-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Efremidis, Konstantinos Letsas, Georgios Giannopoulos, Louisa Lioni, Konstantinos Vlachos, Dimitrios Asvestas, Dimitrios Karlis, Vasileios Kareliotis, Hrysoula Geladari, Antonios Sideris, Spyridon Deftereos, Early pulmonary vein reconnection as a predictor of left atrial ablation outcomes for paroxysmal atrial fibrillation, EP Europace, Volume 17, Issue 5, May 2015, Pages 741–746, https://doi.org/10.1093/europace/euu216
Close - Share Icon Share
Abstract
The objective of the study was to investigate whether early pulmonary vein reconnection (PVR) is a predictor of late arrhythmia recurrence after a single ablation procedure for paroxysmal atrial fibrillation (AF). Further ablation was delivered to patients with acute PVR to test whether this strategy could reduce recurrences.
One hundred and forty-four consecutive patients with symptomatic, drug-refractory paroxysmal AF, undergoing pulmonary vein isolation (PVI), were assigned to the ‘PVR30 test’ group, where PVR was monitored for 30 min after initial PVI and further ablation was applied if needed, and compared with a control group of 128 patients, where the procedure was terminated after initial successful isolation. During a mean follow-up of 17.7 months, sinus rhythm was maintained in 101 patients in the ‘PVR30 test’ group (70.1%) vs. 78 in the control group (60.9%) (P = 0.13). Among patients with acute PVR and reablation after 30 min, the recurrence rate was 45.3 vs. 39.1% in the control group (P = 0.47). Multivariable logistic regression analysis showed that PVR was independently associated with AF recurrence (adjusted hazard ratio 4.7, 95% confidence interval 1.8–12.2), along with left atrial diameter (adjusted hazard ratio 1.3/mm of higher diameter, 95% confidence interval 1.2–1.4).
In patients with paroxysmal AF undergoing a single ablation procedure, PVR 30 min after the initial PVI is associated with late AF recurrence. However, the strategy of 30 min waiting and reablating does not appear to be superior to immediate termination of the procedure after initial PVI.
In agreement with previous studies, about half of the patients undergoing pulmonary vein isolation presented electrical pulmonary vein reconnection with the left atrium within 30 min after initial vein isolation.
Acute reconnection was associated with arrhythmia recurrence over a follow-up of close to 1.5 year.
Despite intensive effort with additional ablation in patients who had reconnection at 30 min, a non-significant effect was observed in terms of improved post-ablation sinus rhythm maintenance rates.
Further research is warranted into more effective ways of inducing sustained electrical isolation of the pulmonary veins.
Introduction
Pulmonary vein isolation (PVI) is the treatment of choice in patients with drug-refractory symptomatic paroxysmal atrial fibrillation (AF).1,2 The aim of PVI is abolishment of all conducted electrical activity beyond the isolating lesions. However, some of the created lesions do not result in permanent conduction block, resulting in gaps after the left atrial ablation is completed. Pulmonary vein recovery of conduction has been verified in up to 80% in at least one vein among patients who return for a second AF ablation, and seems to be the dominant mechanism of AF recurrence3 in patients with paroxysmal AF.
Intravenous adenosine with or without isoproterenol and monitoring of PVI for a period of time have been used to unmask latent conduction between pulmonary veins (PVs) and the left atrium.4–6
The main objective of this study was to investigate whether early PV reconnection (PVR) is a predictor of late arrhythmia recurrence after a single ablation procedure for paroxysmal AF and to test whether the wait-and-reablate strategy could improve outcomes.
Methods
Patients
We studied 272 patients with symptomatic, drug-refractory paroxysmal AF who underwent PVI. Patients were classified as having paroxysmal AF according to current guidelines.7 Exclusion criteria included left atrial diameter (LAD) >50 mm, intracardiac thrombi documented by transesophageal echocardiography, systolic heart failure (left ventricular ejection fraction <45% and NYHA III–IV), previous ablation for AF, uncontrolled thyroid disorders, moderate-to-severe or severe valve disease, inadequate follow-up, and/or inability to provide informed consent. Demographic and clinical characteristics as well as blood samples were collected from all the participants. Transthoracic and transeshophageal echocardiograms were performed in all subjects. Patients underwent PVI followed by observation for PVR and further ablation if necessary (‘PVR30 test’ group, n = 144) and were compared with PVI without monitoring for PVR at the end of the procedure (control group, n = 128). Control patients were selected from our records of PVI cases (last 2 years), using a random selection algorithm, after stratification for known predictors of recurrence, including age, sex, diabetes, hypertension, and LAD. The study protocol was approved by our institutional ethics committee and written informed consent was obtained from all patients.
