-
PDF
- Split View
-
Views
-
Cite
Cite
Gijs E. De Maat, Alberto Pozzoli, Marcoen F. Scholten, Isabelle C. Van Gelder, Yuri Blaauw, Bart A. Mulder, Paolo Della Bella, Ottavio R. Alfieri, Stefano Benussi, Massimo A. Mariani, Long-term results of surgical minimally invasive pulmonary vein isolation for paroxysmal lone atrial fibrillation, EP Europace, Volume 17, Issue 5, May 2015, Pages 747–752, https://doi.org/10.1093/europace/euu287
Close - Share Icon Share
Abstract
Transcatheter pulmonary vein ablation is the current treatment of choice for symptomatic drug-refractory atrial fibrillation (AF). Video-assisted surgical pulmonary vein isolation (sPVI) is an alternative therapy to percutaneous ablation for the treatment of AF. Long-term results of sPVI are currently unknown. The aim of this study was to report on the long-term efficacy and safety of sPVI in patients with paroxysmal AF.
The study design was observational and retrospective. From July 2005 to January 2011, 42 patients with drug-refractory paroxysmal AF underwent video-assisted sPVI in two different centres. Patients were eligible for sPVI when suffering from symptomatic, drug-refractory paroxysmal AF and they agreed to the alternative of sPVI. The median preoperative AF duration was 24 months (range 3–200). Success was defined as the absence of AF on 24 h or 96 h Holter monitoring during follow-up, off antiarrhythmic drugs (AAD). Adverse events and follow-up monitoring were based on the Heart Rhythm Society Consensus Statement 2012 for the catheter and surgical ablation of AF. Mean age was 55 ± 10 years, and 76% were males. After a mean follow-up of 5 years (SD 1.7), 69% of all patients were free from atrial arrhythmias without the use of AAD, and 83% with the use of AAD. Major peri-procedural adverse events occurred in four (9.5%) patients, no strokes or mortalities were registered during long-term follow-up.
This retrospective study shows that sPVI for the treatment of paroxysmal AF is effective and that the outcomes are maintained at long-term follow-up.
The present study is the first to our knowledge to report on real world, very long-term follow-up up to 7 years after surgical minimally invasive pulmonary vein isolation (sPVI) in patients with paroxysmal lone AF.
Surgical PVI is an effective and reproducible treatment strategy for lone paroxysmal AF.
No additional ablation lines were applied on the left atrium, therefore we investigated solely the efficacy of the surgical PVI technique.
No late complications were observed and during long-term follow-up, none of the patients suffered from stroke.
According to patient's preference, sPVI might be considered as the first ablation strategy in young, highly symptomatic patients.
Introduction
Atrial fibrillation (AF) is the most frequent cardiac rhythm disorder with an increasing prevalence and is responsible for substantial morbidity, mortality, and use of healthcare resources.1 Currently, the first choice in treating AF is pharmacological therapy with anti-arrhythmic drugs (AADs), which has shown however to be effective in <40% of patients.2 In addition to AAD, transcatheter pulmonary vein isolation (PVI) has gained popularity in younger patients suffering from paroxysmal AF.3,4 Unfortunately, the single procedure transcatheter technology showed disappointing long-term results.5,6 Technical difficulty of achieving transmural lesions and complete electrical isolation causes re-conduction to occur in 29% of all patients.7 Therefore, approximately one-third to one-half of the patients require multiple procedures to achieve stable sinus rhythm.5,6 As an alternative therapy, surgical minimally invasive PVI (sPVI) was introduced in 2005.8 This technique has rapidly evolved to a complete thoracoscopic procedure.9 Short- and mid-term results of sPVI have shown promising results ranging from 64 to 90% freedom from AF and AAD after a single procedure.10–16 Unfortunately, the current literature reports a large variety in patient selection, lesion sets and the long-term outcomes of the sPVI remain largely unknown. In the present observational and retrospective study, we report on the long-term results of bilateral sPVI in patients with paroxysmal lone AF.
