-
PDF
- Split View
-
Views
-
Cite
Cite
Mateusz K. Hołda, Wiesława Klimek-Piotrowska, Mateusz Koziej, Małgorzata Mazur, Anatomical variations of the coronary sinus valve (Thebesian valve): implications for electrocardiological procedures, EP Europace, Volume 17, Issue 6, June 2015, Pages 921–927, https://doi.org/10.1093/europace/euu397
Close - Share Icon Share
Abstract
The Thebesian valve (TV) can be a significant obstacle to coronary sinus (CS) cannulation. The aim of this study was to evaluate the characteristic features of the CS valve—TV anatomy. In particular, emphasis was placed on identifying specific structures of the TV that could potentially complicate CS cannulation.
We examined 273 autopsied human hearts. The height of the TV and the diameter of the CS were measured. The valves were classified according to their shape into five types: remnant, semilunar, fold, cord, and mesh and fenestrated. The mean transverse CS ostium (CSO) diameter was 12.2 ± 3.5 mm. The TV was present in 224 (82.1%) cases. The most common type of TV was semilunar: 32.6%; followed by remnant: 25.5%; fold: 17.4%; cord: 14.3%; and lastly mesh and fenestrated: 10.3%. The mean TV height for remnant–semilunar–fold types was 5.8 ± 3.0 mm. In seven cases, the present TV (2.6%) covered the entire orifice of the CS. Hearts with larger CSO diameter had lower TV height (P < 0.001).
We propose a new classification of the TV shapes based on the largest sample to date. We assessed that only in 2.6% of all 273 cases the presence of an obstructive TV can cause unsuccessful cannulation. The height of the TV was inversely correlated to the CSO diameter (r = −0.33; P < 0.001).
We propose a new classification of the Thebesian valve (TV) shapes based on the largest sample to date.
Only the TVs that cover >100% of coronary sinus ostium (CSO) can be established as obstructive TVs can make CS cannulation difficult or even impossible (2.6% of all cases).
The height of the TV was inversely correlated to the CSO diameter. Hearts with larger CSO diameters had lower TV height (r = −0.33; P < 0.001).
Introduction
The coronary sinus (CS) is the largest cardiac venous structure, which collects ∼60% of the outflow of the venous blood from the heart to the right atrium. The CS opens into the right atrium posteromedially between the inferior vena cava and the right atrioventricular orifice. It serves as an anatomic landmark as well as a conduit for diagnostic and therapeutic procedures. The biggest obstacle to communication between the right atrium and the CS is the valve of the CS, first described by Adam Christian Thebesius in 1708 in his monograph ‘De circulo sanguinis in corde’. The Thebesian valve (TV) is an embryological caudal remnant of the sinoatrial valves. It is a fold of endocardial tissue that guards the coronary sinus ostium (CSO) and is quite variable in shape. Despite the passage of more than three centuries from its first description, the role of the TV in normal physiology remains unclear and still intrigues many researchers and clinicians.1
Previously, the cardiac venous system had been given modest attention in research on the function and anatomy of the heart. The rapid development of electrophysiology reversed this trend.2 The CS is a commonly used gateway to the left atrial and left ventricular epicardium.3 Cardiac resynchronization therapy, catheter ablation of cardiac arrhythmias, defibrillation, perfusion therapy, mitral valve annuloplasty, targeted drug delivery, and retrograde cardioplegia administration are the commonly used therapeutic methods involving the CS. Equally, the role of the cardiac venous system in providing a potential conduit to bypass coronary artery stenosis (venous arterialization) and to the delivery of stem cells to myocardium after infarction are currently under investigation. Three elements of the CSO are related to successful cannulation of the CS: the size of the CSO, its entrance from the right atrium, and the presence of TV.4
The aim of this study was to evaluate the characteristic features of the TV anatomy with regards to its shape, size, and extent of coverage of the CSO. In particular, emphasis was placed on identifying specific structures of the TV which could potentially complicate electrophysiological and invasive cardiologic procedures. An additional aim was to create a new classification of the TV's shapes.
Methods
Study population
The study was conducted by the Department of Anatomy, Jagiellonian University Medical College in Cracow, Poland. We examined the CSO and TV, when present, in 273 autopsied human hearts of both sexes aged from 6 months to 94 years old. Specimens were specifically collected for this study during routine forensic medical autopsies performed at the Department of Forensic Medicine, Jagiellonian University Medical College. The hearts were removed together with the proximal portions of the great vessels: the ascending aorta, pulmonary trunk, superior vena cava, inferior vena cava, and all the pulmonary veins.
Exclusion criteria include myocardial infraction, severe anatomical defects, states after operations and grafts on the heart, obvious severe macroscopic pathology of the heart or vascular system found during the autopsy (aneurysms, storage diseases), heart trauma, and macroscopic signs of decomposition of cadavers. Other conditions such as arterial and pulmonary hypertension, cardiomyopathy, heart failure, and arrhythmias were not recognized as exclusion criteria. After dissection all hearts were fixed in 10% formalin for a maximum of two months to the time of measurement. The demographic data were available for all 273 specimens.