Catheter ablation procedure
Oral anticoagulation was stopped 1 day prior to the ablation procedure, and all subjects were anticoagulated with enoxaparin (1 mg/kg twice daily). Antiarrhythmic drug (AAD) treatment was suspended for the day of the ablation procedure and restarted the following day. The ablation procedure has been described in detail elsewhere.8,9 Following a single transeptal puncture, the three-dimensional geometry of the left atrium was reconstructed using the CARTO 3 navigation system (Biosense Webster, Inc.). Wide circumferential lesions for isolation of large atrial areas around both ipsilateral PVs (PV antral isolation) were applied using a 3.5 mm-tip ablation catheter (Thermo Cool Navi-Star, Biosense Webster, Inc.). Typically applied power settings were 40 W for the anterior wall and 35 W for the posterior wall. Each radiofrequency (RF) lesion duration was 45–60 s. Circumferential ablation was performed on the posterior wall >1 cm and on the anterior wall >5 mm away from the defined PV ostia. The endpoint of ablation was absence or dissociation of potentials in the isolated area as documented by the circular mapping catheter (Lasso, Biosense Webster, Inc.). When PV conduction was still present following wide circumferential lesions around both ipsilateral veins, both PVs were mapped sequentially by the circular mapping catheter to localize the earliest PV potentials. Based on the earliest PV potentials recorded by the circular mapping catheter, RF energy was reapplied to close the conduction gap.
Observation for pulmonary vein reconnection and reablation
In both of the patient groups, left atrial ablation was started by isolating left PVs. In the PVR30 test group, entrance and exit block of the left PVs was evaluated 30 min after the initial isolation. Pulmonary veins with conduction recovery were reablated. Additional 30 minute waiting time was given for observation of right PVs reconnection after right PVs isolation. The procedure was considered completed once the right PVs with conduction recovery were reisolated.
Post-ablation care and follow-up
Warfarin was restarted post-ablation and continued for at least 3 months. All subjects underwent ambulatory monitoring the first two post-procedural days. Antiarrhythmic drugs were stopped 3 months after the ablation procedure in all subjects. Recurrences during this blanking period were treated with AADs and/or cardioversion if needed. The patients were seen by the referring cardiologist for 48 h ambulatory monitoring (Holter recordings) at the end of the first, third, sixth, ninth, and twelfth months after the index procedure. Patients were additionally advised to report any symptoms of arrhythmia between scheduled visits. Documented symptomatic or asymptomatic AF episodes lasting >30 s or atrial tachycardias were considered as recurrence after the 3-month blanking period.
Statistical analysis
The study was powered (at a 0.80 power level) to detect a difference of 15 percentage points between the two groups (assuming a 45% failure rate after a single procedure in the control group). Continuous variables are expressed as mean ± standard deviation. Testing for normality was performed with the Kolmogorov–Smirnov test and in case of significant deviation from the normal distribution non-parametric tests (Wilcoxon's and Mann–Whitney, as suited) were applied. Categorical variables are presented as absolute numbers and frequencies. Comparison between categorical variables was performed using Fisher's exact test. Kaplan-Meier analysis was performed to test for differences in time-to-recurrence between patient subsets. The effect of explanatory variables on AF recurrence was evaluated using logistic regression analysis. All reported P values were based on two-sided tests and compared with a significance level of 0.05. All analyses were performed using the SPSS software (version 17.0; SPSS, Inc.).