Methods
Patient population
This observational and retrospective study was performed on a series of 42 consecutive patients who were treated with sPVI between July 2005 and January 2011, in two different centres (San Raffaele University Hospital and Medisch Spectrum Twente) by two surgeons (M.A.M. and S.B.). Inclusion criteria were symptomatic paroxysmal AF, without concomitant cardiac structural disease, refractory to Class I and/or Class III AAD or failed transcatheter PVI. Exclusion criteria for sPVI were left atrial size >55 mm (parasternal echocardiographic view), prior heart or lung surgery, significant coronary disease or previous myocardial infarction, left ventricle hypertrophy >12 mm, previous hospitalization for heart failure, left ventricular dysfunction (ejection fraction <50%), moderate or severe mitral- or aortic valve disease, or lung disease (prior tuberculosis or COPD Gold Class III–IV). Definitions of paroxysmal AF, success and failure of ablation, adverse events, and follow-up monitoring were based on the Heart Rhythm Society Consensus Statement for the catheter and surgical ablation of AF.4
Pre-surgery management
All patients provided written informed consent to the ablation procedure. To exclude significant (cardiac) disease, several examinations were performed; routine laboratory testing of thyroid-stimulating hormone and transthoracic echocardiography was performed within the month prior to surgery. To rule out coronary disease, patients routinely underwent an exercise stress test or a coronary angiography if patients were >50 years of age. Patients were admitted to the hospital 2 days prior to sPVI. Preoperatively, transoesophageal echocardiography was performed to exclude atrial thrombi.
Surgical technique
During the first 10 (24%) sPVI procedures, the pulmonary veins (PVs) were isolated through bilateral video-assisted mini-thoracotomy, using a dedicated bipolar radiofrequency clamp (Isolator; AtriCure Inc., Cincinnati).8 From 2007, the procedure was modified to a complete thoracoscopic approach. Patients were operated in the supine decubitus position, with general aneasthesia and double-lumen endotracheal intubation. The procedure's description was previously published.9,12 Briefly; the PVs were firstly targeted on the right side, then on the left side, taking care not to injure the phrenic nerve. Ancillary procedure was the division of the Marshall's ligament on the left side. The ablation lesions were repeated three to six times before testing of exit block, on each side. To confirm PVI, in all patients direct pacing (120 bpm, 20 mV output, 200 Hz) was applied on multiple sites of the pulmonary veins (Estech Affirm or Atricure Isolator multifunctional pen). In case of ‘capture’ on the left atrium, additional ablations were performed until the exit block was achieved. No additional ablation lines were applied on the atria and no ganglionic plexi were targeted. When feasible, the left atrial appendage (LAA) was excised by a stapler or mechanically excluded by the Atriclip device (AtriCure, Inc.).
Medication
Oral anticoagulation was discontinued 2 days before the procedure and replaced by full-dose low-molecular-weight (LMW) heparin. Oral anti-coagulation was restarted after surgery and LMW heparin was stopped when INR >2.0 was reached. Oral anticoagulation treatment was determined based on the 2006 guidelines for the management of AF,17 and since 2010 based on the CHA2DS2-VASC score.3 Also, the status of the LAA was included in the decision making to stop long-term OAC, this was left at the discretion of the treating cardiologist (and patient). Three months after sPVI the AAD were routinely discontinued.
Follow-up
According to the institutional protocols, the patients included in this study visited the outpatient clinic at 3, 6, and 12 months and underwent Holter monitoring. The duration of scheduled Holter monitoring was 24 h, except for the 6-month follow-up, then the duration was 96 h. After the first year, patients were scheduled for 24 h Holter monitoring and physical examination every year. In case of symptom recurrence, patients were invited for an additional visit, including Holter examination, at the outback patient clinic. Due to the observational nature of the study, no further specific investigation was requested to the patients.
Endpoints
Primary efficacy endpoint was defined as freedom from atrial arrhythmias, i.e. no evidence of AF, atrial flutter, or other atrial arrhythmias with a duration of >30 s, as documented by Holter monitoring, or PM interrogation, off Class I and III AAD. This was according to the definitions of the Expert Consensus Statement for the catheter and surgical ablation of AF 2012.4 Secondary efficacy endpoint was freedom from atrial arrhythmias with the use of AAD. Patients who underwent transcatheter PVI after sPVI were considered failures in both primary and secondary endpoints.