The study was approved by the Bioethical Committee of the Jagiellonian University Medical College, Cracow.
Dissection and measurements
All 273 heart specimens were opened in the usual routine manner by an incision extending from the orifice of the superior vena cava to the orifice of the inferior vena cava with the exception that the Eustachian valve was usually not sectioned. All the samples had an intact area containing the CSO and TV when present. The CS was opened longitudinally along its free wall to allow easy measurement of the diameter of the CSO without damaging present TV. All descriptions and measurements were undertaken with the heart held in anatomical position.
The following measurements were made: the height and width of TV and diameter of the CSO. All measurements were conducted by 0.03 mm precision electronic caliper YATO (YT-7201). Measurements were performed twice to reduce the chance of error. The mean of the two measurements was calculated rounding to the one decimal place. The TV height measurements were taken between the free edge of the valve and its attachment site to the right atrium as the shortest dimension passing through the middle of the free edge parallel to the transverse diameter of the CSO. The transverse diameter of the CSO was measured through the incision in the CS as the largest dimension up to the first point of resistance.
Until now, no one has proposed a uniform and unambiguous classification of TV types because of their shape. Based on previous observations performed on small samples,3,5–10 we created our own division and classified TV as a remnant (Type I), semilunar (Type II), fold (Type III), cord (Type IV), and mesh and fenestrated (Type V) (Table 1).
| Type . | Name . | Characteristic of the shape . |
|---|---|---|
| I | Remnant | Small hem of endocardium which not significantly protruding into the lumen of the CSO |
| II | Semilunar | Significantly protruding valve with a characteristic semilunar shape of the free edge |
| III | Fold | A large ‘veil’ with the non-semilunar edge, almost completely covering the whole CSO |
| IV | Cord | Single thick strand of the endocardium, mostly localized midline |
| V | Mesh and fenestrated | Fenestrated valves in shape from I to III type; net-like valves and multiple cords |
| Type . | Name . | Characteristic of the shape . |
|---|---|---|
| I | Remnant | Small hem of endocardium which not significantly protruding into the lumen of the CSO |
| II | Semilunar | Significantly protruding valve with a characteristic semilunar shape of the free edge |
| III | Fold | A large ‘veil’ with the non-semilunar edge, almost completely covering the whole CSO |
| IV | Cord | Single thick strand of the endocardium, mostly localized midline |
| V | Mesh and fenestrated | Fenestrated valves in shape from I to III type; net-like valves and multiple cords |
CSO, coronary sinus ostium.
| Type . | Name . | Characteristic of the shape . |
|---|---|---|
| I | Remnant | Small hem of endocardium which not significantly protruding into the lumen of the CSO |
| II | Semilunar | Significantly protruding valve with a characteristic semilunar shape of the free edge |
| III | Fold | A large ‘veil’ with the non-semilunar edge, almost completely covering the whole CSO |
| IV | Cord | Single thick strand of the endocardium, mostly localized midline |
| V | Mesh and fenestrated | Fenestrated valves in shape from I to III type; net-like valves and multiple cords |
| Type . | Name . | Characteristic of the shape . |
|---|---|---|
| I | Remnant | Small hem of endocardium which not significantly protruding into the lumen of the CSO |
| II | Semilunar | Significantly protruding valve with a characteristic semilunar shape of the free edge |
| III | Fold | A large ‘veil’ with the non-semilunar edge, almost completely covering the whole CSO |
| IV | Cord | Single thick strand of the endocardium, mostly localized midline |
| V | Mesh and fenestrated | Fenestrated valves in shape from I to III type; net-like valves and multiple cords |
CSO, coronary sinus ostium.
The valves that were shaped by a small hem of endocardium which did not significantly protrude into the lumen of the CSO were defined as Type I—remnant (Figure 1A). In contrast, significantly protruding valves with a characteristic semilunar shape of the free edge were designated as Type II—semilunar (Figure 1B). Fold—Type III was established as a large ‘veil’ with the non-semilunar edge, almost completely covering the whole CSO in many cases (Figure 1C). Type IV—cord, includes all valves that present as a single thick strand of the endocardium mostly localized in the midline (Figure 2A). Due to the special shape of the Type IV valve, its height was not measured. The width of the cord was measured as the shortest distance between two of its free edges in its central part. Type V mesh and fenestrated valves included all the valves which did not fulfil the criteria of the other types, including fenestrated valves from Type I–III, net-like valves, and multiple cords (Figure 2B and C). Due to the complicated morphology of the Type V valve, the height of this TV was not measured.