Results
The ‘PVR30 test’ and the control group consisted of 144 and 128 patients, respectively, with paroxysmal AF. The demographic, clinical, echocardiographic, laboratory, and procedural data of both cohorts are summarized in Table 1. There were no significant differences between the ‘PVR30 test’ group and the control group in terms of population characteristics. The total procedure time, expectedly, was significantly higher in the ‘PVR30 test’ group compared with the control group (P < 0.01).
| Variable . | PVR30 test group (n = 144) . | Control group (n = 128) . | P value . |
|---|---|---|---|
| Age (years) | 56.8 ± 12.3 | 58.1 ± 10.7 | 0.37 |
| Gender (male) (%) | 95 (66.0) | 86 (67.2) | 0.89 |
| Body mass index (kg/m2) | 27.7 ± 4.3 | 28.2 ± 4.0 | 0.31 |
| Hypertension (%) | 62 (43.1) | 57 (44.5) | 0.81 |
| Diabetes (%) | 23 (16.0) | 21 (16.4) | 1.00 |
| Dyslipidaemia/statin use (%) | 59 (41.0) | 55 (43.0) | 0.81 |
| CAD (%) | 10 (6.9) | 12 (9.4) | 0.51 |
| AF duration (years) | 4.9 ± 4.3 | 5.1 ± 4.6 | 0.73 |
| AADs after AF ablationa | |||
| Class I (%) | 9 (6.3) | 6 (4.7) | 0.61 |
| Class III (%) | 61 (42.4) | 67 (52.3) | 0.11 |
| LAD (mm) | 39.3 ± 5.5 | 40.1 ± 4.7 | 0.19 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.34 |
| White blood cell count (/µL) | 8949 ± 2352 | 8438 ± 2234 | 0.07 |
| Fluoroscopy time | 13.2 ± 7.5 | 13.1 ± 6.9 | 0.91 |
| Procedure time | 209.3 ± 48.6 | 183.1 ± 15.4 | <0.01 |
| TIA (%) | 1 (0.7) | 2 (1.6) | 0.60 |
| Tamponade (%) | 2(1.4) | 1(0.8) | 1.00 |
| PVR 30 min (%) | 64 (44.4) | N/A | |
| Follow-up duration (months) | 16.5 ± 7.0 | 17.7 ± 6.2 | 0.14 |
| Variable . | PVR30 test group (n = 144) . | Control group (n = 128) . | P value . |
|---|---|---|---|
| Age (years) | 56.8 ± 12.3 | 58.1 ± 10.7 | 0.37 |
| Gender (male) (%) | 95 (66.0) | 86 (67.2) | 0.89 |
| Body mass index (kg/m2) | 27.7 ± 4.3 | 28.2 ± 4.0 | 0.31 |
| Hypertension (%) | 62 (43.1) | 57 (44.5) | 0.81 |
| Diabetes (%) | 23 (16.0) | 21 (16.4) | 1.00 |
| Dyslipidaemia/statin use (%) | 59 (41.0) | 55 (43.0) | 0.81 |
| CAD (%) | 10 (6.9) | 12 (9.4) | 0.51 |
| AF duration (years) | 4.9 ± 4.3 | 5.1 ± 4.6 | 0.73 |
| AADs after AF ablationa | |||
| Class I (%) | 9 (6.3) | 6 (4.7) | 0.61 |
| Class III (%) | 61 (42.4) | 67 (52.3) | 0.11 |
| LAD (mm) | 39.3 ± 5.5 | 40.1 ± 4.7 | 0.19 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.34 |
| White blood cell count (/µL) | 8949 ± 2352 | 8438 ± 2234 | 0.07 |
| Fluoroscopy time | 13.2 ± 7.5 | 13.1 ± 6.9 | 0.91 |
| Procedure time | 209.3 ± 48.6 | 183.1 ± 15.4 | <0.01 |
| TIA (%) | 1 (0.7) | 2 (1.6) | 0.60 |
| Tamponade (%) | 2(1.4) | 1(0.8) | 1.00 |
| PVR 30 min (%) | 64 (44.4) | N/A | |
| Follow-up duration (months) | 16.5 ± 7.0 | 17.7 ± 6.2 | 0.14 |
AF, atrial fibrillation; CAD, coronary artery disease; AADs, antiarrhythmic drugs; LAD, left atrial diameter; WBC, white blood cells; PVR 30 min, pulmonary vein reconnection 30 min after ablation; TIA, transient cerebral ischemic attack.