Safety endpoint was the occurrence of procedural and post-procedural adverse events. Adverse events were defined as an event that resulted in death or permanent injury, in temporary injury that required intervention or specific treatment (e.g. stroke, transient ischaemic attack, major bleeding requiring surgery or blood transfusion or cardiac tamponade and/or perforation, significant/symptomatic PV stenosis >70%, pericarditis and/or pericardial effusion, acute coronary syndrome, myocardial infarction, nervus phrenicus lesion, pneumothorax, wound infections, empyema, pneumonia, peri-procedural conversion to thoracotomy, and other not pre-defined events).18
Statistics
Baseline descriptive statistics are presented as mean ± standard deviation or median (range) for continuous variables, as appropriate, and counts with percentages for categorical variables. Differences between subgroups, in terms of patient characteristics at baseline, different follow-up moments, and end of study were evaluated by Student's t test or the Mann–Whitney U test, depending on normality of the data. Chi-square or Fisher's exact test were used for comparison of categorical variables. Follow-up data were censored for patients who had a first recurrence of AF or had been followed through 1 February 2014. The observation time was calculated as the time from ablation until either the occurrence of AF or the moment of censoring. The statistical software package SPSS 20 was used for analysis.
Results
Patient population
A total of 42 patients were treated with sPVI in two different centres. Twenty-two patients were treated at San Raffaele University Hospital and 20 patients were treated at Medisch Spectrum Twente, respectively. Mean age was 55 ± 10, 32 (76%) patients were males. The EHRA score was two in 48% and three in 52% of all patients. Hypertension was present in 33% of patients, the CHA2DS2-VASC was two or more in 19% and underlying heart disease was excluded in all patients as described previously. Atrial fibrillation was present for a median of 24 months (3–200) before the SPVI procedure. Previous transcatheter PVI were performed in nine (21%) patients. Preoperatively, three patients had a pacemaker due to significant AAD-related bradycardia. Patient baseline characteristics of the groups are illustrated in detail below (Table 1).
| . | SPVI group (n = 42) . | Centre 1 HSR (n = 22) . | Centre 2 MST (n = 20) . | P-value . |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 57 ± 8 | 54 ± 12 | 0.099 |
| Male, n (%) | 32 (76%) | 19 (86%) | 13 (65%) | 0.152 |
| Median AF history, months (range) | 24 [3–200] | 24 [12–200] | 19 [3–144] | 0.165 |
| Previous transcatheter PVI | 9 (21%) | 7 (32%) | 2 (10%) | 0.135 |
| EHRA-score | 0.769 | |||
| II, n (%) | 20 (48%) | 10 (45%) | 10 (50%) | |
| III, n (%) | 22 (52%) | 12 (55%) | 10 (50%) | |
| NYHA-score | 0.945 | |||
| II, n (%) | 40 (95%) | 21 (95%) | 19 (95%) | |
| III, n (%) | 2 (5%) | 1 (5%) | 1 (5%) | |
| CHA2DS2-VASC | 0.176 | |||
| 0, n (%) | 18 (42%) | 10 (45%) | 8 (40%) | |
| 1, n (%) | 16 (37%) | 8 (36%) | 8 (40%) | |
| ≥2, n (%) | 8 (19%) | 4 (18%) | 4 (20%) | |
| BMI (kg/m²) | 27 ± 4 | 26 ± 3 | 27 ± 4 | 0.489 |
| Hypertension, n (%) | 14 (33%) | 8 (43%) | 6 (30%) | 0.750 |
| Echocardiographic findings | ||||
| LV ejection fraction (mm) | 58 ± 6 | 59 ± 3 | 56 ± 8 | 0.240 |
| LA parasternal diameter (mm) | 42 ± 5 | 43 ± 5 | 41 ± 5 | 0.658 |