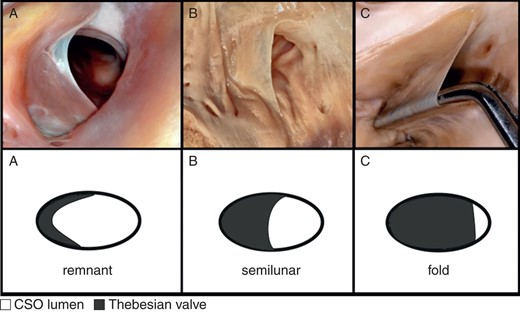
Photograph of cadaveric heart specimens and schematic pictures with examples of: (A) Type I of TV—remnant; (B) Type II of TV—semilunar; (C) Type III of TV—fold.
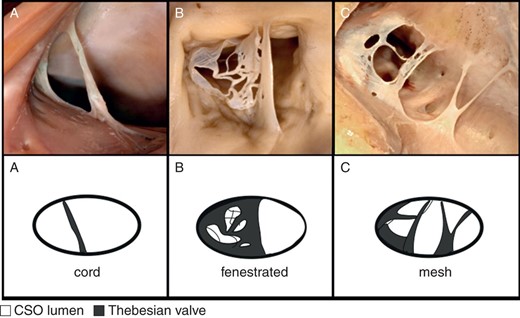
Photograph of cadaveric heart specimens and schematic pictures with examples of: (A) Type IV of TV—cord; (B), (C) Type V of TV—mesh and fenestrated.
Manipulations with standard electrocardiological catheters (SAPIRE BLU Irrigated Ablation Catheter 7F 4 mm Medium SWEEP, tip diameter = 2.38 mm) were performed in hearts with TV in Types III (fold) and V (mesh and fenestrated). The purpose of this manipulation was the introduction of a catheter into the CS through the CSO guarded by the TV.
Statistical analysis
Data are presented as mean values and corresponding standard deviations. StatSoft Statistica 10.0 for Windows was used for all statistical analyses. P-values <0.05 were considered to be statistically significant. Student's t-tests and the Mann–Whitney U tests were executed for statistical comparison in the CSO diameter and the TV height between sexes. The Kruskal–Wallis one-way analysis of variance was performed to find out significant difference in the CSO diameter and the TV shape in I–V types and hearts without TV. Correlation coefficients were calculated to measure statistical dependence.
Results
A total of 273 hearts were measured and assessed. The mean age of the cadavers from which the specimens were obtained was 48.7 ± 15.8 years (range 0.5–94 years) and 59 (21.6%) of those specimens were from female cadavers. In all hearts the CSO was elliptic in shape. Its transverse diameter was 12.2 ± 3.5 mm (min = 2.1 mm; max = 18.3 mm) and it was independent of age and sex.
The TV was present in 224 (82.1%) of the examined hearts. We observed considerable variations in the morphology of the TV. According to the shape, semilunar—Type II was the most common type of TV and was observed in 73 (73/224; 32.6%) hearts, followed by remnant—25.5%; fold—17.4%; cord—14.3%; and lastly mesh and fenestrated—10.3%. There were no relations between the prevalence of the different types of TV and age, and sex.
The overwhelming majority of the TVs took its origin from the right margin of the CSO, then further extending on its caudal and cranial edge. We noted that the TVs were never attached to the left margin of the CS orifice. This area was always free of any point of attachment in all cases. Furthermore, seven valves (2.9% of all TVs; 4 in Type III and 3 in Type V) covered the whole CS orifice going well beyond the CSO contour (Figure 3). The range of CSO coverage varies in these cases from 105 to 290% (calculated as the ratio of the TV height to the transverse CSO diameter). This indicates that these valves covered the whole CSO and that their free edges significantly extended beyond the CSO contour.
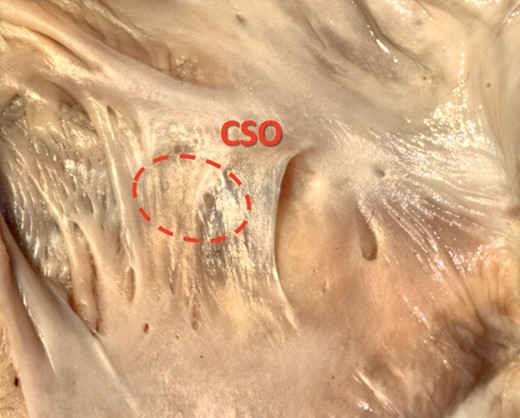
Photograph of cadaveric heart specimen showing a TV (Type III—fold) which covers the whole coronary sinus ostium (CSO), going well beyond the CSO contour.
The mean TV height for Types I–III was 5.8 ± 3.0 mm (I = 2.9 ± 0.9 mm; II = 6.5 ± 2.1 mm; III = 8.8 ± 2.7 mm). The mean width of Type IV—cord was 1.8 ± 0.9 mm. The height and width of the TV and its shape did not depend on age and sex. The mean percentage coverage of the CS by the valve was:Type I—24 ± 8%; II—58 ± 11%; III—81 ± 5.5% (with the exception of the TV covering the entire CSO).