P values lower than 0.05 in bold.
aAll AADs were discontinued at the end of the 3-month blanking period.
| Variable . | PVR30 test group (n = 144) . | Control group (n = 128) . | P value . |
|---|---|---|---|
| Age (years) | 56.8 ± 12.3 | 58.1 ± 10.7 | 0.37 |
| Gender (male) (%) | 95 (66.0) | 86 (67.2) | 0.89 |
| Body mass index (kg/m2) | 27.7 ± 4.3 | 28.2 ± 4.0 | 0.31 |
| Hypertension (%) | 62 (43.1) | 57 (44.5) | 0.81 |
| Diabetes (%) | 23 (16.0) | 21 (16.4) | 1.00 |
| Dyslipidaemia/statin use (%) | 59 (41.0) | 55 (43.0) | 0.81 |
| CAD (%) | 10 (6.9) | 12 (9.4) | 0.51 |
| AF duration (years) | 4.9 ± 4.3 | 5.1 ± 4.6 | 0.73 |
| AADs after AF ablationa | |||
| Class I (%) | 9 (6.3) | 6 (4.7) | 0.61 |
| Class III (%) | 61 (42.4) | 67 (52.3) | 0.11 |
| LAD (mm) | 39.3 ± 5.5 | 40.1 ± 4.7 | 0.19 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.34 |
| White blood cell count (/µL) | 8949 ± 2352 | 8438 ± 2234 | 0.07 |
| Fluoroscopy time | 13.2 ± 7.5 | 13.1 ± 6.9 | 0.91 |
| Procedure time | 209.3 ± 48.6 | 183.1 ± 15.4 | <0.01 |
| TIA (%) | 1 (0.7) | 2 (1.6) | 0.60 |
| Tamponade (%) | 2(1.4) | 1(0.8) | 1.00 |
| PVR 30 min (%) | 64 (44.4) | N/A | |
| Follow-up duration (months) | 16.5 ± 7.0 | 17.7 ± 6.2 | 0.14 |
| Variable . | PVR30 test group (n = 144) . | Control group (n = 128) . | P value . |
|---|---|---|---|
| Age (years) | 56.8 ± 12.3 | 58.1 ± 10.7 | 0.37 |
| Gender (male) (%) | 95 (66.0) | 86 (67.2) | 0.89 |
| Body mass index (kg/m2) | 27.7 ± 4.3 | 28.2 ± 4.0 | 0.31 |
| Hypertension (%) | 62 (43.1) | 57 (44.5) | 0.81 |
| Diabetes (%) | 23 (16.0) | 21 (16.4) | 1.00 |
| Dyslipidaemia/statin use (%) | 59 (41.0) | 55 (43.0) | 0.81 |
| CAD (%) | 10 (6.9) | 12 (9.4) | 0.51 |
| AF duration (years) | 4.9 ± 4.3 | 5.1 ± 4.6 | 0.73 |
| AADs after AF ablationa | |||
| Class I (%) | 9 (6.3) | 6 (4.7) | 0.61 |
| Class III (%) | 61 (42.4) | 67 (52.3) | 0.11 |
| LAD (mm) | 39.3 ± 5.5 | 40.1 ± 4.7 | 0.19 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.34 |
| White blood cell count (/µL) | 8949 ± 2352 | 8438 ± 2234 | 0.07 |
| Fluoroscopy time | 13.2 ± 7.5 | 13.1 ± 6.9 | 0.91 |
| Procedure time | 209.3 ± 48.6 | 183.1 ± 15.4 | <0.01 |
| TIA (%) | 1 (0.7) | 2 (1.6) | 0.60 |
| Tamponade (%) | 2(1.4) | 1(0.8) | 1.00 |
| PVR 30 min (%) | 64 (44.4) | N/A | |
| Follow-up duration (months) | 16.5 ± 7.0 | 17.7 ± 6.2 | 0.14 |
AF, atrial fibrillation; CAD, coronary artery disease; AADs, antiarrhythmic drugs; LAD, left atrial diameter; WBC, white blood cells; PVR 30 min, pulmonary vein reconnection 30 min after ablation; TIA, transient cerebral ischemic attack.