| . | SPVI group (n = 42) . | Centre 1 HSR (n = 22) . | Centre 2 MST (n = 20) . | P-value . |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 57 ± 8 | 54 ± 12 | 0.099 |
| Male, n (%) | 32 (76%) | 19 (86%) | 13 (65%) | 0.152 |
| Median AF history, months (range) | 24 [3–200] | 24 [12–200] | 19 [3–144] | 0.165 |
| Previous transcatheter PVI | 9 (21%) | 7 (32%) | 2 (10%) | 0.135 |
| EHRA-score | 0.769 | |||
| II, n (%) | 20 (48%) | 10 (45%) | 10 (50%) | |
| III, n (%) | 22 (52%) | 12 (55%) | 10 (50%) | |
| NYHA-score | 0.945 | |||
| II, n (%) | 40 (95%) | 21 (95%) | 19 (95%) | |
| III, n (%) | 2 (5%) | 1 (5%) | 1 (5%) | |
| CHA2DS2-VASC | 0.176 | |||
| 0, n (%) | 18 (42%) | 10 (45%) | 8 (40%) | |
| 1, n (%) | 16 (37%) | 8 (36%) | 8 (40%) | |
| ≥2, n (%) | 8 (19%) | 4 (18%) | 4 (20%) | |
| BMI (kg/m²) | 27 ± 4 | 26 ± 3 | 27 ± 4 | 0.489 |
| Hypertension, n (%) | 14 (33%) | 8 (43%) | 6 (30%) | 0.750 |
| Echocardiographic findings | ||||
| LV ejection fraction (mm) | 58 ± 6 | 59 ± 3 | 56 ± 8 | 0.240 |
| LA parasternal diameter (mm) | 42 ± 5 | 43 ± 5 | 41 ± 5 | 0.658 |
AAD, anti-arrhythmic drugs; TEE, thrombo-embolic; LA, left atrium.
| . | SPVI group (n = 42) . | Centre 1 HSR (n = 22) . | Centre 2 MST (n = 20) . | P-value . |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 57 ± 8 | 54 ± 12 | 0.099 |
| Male, n (%) | 32 (76%) | 19 (86%) | 13 (65%) | 0.152 |
| Median AF history, months (range) | 24 [3–200] | 24 [12–200] | 19 [3–144] | 0.165 |
| Previous transcatheter PVI | 9 (21%) | 7 (32%) | 2 (10%) | 0.135 |
| EHRA-score | 0.769 | |||
| II, n (%) | 20 (48%) | 10 (45%) | 10 (50%) | |
| III, n (%) | 22 (52%) | 12 (55%) | 10 (50%) | |
| NYHA-score | 0.945 | |||
| II, n (%) | 40 (95%) | 21 (95%) | 19 (95%) | |
| III, n (%) | 2 (5%) | 1 (5%) | 1 (5%) | |
| CHA2DS2-VASC | 0.176 | |||
| 0, n (%) | 18 (42%) | 10 (45%) | 8 (40%) | |
| 1, n (%) | 16 (37%) | 8 (36%) | 8 (40%) | |
| ≥2, n (%) | 8 (19%) | 4 (18%) | 4 (20%) | |
| BMI (kg/m²) | 27 ± 4 | 26 ± 3 | 27 ± 4 | 0.489 |
| Hypertension, n (%) | 14 (33%) | 8 (43%) | 6 (30%) | 0.750 |
| Echocardiographic findings | ||||
| LV ejection fraction (mm) | 58 ± 6 | 59 ± 3 | 56 ± 8 | 0.240 |
| LA parasternal diameter (mm) | 42 ± 5 | 43 ± 5 | 41 ± 5 | 0.658 |
| . | SPVI group (n = 42) . | Centre 1 HSR (n = 22) . | Centre 2 MST (n = 20) . | P-value . |
|---|---|---|---|---|
| Age, years | 55 ± 10 | 57 ± 8 | 54 ± 12 | 0.099 |
| Male, n (%) | 32 (76%) | 19 (86%) | 13 (65%) | 0.152 |
| Median AF history, months (range) | 24 [3–200] | 24 [12–200] | 19 [3–144] | 0.165 |
| Previous transcatheter PVI | 9 (21%) | 7 (32%) | 2 (10%) | 0.135 |
| EHRA-score | 0.769 | |||
| II, n (%) | 20 (48%) | 10 (45%) | 10 (50%) | |
| III, n (%) | 22 (52%) | 12 (55%) | 10 (50%) | |
| NYHA-score | 0.945 | |||
| II, n (%) | 40 (95%) | 21 (95%) | 19 (95%) | |
| III, n (%) | 2 (5%) | 1 (5%) | 1 (5%) | |
| CHA2DS2-VASC | 0.176 | |||
| 0, n (%) | 18 (42%) | 10 (45%) | 8 (40%) | |
| 1, n (%) | 16 (37%) | 8 (36%) | 8 (40%) | |
| ≥2, n (%) | 8 (19%) | 4 (18%) | 4 (20%) | |
| BMI (kg/m²) | 27 ± 4 | 26 ± 3 | 27 ± 4 | 0.489 |
| Hypertension, n (%) | 14 (33%) | 8 (43%) | 6 (30%) | 0.750 |
| Echocardiographic findings | ||||
| LV ejection fraction (mm) | 58 ± 6 | 59 ± 3 | 56 ± 8 | 0.240 |
| LA parasternal diameter (mm) | 42 ± 5 | 43 ± 5 | 41 ± 5 | 0.658 |
AAD, anti-arrhythmic drugs; TEE, thrombo-embolic; LA, left atrium.