It was found that TV shape or its absence has a significant influence on the size of the CSO diameter. Hearts without a TV had a larger diameter (15.1 ± 3.1 mm) than those with a valve present (11.6 ± 3.3 mm; P < 0.001). Furthermore, the height of the TV's was inversely correlated to the CSO diameter. Hearts with larger CSO diameters had lower TV height (r = −0.33; P < 0.001).
Discussion
Clinical data suggest that CS cannulation is unsuccessful in 5–10% of the patients undergoing invasive cardiac procedures.3 Gras et al.11 noted that 3.7% of CS cannulation were unsuccessful due to failure to catheterize the CS. A similar percentage of failures (2.87%) were attributed to the inability to locate the CSO by Azizi et al.12 There are several studies using different models seeking to explore the anatomy of the TV and to determine what type and percentage of presentations can hinder cannulation procedure (Table 2). The definitions of an obstructive TV that can potentially complicate cannulation shown in Table 2 demonstrate that the authors focused mainly on a percentage determination of the degree of coverage of the CS by the valve. In most cases, this limit is established at 75% coverage by which they determined that in ∼15% of cases the TV is a potential obstacle to the cannulation. According to these assumptions, we can conclude that all valves which we classified in our study as Type III—folds (17.4% of all TVs and 14.3% of all specimens) cover >75% of CSO and may be considered as obstructive.
Comparison between results of the present study and those conducted previously
| Name of authors . | n . | Type of materials . | No. of hearts with present TV . | Definition of TV which can potentially complicating cannulation (obstructive) . | No. of cases in which TV can complicate cannulation . | Diameters of the coronary sinus (mean ± SD) (mm) . | |
|---|---|---|---|---|---|---|---|
| Without TV . | With TV . | ||||||
| Present study | 273 | Cadaveric | 224 (82%) | Type IV—fold | 39(14.3%) | T: 15.1 ± 3.1 | T: 11.6 ± 3.3 |
| Gami et al.13 | 560 | Cadaveric | 348 (62%) | a. Circumferential (covered all 4 quadrants of CSO) b. Covered 3 quadrants of CSO | a. 3(0.5%) b. 68(12%) | — | |
| Mlynarski et al.4 | 150 | Multislice computed tomography | 69 (46%) | — | — | T: 13.8 ± 3.3 | T: 14.6 ± 3.7 |
| Ghosh et al.8 | 150 | Cadaveric | 118 (79%) | Non-fenestrated, non-membranous TV covering >75% of CSO | 27(18%) | CD: 11.2 ± 1.4 T: 9.6 ± 0.8 | CD: 9.4 ± 2.1 T: 7.15 ± 1.5 |
| Pejković et al.14 | 150 | Cadaveric | 120 (80%) | — | — | CD: 8.0 (range 3–15) T: 6.6 (range 3–15) | |
| Hellerstein and Orbison15 | 150 | Cadaveric | 128 (85%) | CSO completely covered by a TV membrane | 37(24.7%) | 11.1 (range 7–19) | 8.6 (range 5–13) |
| Anh et al.6 | 98 | In vivo | 53 (54%) | TV covering >70% of CSO | 11(11%) | — | |
| Mak et al.3 | 75 | Cadaveric | 55 (73%) | Non-fenestrated, fibrous, fibromuscular or muscular TV covering >75% of CSO | 12(16%) | CD: 9.3 ± 2.9 T: 9.4 ± 2.9 | CD: 7.9 ± 2.7 T: 7.3 ± 2.8 |
| Karaca et al.9 | 52 | Cadaveric | 35 (67%) | fenestrated or band shaped TV | 4(8%) | 9.47 | |
| Katti and Patil16 | 50 | Cadaveric | 44 (80%) | TV covering >65% of CSO | 10(20%) | CD: 8.0 ± 2.5 T: 6.5 ± 1.9 | |
| Christiaens et al.17 | 50 | Multidetector row computed tomography | 18 (36%) | TV covering >60% of CSO | 1(2%) | CD: 15.3 ± 3.7 | |
| Randhawa et al.5 | 50 | Cadaveric | 32 (64%) | TV covering ≥75% of CSO | 8(16%) | CD: 5.9 ± 1.3 3.6 ± 0.8 | CD: 5.7 ± 1.3 T: 3.4 ± 1.1 |
| Name of authors . | n . | Type of materials . | No. of hearts with present TV . | Definition of TV which can potentially complicating cannulation (obstructive) . | No. of cases in which TV can complicate cannulation . | Diameters of the coronary sinus (mean ± SD) (mm) . | |
|---|---|---|---|---|---|---|---|
| Without TV . | With TV . | ||||||
| Present study | 273 | Cadaveric | 224 (82%) | Type IV—fold | 39(14.3%) | T: 15.1 ± 3.1 | T: 11.6 ± 3.3 |