P values lower than 0.05 in bold.
aAll AADs were discontinued at the end of the 3-month blanking period.
Pulmonary vein reconnection 30 min after ablation was present in 64 (44.4%) patients of the ‘PVR30 test’ group. Pulmonary vein reconnection was observed in 86 (14.9%) PVs; it was more frequently observed at the left superior PV (LSPV) 39 (27.1% of LSPVs) and the right superior PV (RSPV) 31 (21.5% of RSPVs) when compared with the right inferior PV (RIPV) 4 (2.8% of RIPVs) and the left inferior PV (LIPV) 12 (8.3% of LIPVs). The most frequent sites of PVR in each vein were: LSPV, 65% at the region between the left atrial appendage and LSPV; LIPV, 47% at the anterior aspect and 32% at the interpulmonary carina; RSPV, 62% at the anterosuperior aspect; RIPV, 54% at the anteroinferior aspect.
Over the 17.7 ± 6.2 months follow-up, 43 (29.9%) and 50 (39.1%) patients experienced recurrent AF in the ‘PVR30 test’ and control groups, respectively. Twenty-nine out of 64 (45.3%) patients with PVR (all of whom received further ablation after initial PVI) experienced AF recurrence during follow-up, when compared with 50 out of 128 (39.1%) patients in the control group. In comparison, 14 out of 80 (17.5%) patients who did not demonstrate PVR in the ‘PVR30 test’ group experienced AF recurrence (Figure 1A). Similar were the findings of the time-dependent analysis (Figure 1B).
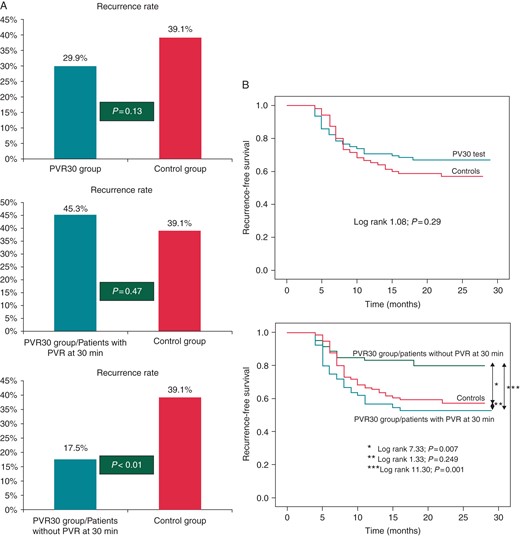
(A) Atrial fibrillation recurrence rates after a single ablation procedure in the ‘wait-see-and-reablate-as-needed’ PVR30 test group (upper panel), as well as in the subgroups of patients with (middle panel) and without (lower panel) PV reconnection at 30 min, when compared with the control group. (B) Kaplan–Meier curves of recurrence-free survival with patients stratified into two groups (PVR30 test vs. control) (upper panel) and with the PVR30 test group broken down into two subsets: those with and without reconnection at 30 min (lower panel).
Procedural complications in both groups included: cardiac tamponade in three patients (1.1%) who were effectively treated with pericardiocentesis without the need for surgical intervention. Moreover, transient cerebral ischaemic attack occurred in three patients (1.1%).
As shown in Table 2, ‘PVR30 test’ group patients with AF recurrence displayed increased LAD (42.2 ± 5.1 vs. 38.1 ± 5.1 mm, P < 0.01), higher prevalence of diabetes (P = 0.02), dyslipidaemia/statin use (P = 0.02), coronary artery disease (P < 0.01), and PVR 30 min after PV antral isolation (P < 0.01). Logistic regression analysis showed that PVR (adjusted hazard ratio 4.7, 95% confidence interval 1.8–12.2; P < 0.01), LAD as a continuous parameter (adjusted hazard ratio 1.3/mm of higher LAD, 95% confidence interval 1.2–1.4; P < 0.01), and dyslipidaemia/statin use (adjusted hazard ratio 8.1, 95% confidence interval 2.6–25.6; P < 0.01) were significant independent predictors of AF recurrence.