Surgical procedure
The sPVI procedure was completed in all patients. In all patients, an exit block could be confirmed. No additional (linear) ablation lines were applied and no additional ablation of the ganglionic plexi was performed. The LAA was excluded or closed by the Atriclip device in 33 (79%) patients. In nine (21%) patients, the LAA was intentionally unaddressed. The mean procedural time was 194 ± 50 min and post-operative hospitalization was 7 ± 2 days.
Efficacy endpoints
After a mean follow-up of 5 years (SD 1.7, range 3–8), 29 (69%) of all patients were free from atrial arrhythmias without the use of AAD. Success was higher when AAD were taken into account, with 35 (83%) patients free from atrial arrhythmias at long-term follow-up (Figure 1). Of all scheduled Holter recordings, a total of 93% were performed successfully.
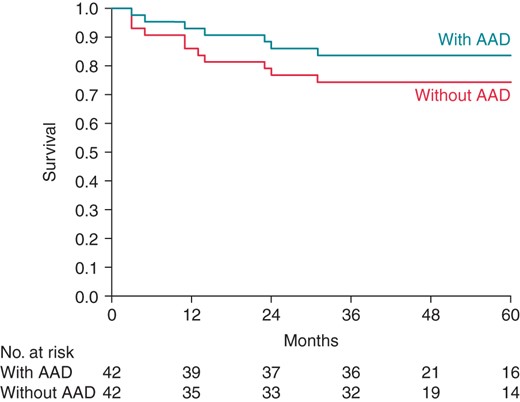
Kaplan–Meier survival curve freedom from arrhythmia at long-term follow-up with and without AADs.
Over time, the percentage of patients free from atrial arrhythmias without AAD use was 83, 79, 76, 70, 74% at 12, 24, 36, 48, and 60 months, respectively. At these time intervals, freedom from atrial arrhythmias with the use of AAD was 93, 86, 86, 78 and 84% (Figure 2). There were no significant differences in outcome between the two centres.
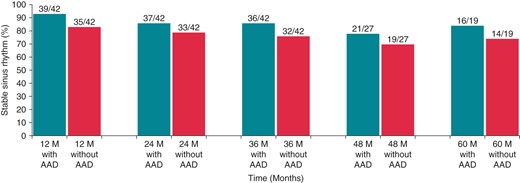
Freedom from arrhythmia at 12, 24, 36, 48, and 60 months follow-up, with and without AADs.
Of the 13 patients with recurrent atrial arrhythmia after sPVI, 1 patient relapsed with right atrial flutter while all the others had recurrent AF. Of these patients, six (46%) underwent additional transcatheter ablation. In two of these patients, this resulted in stable sinus rhythm without AAD and in one this resulted in stable sinus rhythm with AADs. (Table 2).