| Gami et al.13 | 560 | Cadaveric | 348 (62%) | a. Circumferential (covered all 4 quadrants of CSO) b. Covered 3 quadrants of CSO | a. 3(0.5%) b. 68(12%) | — | |
| Mlynarski et al.4 | 150 | Multislice computed tomography | 69 (46%) | — | — | T: 13.8 ± 3.3 | T: 14.6 ± 3.7 |
| Ghosh et al.8 | 150 | Cadaveric | 118 (79%) | Non-fenestrated, non-membranous TV covering >75% of CSO | 27(18%) | CD: 11.2 ± 1.4 T: 9.6 ± 0.8 | CD: 9.4 ± 2.1 T: 7.15 ± 1.5 |
| Pejković et al.14 | 150 | Cadaveric | 120 (80%) | — | — | CD: 8.0 (range 3–15) T: 6.6 (range 3–15) | |
| Hellerstein and Orbison15 | 150 | Cadaveric | 128 (85%) | CSO completely covered by a TV membrane | 37(24.7%) | 11.1 (range 7–19) | 8.6 (range 5–13) |
| Anh et al.6 | 98 | In vivo | 53 (54%) | TV covering >70% of CSO | 11(11%) | — | |
| Mak et al.3 | 75 | Cadaveric | 55 (73%) | Non-fenestrated, fibrous, fibromuscular or muscular TV covering >75% of CSO | 12(16%) | CD: 9.3 ± 2.9 T: 9.4 ± 2.9 | CD: 7.9 ± 2.7 T: 7.3 ± 2.8 |
| Karaca et al.9 | 52 | Cadaveric | 35 (67%) | fenestrated or band shaped TV | 4(8%) | 9.47 | |
| Katti and Patil16 | 50 | Cadaveric | 44 (80%) | TV covering >65% of CSO | 10(20%) | CD: 8.0 ± 2.5 T: 6.5 ± 1.9 | |
| Christiaens et al.17 | 50 | Multidetector row computed tomography | 18 (36%) | TV covering >60% of CSO | 1(2%) | CD: 15.3 ± 3.7 | |
| Randhawa et al.5 | 50 | Cadaveric | 32 (64%) | TV covering ≥75% of CSO | 8(16%) | CD: 5.9 ± 1.3 3.6 ± 0.8 | CD: 5.7 ± 1.3 T: 3.4 ± 1.1 |
TV, Thebesian valve; SD, standard deviations; T, transverse diameter; CD, craniocaudal diameter; CSO, coronary sinus ostium.
Comparison between results of the present study and those conducted previously
| Name of authors . | n . | Type of materials . | No. of hearts with present TV . | Definition of TV which can potentially complicating cannulation (obstructive) . | No. of cases in which TV can complicate cannulation . | Diameters of the coronary sinus (mean ± SD) (mm) . | |
|---|---|---|---|---|---|---|---|
| Without TV . | With TV . | ||||||
| Present study | 273 | Cadaveric | 224 (82%) | Type IV—fold | 39(14.3%) | T: 15.1 ± 3.1 | T: 11.6 ± 3.3 |
| Gami et al.13 | 560 | Cadaveric | 348 (62%) | a. Circumferential (covered all 4 quadrants of CSO) b. Covered 3 quadrants of CSO | a. 3(0.5%) b. 68(12%) | — | |
| Mlynarski et al.4 | 150 | Multislice computed tomography | 69 (46%) | — | — | T: 13.8 ± 3.3 | T: 14.6 ± 3.7 |
| Ghosh et al.8 | 150 | Cadaveric | 118 (79%) | Non-fenestrated, non-membranous TV covering >75% of CSO | 27(18%) | CD: 11.2 ± 1.4 T: 9.6 ± 0.8 | CD: 9.4 ± 2.1 T: 7.15 ± 1.5 |
| Pejković et al.14 | 150 | Cadaveric | 120 (80%) | — | — | CD: 8.0 (range 3–15) T: 6.6 (range 3–15) | |
| Hellerstein and Orbison15 | 150 | Cadaveric | 128 (85%) | CSO completely covered by a TV membrane | 37(24.7%) | 11.1 (range 7–19) | 8.6 (range 5–13) |
| Anh et al.6 | 98 | In vivo | 53 (54%) | TV covering >70% of CSO | 11(11%) | — | |
| Mak et al.3 | 75 | Cadaveric | 55 (73%) | Non-fenestrated, fibrous, fibromuscular or muscular TV covering >75% of CSO | 12(16%) | CD: 9.3 ± 2.9 T: 9.4 ± 2.9 | CD: 7.9 ± 2.7 T: 7.3 ± 2.8 |
| Karaca et al.9 | 52 | Cadaveric | 35 (67%) | fenestrated or band shaped TV | 4(8%) | 9.47 | |
| Katti and Patil16 | 50 | Cadaveric | 44 (80%) | TV covering >65% of CSO | 10(20%) | CD: 8.0 ± 2.5 T: 6.5 ± 1.9 | |
| Christiaens et al.17 | 50 | Multidetector row computed tomography | 18 (36%) | TV covering >60% of CSO | 1(2%) | CD: 15.3 ± 3.7 | |
| Randhawa et al.5 | 50 | Cadaveric | 32 (64%) | TV covering ≥75% of CSO | 8(16%) | CD: 5.9 ± 1.3 3.6 ± 0.8 | CD: 5.7 ± 1.3 T: 3.4 ± 1.1 |
| Name of authors . | n . | Type of materials . | No. of hearts with present TV . | Definition of TV which can potentially complicating cannulation (obstructive) . | No. of cases in which TV can complicate cannulation . | Diameters of the coronary sinus (mean ± SD) (mm) . | |
|---|---|---|---|---|---|---|---|