Demographic, clinical, echocardiographic, laboratory, and procedural data in patients with and without AF recurrence following catheter ablation in the ‘PVR30 test’ group
| Variable . | Free from AF recurrence (n = 101) . | AF recurrence (n = 43) . | P value . |
|---|---|---|---|
| Age (years) | 56.0 ± 12.5 | 58.8 ± 11.6 | 0.20 |
| Gender (male) (%) | 66 (65.3) | 29 (67.4) | 0.85 |
| Body mass index (kg/m2) | 28.0 ± 4.3 | 26.9 ± 4.2 | 0.13 |
| Hypertension (%) | 39 (38.6) | 23 (53.5) | 0.14 |
| Diabetes mellitus (%) | 11 (10.9) | 12 (27.9) | 0.02 |
| Dyslipidaemia/statin use (%) | 35 (34.7) | 24 (55.8) | 0.02 |
| CAD (%) | 3 (3.0) | 7 (16.3) | <0.01 |
| Duration of history of AF episodes (years) | 4.9 ± 4.4 | 4.9 ± 4.4 | 0.97 |
| White blood cell count (/µL) | 8979 ± 2474 | 8878 ± 2063 | 0.81 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.98 |
| AADs after AF ablation | |||
| Class I (%) | 5 (5.0) | 4 (9.3) | 0.45 |
| Class III (%) | 46 (45.5) | 15 (34.9) | 0.27 |
| LAD (mm) | 38.1 ± 5.1 | 42.2 ± 5.1 | <0.01 |
| Procedure time (min) | 211.4 ± 52.3 | 204.4 ± 38.7 | 0.43 |
| Fluoroscopy time (min) | 13.5 ± 7.9 | 12.4 ± 6.6 | 0.46 |
| PVR 30 min | 35 (34.7) | 29 (67.4) | <0.01 |
| Variable . | Free from AF recurrence (n = 101) . | AF recurrence (n = 43) . | P value . |
|---|---|---|---|
| Age (years) | 56.0 ± 12.5 | 58.8 ± 11.6 | 0.20 |
| Gender (male) (%) | 66 (65.3) | 29 (67.4) | 0.85 |
| Body mass index (kg/m2) | 28.0 ± 4.3 | 26.9 ± 4.2 | 0.13 |
| Hypertension (%) | 39 (38.6) | 23 (53.5) | 0.14 |
| Diabetes mellitus (%) | 11 (10.9) | 12 (27.9) | 0.02 |
| Dyslipidaemia/statin use (%) | 35 (34.7) | 24 (55.8) | 0.02 |
| CAD (%) | 3 (3.0) | 7 (16.3) | <0.01 |
| Duration of history of AF episodes (years) | 4.9 ± 4.4 | 4.9 ± 4.4 | 0.97 |
| White blood cell count (/µL) | 8979 ± 2474 | 8878 ± 2063 | 0.81 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.98 |
| AADs after AF ablation | |||
| Class I (%) | 5 (5.0) | 4 (9.3) | 0.45 |
| Class III (%) | 46 (45.5) | 15 (34.9) | 0.27 |
| LAD (mm) | 38.1 ± 5.1 | 42.2 ± 5.1 | <0.01 |
| Procedure time (min) | 211.4 ± 52.3 | 204.4 ± 38.7 | 0.43 |
| Fluoroscopy time (min) | 13.5 ± 7.9 | 12.4 ± 6.6 | 0.46 |
| PVR 30 min | 35 (34.7) | 29 (67.4) | <0.01 |
AF, atrial fibrillation; CAD, coronary artery disease; AADs, antiarrhythmic drugs; LAD, left atrial diameter; PVR 30 min, pulmonary vein reconnection 30 min after ablation.
P values lower than 0.05 in bold.