| . | SPVI (n = 42) . |
|---|---|
| Mean follow-up, years (range) | 5 (SD 1.7, range 3–8) |
| FFAs without AAD (n, %) | 29 (69%) |
| FFAs with AAD (n, %) | 35 (83%) |
| FFA with additional transcatheter ablation, no AAD | 31 (74%) |
| FFA with additional transcatheter ablation and AAD | 36 (86%) |
| Freedom from anti arrhythmic drugs | 32 (76%) |
| Freedom from oral anticoagulants | 39 (93%) |
| Mortality at follow-up (n, %) | 0 (0%) |
| Late stroke (>30 day), (n, %) | 0 (0%) |
| . | SPVI (n = 42) . |
|---|---|
| Mean follow-up, years (range) | 5 (SD 1.7, range 3–8) |
| FFAs without AAD (n, %) | 29 (69%) |
| FFAs with AAD (n, %) | 35 (83%) |
| FFA with additional transcatheter ablation, no AAD | 31 (74%) |
| FFA with additional transcatheter ablation and AAD | 36 (86%) |
| Freedom from anti arrhythmic drugs | 32 (76%) |
| Freedom from oral anticoagulants | 39 (93%) |
| Mortality at follow-up (n, %) | 0 (0%) |
| Late stroke (>30 day), (n, %) | 0 (0%) |
FFA, freedom from atrial arrhythmias.
| . | SPVI (n = 42) . |
|---|---|
| Mean follow-up, years (range) | 5 (SD 1.7, range 3–8) |
| FFAs without AAD (n, %) | 29 (69%) |
| FFAs with AAD (n, %) | 35 (83%) |
| FFA with additional transcatheter ablation, no AAD | 31 (74%) |
| FFA with additional transcatheter ablation and AAD | 36 (86%) |
| Freedom from anti arrhythmic drugs | 32 (76%) |
| Freedom from oral anticoagulants | 39 (93%) |
| Mortality at follow-up (n, %) | 0 (0%) |
| Late stroke (>30 day), (n, %) | 0 (0%) |
| . | SPVI (n = 42) . |
|---|---|
| Mean follow-up, years (range) | 5 (SD 1.7, range 3–8) |
| FFAs without AAD (n, %) | 29 (69%) |
| FFAs with AAD (n, %) | 35 (83%) |
| FFA with additional transcatheter ablation, no AAD | 31 (74%) |
| FFA with additional transcatheter ablation and AAD | 36 (86%) |
| Freedom from anti arrhythmic drugs | 32 (76%) |
| Freedom from oral anticoagulants | 39 (93%) |
| Mortality at follow-up (n, %) | 0 (0%) |
| Late stroke (>30 day), (n, %) | 0 (0%) |
FFA, freedom from atrial arrhythmias.
Safety endpoints
In four (9.5%) patients peri-procedural adverse events occurred. In one case, bleeding of the LAA required conversion to median sternotomy. Another patient suffered from unilateral paralysis of the diaphragm, which was resolved at 3 months follow-up. Two patients developed a pneumothorax during the postoperative course, which was treated with chest drainage. All patients recovered completely. No 30-day or in-hospital mortality was observed in this cohort of paroxysmal AF patients. There were no cases of re-hospitalization. During long-term follow-up, none of the patients included in this study suffered from stroke based on clinical observation and no mortality occurred during follow-up.
Discussion
Main findings
We show that at long-term follow-up, freedom from recurrent AF without the use of AADs was 69% and freedom from recurrent AF with AAD was 84% in patients with paroxysmal AF undergoing sPVI. The occurrence of adverse events was 9.5%. No unforeseen late complications of sPVI were detected. We report the longest follow-up to date of any minimally invasive sPVI procedure for the treatment of paroxysmal lone AF. Our previous study reported 73% of freedom from arrhythmia without the use of AAD, 24 months after the procedure.12 This study shows slightly better results in a ‘lone’ paroxysmal AF population and demonstrates that the previously reported results are maintained at long-term follow-up. The use of bipolar radiofrequency ablation clamp (integrating an automatic transmurality algorithm with impedance feedback) and systematic verification of exit block offer certainty regarding effective isolation of the PVs, even in the thickest region surrounding the PVs.19
Comparison with mid-term outcomes surgical pulmonary vein isolation
In recent years, several small studies have reported on short- and mid-term outcomes of sPVI, showing successful outcome ranging from 64 to 90%.10–14 The report by Weimar et al. showed a comparable freedom from atrial arrhythmia at 2 years follow-up, ∼82% (19 patients available).13 All studies mentioned above report results of sPVI in heterogeneous AF populations. In lone paroxysmal AF previous reported results remain largely unknown. The literature also reports a large variety of lesion sets. Since in ∼90% of all cases, the trigger for paroxysmal AF originates from the region of the PVs, addressing the PVs is essential and in most cases sufficient to cure paroxysmal AF. However, using this lesion set, triggers in non-PV sites may lead to AF recurrences. In our study, sPVI was performed without any additional linear ablations and without addressing the ganglionic plexi. The additional value of additional (linear) ablation lines is still unclear. An advantage of the ‘lone’ sPVI in our patient population might be that the risk of left atrial flutters or atrial tachycardia is low, compared with additional lesion sets using unipolar ablation. This is also confirmed by our data. Interestingly, in our series, recurrences of arrhythmia were mostly recorded during the first year after ablation. In the follow-up period afterwards, we noted that the rate of decline in freedom from AF after the first intervention stabilized after 24 months, although it did not entirely reached a plateau. This is comparable with long-term outcome after MAZE surgery.