| Without TV . | With TV . | ||||||
| Present study | 273 | Cadaveric | 224 (82%) | Type IV—fold | 39(14.3%) | T: 15.1 ± 3.1 | T: 11.6 ± 3.3 |
| Gami et al.13 | 560 | Cadaveric | 348 (62%) | a. Circumferential (covered all 4 quadrants of CSO) b. Covered 3 quadrants of CSO | a. 3(0.5%) b. 68(12%) | — | |
| Mlynarski et al.4 | 150 | Multislice computed tomography | 69 (46%) | — | — | T: 13.8 ± 3.3 | T: 14.6 ± 3.7 |
| Ghosh et al.8 | 150 | Cadaveric | 118 (79%) | Non-fenestrated, non-membranous TV covering >75% of CSO | 27(18%) | CD: 11.2 ± 1.4 T: 9.6 ± 0.8 | CD: 9.4 ± 2.1 T: 7.15 ± 1.5 |
| Pejković et al.14 | 150 | Cadaveric | 120 (80%) | — | — | CD: 8.0 (range 3–15) T: 6.6 (range 3–15) | |
| Hellerstein and Orbison15 | 150 | Cadaveric | 128 (85%) | CSO completely covered by a TV membrane | 37(24.7%) | 11.1 (range 7–19) | 8.6 (range 5–13) |
| Anh et al.6 | 98 | In vivo | 53 (54%) | TV covering >70% of CSO | 11(11%) | — | |
| Mak et al.3 | 75 | Cadaveric | 55 (73%) | Non-fenestrated, fibrous, fibromuscular or muscular TV covering >75% of CSO | 12(16%) | CD: 9.3 ± 2.9 T: 9.4 ± 2.9 | CD: 7.9 ± 2.7 T: 7.3 ± 2.8 |
| Karaca et al.9 | 52 | Cadaveric | 35 (67%) | fenestrated or band shaped TV | 4(8%) | 9.47 | |
| Katti and Patil16 | 50 | Cadaveric | 44 (80%) | TV covering >65% of CSO | 10(20%) | CD: 8.0 ± 2.5 T: 6.5 ± 1.9 | |
| Christiaens et al.17 | 50 | Multidetector row computed tomography | 18 (36%) | TV covering >60% of CSO | 1(2%) | CD: 15.3 ± 3.7 | |
| Randhawa et al.5 | 50 | Cadaveric | 32 (64%) | TV covering ≥75% of CSO | 8(16%) | CD: 5.9 ± 1.3 3.6 ± 0.8 | CD: 5.7 ± 1.3 T: 3.4 ± 1.1 |
TV, Thebesian valve; SD, standard deviations; T, transverse diameter; CD, craniocaudal diameter; CSO, coronary sinus ostium.
However, comparing the results of theoretical studies3,5,6,8,13,15,16 with clinical data presented above,11,12 we can conclude that they are about six times overstated. It suggests that the grounds on which the definitions of obstructive TV were based may be misleading. Accordingly, in the present study we reveal that seven valves (4 in Type III—fold and 3 in Type V—mesh or fenestrated) covered the whole CSO, going well beyond the CSO contour (Figure 3). Cannulation of the CS in these 2.6% of all study cases (7/273) appears to be extremely difficult, which corresponds with the prevalence of cannulation failures found in clinical data. Therefore, we propose that only TV which completely overlap CSO and significantly extend beyond its contour (TV height to CSO diameter ratio >100%) should be established as obstructive TV. Such valves can make CS cannulation difficult or even impossible.
The presence of a large TV that covers the entire CSO can in fact completely prevent the passage of a catheter from the right atrium into the CS which is confirmed by several presented in literature cases. On the one hand, mesh and fenestrated TVs (Type V) can facilitate the passage of standard electrocardiological catheter through the CSO but may also hinder manipulations of the catheter in the further parts of the cardiac venous system. Larger diameter catheters, with additional equipment (e.g. for coronary vein angioplasty) may not pass through tiny fenestrations.
Moreover, it is important to consider the ability of coronary blood flow to preserve on the integrity and shape of the TV. In the present study, it has been shown that the CSO diameter is correlated with TV shape (P < 0.001). Furthermore, hearts with a larger CSO diameter had lower TV heights. According to the authors one of the reasons of this stage may be the increased blood flow in the CS owing to an increased diameter of the CSO that causes atrophy of the valve. This postulate is also issued on the basis of the observation of the CS in the hearts with the persistent left superior vena cava. In the cases of such anatomical variation, we observed the absence of all valves within cardiac venous system.