Demographic, clinical, echocardiographic, laboratory, and procedural data in patients with and without AF recurrence following catheter ablation in the ‘PVR30 test’ group
| Variable . | Free from AF recurrence (n = 101) . | AF recurrence (n = 43) . | P value . |
|---|---|---|---|
| Age (years) | 56.0 ± 12.5 | 58.8 ± 11.6 | 0.20 |
| Gender (male) (%) | 66 (65.3) | 29 (67.4) | 0.85 |
| Body mass index (kg/m2) | 28.0 ± 4.3 | 26.9 ± 4.2 | 0.13 |
| Hypertension (%) | 39 (38.6) | 23 (53.5) | 0.14 |
| Diabetes mellitus (%) | 11 (10.9) | 12 (27.9) | 0.02 |
| Dyslipidaemia/statin use (%) | 35 (34.7) | 24 (55.8) | 0.02 |
| CAD (%) | 3 (3.0) | 7 (16.3) | <0.01 |
| Duration of history of AF episodes (years) | 4.9 ± 4.4 | 4.9 ± 4.4 | 0.97 |
| White blood cell count (/µL) | 8979 ± 2474 | 8878 ± 2063 | 0.81 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.98 |
| AADs after AF ablation | |||
| Class I (%) | 5 (5.0) | 4 (9.3) | 0.45 |
| Class III (%) | 46 (45.5) | 15 (34.9) | 0.27 |
| LAD (mm) | 38.1 ± 5.1 | 42.2 ± 5.1 | <0.01 |
| Procedure time (min) | 211.4 ± 52.3 | 204.4 ± 38.7 | 0.43 |
| Fluoroscopy time (min) | 13.5 ± 7.9 | 12.4 ± 6.6 | 0.46 |
| PVR 30 min | 35 (34.7) | 29 (67.4) | <0.01 |
| Variable . | Free from AF recurrence (n = 101) . | AF recurrence (n = 43) . | P value . |
|---|---|---|---|
| Age (years) | 56.0 ± 12.5 | 58.8 ± 11.6 | 0.20 |
| Gender (male) (%) | 66 (65.3) | 29 (67.4) | 0.85 |
| Body mass index (kg/m2) | 28.0 ± 4.3 | 26.9 ± 4.2 | 0.13 |
| Hypertension (%) | 39 (38.6) | 23 (53.5) | 0.14 |
| Diabetes mellitus (%) | 11 (10.9) | 12 (27.9) | 0.02 |
| Dyslipidaemia/statin use (%) | 35 (34.7) | 24 (55.8) | 0.02 |
| CAD (%) | 3 (3.0) | 7 (16.3) | <0.01 |
| Duration of history of AF episodes (years) | 4.9 ± 4.4 | 4.9 ± 4.4 | 0.97 |
| White blood cell count (/µL) | 8979 ± 2474 | 8878 ± 2063 | 0.81 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.98 |
| AADs after AF ablation | |||
| Class I (%) | 5 (5.0) | 4 (9.3) | 0.45 |
| Class III (%) | 46 (45.5) | 15 (34.9) | 0.27 |
| LAD (mm) | 38.1 ± 5.1 | 42.2 ± 5.1 | <0.01 |
| Procedure time (min) | 211.4 ± 52.3 | 204.4 ± 38.7 | 0.43 |
| Fluoroscopy time (min) | 13.5 ± 7.9 | 12.4 ± 6.6 | 0.46 |
| PVR 30 min | 35 (34.7) | 29 (67.4) | <0.01 |
AF, atrial fibrillation; CAD, coronary artery disease; AADs, antiarrhythmic drugs; LAD, left atrial diameter; PVR 30 min, pulmonary vein reconnection 30 min after ablation.
P values lower than 0.05 in bold.
Discussion
The following observations summarize the main findings of the present study: (i) PVR 30 min after PVI was present in almost half of the patients, (ii) PVR is an independent prognostic factor of AF recurrence in patients with paroxysmal AF undergoing a single catheter ablation procedure, (iii) of interest, repeat ablation in patients with PVR to eliminate reconduction did not improve sinus rhythm maintenance rates: outcomes in patients who were reablated after the waiting period were not significantly better than in patients where the procedure was terminated immediately after successful PVI.
