interventions, different techniques to reduce radiation should be used. These measures include shielding screens, close detector position, filtration, pulsed fluoroscopy, collimation, and digital fluoro processing.7 With the proper implementation of all these methods electrophysiologists can reduce radiation significantly.6,8 Currently, two non-fluoroscopic mapping systems—Ensite NavX by St Jude Medical and CARTO by Biosense Webster—are common in clinical use. There have been a number of studies demonstrating that these technologies are helpful in our efforts to further reduce fluoroscopy dose.9–11 Despite these undisputable advantages of these 3D-mapping systems, they do not include information obtained by real-time fluoroscopy. This important limitation is eliminated by the new CARTO-UNIVU as it seamlessly combines fluoro images with 3D EAMs into a single accurate 3D view on the conventional CARTO-3 system. With the integration of this technology in our daily work, we were able to dramatically reduce radiation exposure in a wide spectrum of arrhythmias, especially during ablation of complex arrhythmias with an intrinsically high fluoroscopy requirement such as in AF and VT. Notably, this was not associated with longer procedure times, more ablation energy, and periprocedural complications. These results are consistent with current data from another 3D-mapping system that provides real-time catheter tracking in pre-recorded cine loops—MediGuide (St Jude Medical).12,13
In contrast, during the ablation of cardiac arrhythmias with already naturally low fluoroscopy requirements such as in patients with simple accessory pathways or simple PVCs the utilization of this novel technology did not result in further significant decrease in radiation dose. On the other hand, both of these arrhythmias may constitute a clinical situation where difficulties in catheter access and/or delicate neighbouring structures (parahisian APs, aortic bulb PVCs) may necessitate frequent live fluoroscopy to confirm the motion-uncompensated gated catheter tip display. Both mechanisms are likely to add to the observation that overall usage of CARTO-UNIVU did not reduce fluoroscopy exposure for these two arrhythmia entities.
Our procedural analyses of five representative cases of AF ablation nicely illustrates that the reduction of radiation exposure was mainly achieved during EAM acquisition, PV isolation, and confirmation of PV isolation. So obviously, the new system mainly provides essential information during these procedural steps, whereas during placement of the catheter and trans-septal puncture the application of the CARTO-UNIVU system did not lead to a reduction in fluoroscopy burden. It must be mentioned, that in ablation of AF CT image integration was used in case of normal renal function. Of course, this additional CT increases the total fluoroscopy dose for the patients in both treatment groups. But in our opinion the CT image integration helps to plan the ablation procedure like the choice of the best fitting trans-septal sheath, individual trans-septal puncture or ablation of atypical anatomies. Previous studies could show that the CT image integration might be associated with reduced arrhythmia recurrence and increased restoration of sinus rhythm. Additionally the improved visualization of complex LA geometries might improve the safety of AF ablation.
Besides fluoroscopy reduction, the option of a 3D-mapping system to align X-ray images/angiograms with electro-anatomical maps offers further advantages, which are difficult to be described in statistical analyses. For example, during epicardial ablation of VTs, it is possible to overlay the map with an angiogram of the coronary arteries to avoid ablation in the immediate neighbourhood of the large epicardial vessels. Not only these relative specific indications of angiogram overlay, but also the integration of cardiac images during common EP procedures like AF ablation or endocardial VT ablation may significantly improve intracardiac orientation and by that safety and efficacy of the procedure due to permanent visualization of the endocardial chamber surface within a rather rough generic fluoroscopic cardiac anatomy. But clearly, the abovementioned theoretical advantages have to be confirmed by larger studies.
There are some limitations of this study. First, the study was designed as a retrospective registry without randomization or statistical matching of the patients. Further, all patients of the CARTO-3 group were treated before the CARTO-UNIVU group were ablated. This could cause a selection bias of the patients. On the other hand, this study design prevented an intra-procedural bias of the operators. Next, no long time follow-up data of the patients are available.
Conclusions
This study demonstrates that a 3D-mapping system with the option of fluoroscopy integrated catheter tracking is able to dramatically reduce radiation exposure for patients and medical staff during EP studies, especially in the light of the growing demand of complex ablation procedures such as AF and VT.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was not supported by any grant.
Acknowledgements
We thank all staff of the EP laboratory, Heart Center Dresden for supporting this registry.
Conflict of interest: M.C. is lecture honoraria SJM. C.W. reports no conflicts. C.P. is advisory board member SJM, Biotronik, Siemens, Biosense, Imricor. Lecture honoraria is from SJM, Biotronik, Siemens, Biosense. Research support from SJM, Biotronik, Biosense. T.G. is lecture honoraria SJM, Biosense.
References
Author notes
The authors have contributed equally.