Presence of an obstructive TV does not rule out the possibility of successful CS cannulation. Parikh et al.18 confirmed that two-dimensional intracardiac echocardiographic and left coronary angiography with levophase imaging can be extremely helpful in locating and defining CS ostial abnormalities leading to successful CS cannulation. In addition, Worley has described the use of coronary artery interventional techniques to dilate an obstructive TV, thus making CS cannulation possible.19 Further, the use of radiofrequency energy to traverse an occlusive TV may be used as an alternative method.18 Based on our own observations and manipulations, we can confirm earlier reports which stipulate that directing the tip of the catheter towards the cranial margin of the CS ostia under direct vision may lead to its successful cannulation when conventional techniques have failed.8 Inserting the catheter from anterior to posterior and from left to right side with a rotational movement may increase the chance of successful CS cannulation. Imaging of the obstructive TV is easily visible using electron beam computed tomographic angiography, multislice computed tomography, or echocardiography and should be an integral part of planning the CS cannulation procedure in cases where there is a strong suspicion of complications to choose the appropriate technique. Direct visualization of the CSO using an endocardial visualization catheter to locate and cannulate the CS seems to be an invaluable and extremely helpful tool.5,20
Finally, because TVs are very thin and fibrous, fibromuscular, or muscular in composition,3,8 it can be easily damaged and perforated during medical procedures. According to the available literature perioperative complications such as coronary venous dissection and perforation of the CS or cardiac veins occur, respectively, in 2.88 and 1.2% of cases.12 Lesions of the CS are very difficult or even impossible to repair and can be fatal.20 These perforations and dissections may be a direct consequence of the use of excessive force while guiding the catheter through the CSO in which the prominent TV is present and complicating the procedure. The TV is not the only anatomical obstacle for the catheter placed into the venous system of the heart,5,9,21 but it is nonetheless a significant factor to consider during cannulation procedures which can be addressed by knowledge of the variability of the anatomy of this region. Also, the other aspects may preclude CS cannulation, including superior vena cava vs. inferior vena cava approach, catheters characteristics and sizes, and operator's experiences.
Among all the studies carried out on the anatomy of the TV, there are only a few in which the authors try to define the shapes of valve.3,5–10 In each of these works, we can find different terms for various TV shapes (Table 3). Based on the present observations, the previous classification systems appear incomplete and do not fully capture the dynamics of the shape of the valve. Therefore, we found it reasonable to create a new classification of TV shapes. We set clear criteria for the classification (Table 1) and present sample pictures for each TV type (Figure 1 and 2). Two teams before this presented research had studied the area of CSO on a larger sample of hearts, but the shape of the TV was not described in those papers.13,21 Thus, to the best of our knowledge, this study is the biggest one that specifies the shape of the TV.
| Name of authors . | No. of valves . | TVs shape . | % of valves . |
|---|---|---|---|
| Mak et al.3 | 55 | I: semicircular | 65.4 |
| II: strands | 3.6 | ||
| III: band | 12.7 | ||
| IV: remnant | 18.2 | ||
| Randhawa et al.5 | 32 | I: crescent | 40.6 |
| II: semilunar (>50% CSO) | 53.1 | ||
| III: band | 6.3 | ||
| Anh et al.6 | 53 | I: semilunar | 49 |
| II: strands and bands | 34 | ||
| III: fenestrated | 17 | ||
| Pejković et al.7 | 120 | I: semilunar | 75 |
| II: fenestrated | 11 | ||
| III: netlike | 11 | ||
| IV: ribbon | 3 | ||
| Ghosh et al.8 | 118 | I: semilunar | 55.1 |
| II: fenestrated | 30.5 | ||
| III: biconcave band like | 14.5 | ||
| Karaca et al.9 | 35 | I: crescent | 45.7 |
| II: semilunar | 42.9 | ||
| III: fenestrated | 5.7 | ||
| IV: band shaped | 5.7 | ||
| Kuta et al.10 | 94 | I: semilunar | 53.2 |
| II: semilunar perforated | 17 | ||
| III: residual with strands | 19.1 | ||
| IV: strands | 4.3 | ||
| V: border | 6.4 |
| Name of authors . | No. of valves . | TVs shape . | % of valves . |
|---|---|---|---|
| Mak et al.3 | 55 | I: semicircular | 65.4 |
| II: strands | 3.6 | ||
| III: band | 12.7 | ||
| IV: remnant | 18.2 | ||
| Randhawa et al.5 | 32 | I: crescent | 40.6 |
| II: semilunar (>50% CSO) | 53.1 | ||
| III: band | 6.3 | ||
| Anh et al.6 | 53 | I: semilunar | 49 |
| II: strands and bands | 34 | ||
| III: fenestrated | 17 | ||
| Pejković et al.7 | 120 | I: semilunar | 75 |
| II: fenestrated | 11 | ||
| III: netlike | 11 | ||
| IV: ribbon | 3 | ||
| Ghosh et al.8 | 118 | I: semilunar | 55.1 |
| II: fenestrated | 30.5 | ||
| III: biconcave band like | 14.5 | ||
| Karaca et al.9 | 35 | I: crescent | 45.7 |
| II: semilunar | 42.9 | ||
| III: fenestrated | 5.7 | ||
| IV: band shaped | 5.7 | ||
| Kuta et al.10 | 94 | I: semilunar | 53.2 |
| II: semilunar perforated | 17 | ||
| III: residual with strands | 19.1 | ||
| IV: strands | 4.3 | ||
| V: border | 6.4 |
TV, Thebesian valve.