It is well-established that the main cause of paroxysmal AF recurrence after PVI is recovery of PV conduction.3,10 Additional evidence suggests that most patients in sinus rhythm during follow-up exhibit sustained PVI, whereas PVR is verified in most of the patients with AF recurrence.3,11 In the present study, PVR occurred in about half of the patients, within 30 min after the initial PVI procedure. This acute electrical reconnection was a prognostic factor of AF recurrence during a mean 17-month follow-up period despite additional ablation. It seems that patients with acute PVR, even when reablated in order to achieve PVI, constitute a group of patients more vulnerable to late AF recurrence. This may explain the non-superiority of the waiting strategy over immediate termination of the procedure. This finding might be due to failure to create permanent transmural lesions in patients with early PVR, despite reablation, which can be attributed to a number of plausible causes. First, the energy applied in each patient is the same regardless of the thickness of the left atrial wall, which is different in each patient. Secondly, the variable geometry of the PV antrum may prevent adequate catheter contact. Kowalski et al.12 found that PVR was associated with histopathological evidence of nontrasmural lesions along the ablation line, among patients undergoing a surgical Cox maze III procedure for recurrent AF, after an initially successful PVI procedure. In addition, the latter study suggested evidence of complex histology of antral ablation lesions with observation of myocytolysis, which may represent potentially reversible cellular responses to injury. The heterogeneity of ablative lesions has also been shown with magnetic resonance imaging (MRI), suggesting that only a subset of lesions result in nonenhancing areas in late gadolinium enhancement MRI, which are strongly correlated with final scar formation at 3 months post-ablation.13 Of note, nontransmural lesions are noted in some PVs with persistent conduction block (i.e. nontransmurality does not necessarily mean recovery of conduction). However, nontransmural ablation can produce a dynamic cellular substrate with features of reversible injury; reversibility of the thermal injury appears to be an important determinant of recovery of conduction and recovery from injury may explain recurrences of AF after PV isolation.12
The reconduction rate in our study was similar to that reported by Wang et al., who demonstrated PVR in 43.8% of patients 30 min after PVI. Wang concluded that reisolation of recovered gaps reduced the recurrence of AF when compared with patients in whom the procedure was stopped after initial ablation.14 Cheema et al.15 examined the time course of PVR at 30 and 60 min after PVI. Pulmonary vein reconnection was observed in 93% of the patients 60 min after successful isolation of the PVs. Thirty-three percent of the veins showed resumption of conduction at 30 min, while 17% of the veins showed a first recurrence at 60 min. In a randomized study,16 recovery of conduction was found in 50% of the patients 60 min after PVI. In this study, similar to our results, the outcome of AF ablation was not better between the ‘wait and reablate’ group and the ‘stop’ group. Interestingly enough, among the patients who underwent repeat ablation during the follow-up, there was no difference in the number of upcoming gaps per patient between the wait-and-reablate and the stop group.
Limitations
Asymptomatic episodes of AF may not have been recognized because AF recurrence was based on clinical symptoms and ambulatory monitoring for a short period of time. However, monitoring protocols were identical for all patients and, as a result, this fact should not have influenced in any significant way the comparisons between groups. It has been pointed out that inadequate contact force may be responsible for at least some part of suboptimal lesion formation at the antral ablation lines during PVIs and it is reasonable to expect some improvement with the advent of catheter contact calculation modalities (force sensing systems have been integrated into ablation catheters). The present study did not involve the use of novel catheters with catheter tip-to-tissue contact-sensing capability. Another potential limitation is the fact that only a very small number of patients had repeat ablation procedures and, as a result, no conclusions could be drawn on the relationship between acute PVR and late PVR or on the durability of acute PV isolation with additional ablation after initial reconnection.
Conclusions
The tendency of PVs to reconnect with the left atrium, even if they are reablated after a 30 minute waiting period, probably suggests the necessity for a different ablation strategy applied to these early-reconnected PVs. More energy, longer time of ablation, better catheter-tissue contact as well as longer waiting period, or even different equipment altogether might be necessary to pursue better outcomes in terms of postablation AF recurrence rates. Which of these measures, if any, may result in better outcomes is an open issue pending further investigation.
Conflict of interest: none declared.