Comparison with transcatheter pulmonary vein isolation
The majority of our relatively young and healthy paroxysmal AF patient population underwent sPVI as first invasive treatment, according to patients' preference. These patients would normally be directed to transcatheter PVI, according to the current guidelines.4 When compared with the long-term results of multiple transcatheter PVI, following similar follow-up, this group, although small, shows comparable freedom from AF.6,20 In a small sub-group of our patients, transcatheter ablation had been performed prior to sPVI. The recent literature advocates that these patients could also have been proposed for a second transcatheter procedure.6,20 Although, when compared with single transcatheter PVI, sPVI shows significantly higher freedom from AF.5,6 and the recurrence rate after single procedure transcatheter PVI tends to show a faster decay. This indicates that the surgically applied lesion, created by bipolar radiofrequency, offers a higher durability.
When considering the LAA, the sPVI procedure offers a unique advantage of fast, simple, and effective exclusion or closure. While not necessarily being part of the standard sPVI procedure, this could be considered as an important upgrade in higher risk patients, offered with a much shorter procedure time, less risky, and less expensive when compared with a transcatheter closure.
Future perspectives
With the introduction of new transcatheter techniques (e.g. cooltip, RF catheters with pressure sensoring, cryobaloon PVI), the single procedure results tend to improve although long-term results remain unknown.21 In our view, the sPVI and the transcatheter techniques might perform well in a multi-disciplinary context, either performed as one combined procedure or sequentially. Excellent preliminary results have been reported with the hybrid procedures.22 This is also supported by our data, additional transcatheter ablation in our patients with AF recurrence, led to an overall freedom from arrhythmia of 74% without AADs and 86% with AAD at long-term follow-up. Unfortunately, because of the invasive nature of the thoracoscopic approach, the sPVI shows a slightly higher rate of adverse events and longer hospitalization compared with transcatheter PVI.5,18 The invasiveness appears to be one of the major concerns of patients and referring electrophysiologists. Current practice of sPVI in high volume centres, however, shows a trend toward a shorter postoperative hospital stay. Future studies are awaited and may show lower complication rates.
Strengths and limitations
Strength of the present analysis were the long-term follow-up performed by Holter monitoring, the unique patient population with lone paroxysmal AF and the satisfactory long-term results of sPVI. The observational and retrospective nature of this study and limited number of patients means that no definite conclusions can be drawn regarding the procedure efficacy and safety. Also, the patients included in our study were young, non-obese and without structural heart disease. Implantable loop recorders were not used due to the more invasive nature, cost aspects and the battery in these devices lasts only 18 to 24 months. Follow-up using 24 and 96 h Holter monitoring may underestimate the recurrence of AF.
Conclusion
Surgical PVI as treatment for paroxysmal AF is an effective, reproducible treatment strategy with maintained efficacy at long-term follow-up up to 7 years. This observational, retrospective two-centre study shows that sPVI might be considered as the first ablation strategy in young, highly symptomatic patients for lone, paroxysmal AF.
Conflicts of interest: S.B. has a financial relationship with St. Jude Medical Inc., AtriCure Inc., Medtronic Inc., CryoCath Inc., and Edwards Lifesciences Inc. The other authors report no conflicts.
References
Author notes
Both authors have contributed equally.