| Name of authors . | No. of valves . | TVs shape . | % of valves . |
|---|---|---|---|
| Mak et al.3 | 55 | I: semicircular | 65.4 |
| II: strands | 3.6 | ||
| III: band | 12.7 | ||
| IV: remnant | 18.2 | ||
| Randhawa et al.5 | 32 | I: crescent | 40.6 |
| II: semilunar (>50% CSO) | 53.1 | ||
| III: band | 6.3 | ||
| Anh et al.6 | 53 | I: semilunar | 49 |
| II: strands and bands | 34 | ||
| III: fenestrated | 17 | ||
| Pejković et al.7 | 120 | I: semilunar | 75 |
| II: fenestrated | 11 | ||
| III: netlike | 11 | ||
| IV: ribbon | 3 | ||
| Ghosh et al.8 | 118 | I: semilunar | 55.1 |
| II: fenestrated | 30.5 | ||
| III: biconcave band like | 14.5 | ||
| Karaca et al.9 | 35 | I: crescent | 45.7 |
| II: semilunar | 42.9 | ||
| III: fenestrated | 5.7 | ||
| IV: band shaped | 5.7 | ||
| Kuta et al.10 | 94 | I: semilunar | 53.2 |
| II: semilunar perforated | 17 | ||
| III: residual with strands | 19.1 | ||
| IV: strands | 4.3 | ||
| V: border | 6.4 |
| Name of authors . | No. of valves . | TVs shape . | % of valves . |
|---|---|---|---|
| Mak et al.3 | 55 | I: semicircular | 65.4 |
| II: strands | 3.6 | ||
| III: band | 12.7 | ||
| IV: remnant | 18.2 | ||
| Randhawa et al.5 | 32 | I: crescent | 40.6 |
| II: semilunar (>50% CSO) | 53.1 | ||
| III: band | 6.3 | ||
| Anh et al.6 | 53 | I: semilunar | 49 |
| II: strands and bands | 34 | ||
| III: fenestrated | 17 | ||
| Pejković et al.7 | 120 | I: semilunar | 75 |
| II: fenestrated | 11 | ||
| III: netlike | 11 | ||
| IV: ribbon | 3 | ||
| Ghosh et al.8 | 118 | I: semilunar | 55.1 |
| II: fenestrated | 30.5 | ||
| III: biconcave band like | 14.5 | ||
| Karaca et al.9 | 35 | I: crescent | 45.7 |
| II: semilunar | 42.9 | ||
| III: fenestrated | 5.7 | ||
| IV: band shaped | 5.7 | ||
| Kuta et al.10 | 94 | I: semilunar | 53.2 |
| II: semilunar perforated | 17 | ||
| III: residual with strands | 19.1 | ||
| IV: strands | 4.3 | ||
| V: border | 6.4 |
TV, Thebesian valve.
The main limitation of our study is that all the measurements were made on autopsied heart specimens that have been fixed in formalin, which could cause some slight changes in size and shape of the heart. Studies performed on post-mortem material may not directly correlate to the physiology of the tissues in vivo. Therefore, we cannot say anything about the behaviour and dimensions changes of the TV and the area of CSO within the cardiac cycle. Despite these limitations, we believe that they do not impede the morphological analysis of the shape of valve and on the relations between the dimensions of the TV and CSO.
Conclusions
In the present study, the TV was present in 82.1% of the examined hearts. A new classification of the types of TV (Table 1) was proposed on the basis of the morphology observed in the current investigation, which represents one of the largest sample sizes analysed to date. The use of a clear classification system, such as the one proposed in this study, will improve the understanding of the morphology of the TV by providing a standardized way to describe it. We also postulate that the inverse relationship noted between the CS diameter and the height of the valve in the present investigation may be the result of increased blood flow in the CS, causing atrophy of the valve. In accordance with the present research in conjunction with clinical findings, we propose that only TVs that cover >100% of CSO will be established as obstructive TV and can make CS cannulation difficult or even impossible (∼2.6% of all cases). Presence of a defined obstructive TV may be the direct cause of the failure in ∼25% of all unsuccessful CS cannulation performed using conventional techniques. Type III—fold valves may prolong CS cannulation procedural times by impeding access to the CSO. Valves classified as a Type I—remnant, II—semilunar, IV—cord probably have no influence on CS cannulation.
Conflict of interest: none declared.
References
Author notes
These authors contributed equally.



