-
PDF
- Split View
-
Views
-
Cite
Cite
Masateru Takigawa, Taishi Kuwahara, Atsushi Takahashi, Kenji Okubo, Yoshihide Takahashi, Emiko Nakashima, Kazuya Yamao, Yuji Watari, Jun Nakajima, Katsumasa Takagi, Tadashi Fujino, Shigeki Kimura, Hiroyuki Hikita, Kenzo Hirao, Mitsuaki Isobe, Simultaneous isolation of superior and inferior pulmonary veins on both the left and right sides could yield better outcomes in patients with paroxysmal atrial fibrillation, EP Europace, Volume 17, Issue 5, May 2015, Pages 732–740, https://doi.org/10.1093/europace/euu372
Close - Share Icon Share
Abstract
This study investigated whether disappearance patterns of pulmonary vein (PV) potentials (PVPs) during PV isolation (PVI) affect the outcome of catheter ablation (CA) in patients with paroxysmal atrial fibrillation (PAF).
Extensive PVI was performed in 1149 PAF patients (age, 61 ± 10 years). Clinical and demographic characteristics, ablation data, and follow-up outcomes were prospectively collected. During an initial CA, simultaneous disappearance of superior and inferior PVPs in both right and left PVs was observed in 464 (40.4%) patients (Group S). Atrial fibrillation-recurrence free rates at 1, 3, and 5 years after the initial CA in Group S were 78.9, 71.9, and 68.1%, respectively, which were higher than those in Group Non-S (P = 0.004). However, those were similar after the final CA between both groups. The incidence of PV–left atrium (LA) electrical reconnection was significantly lower in Group S than in Group Non-S in the second (Group S, 65.6% vs. Group Non-S, 82.1%; P = 0.004) and third (Group S, 8.3% vs. Group Non-S, 47.6%; P = 0.03) CAs. Furthermore, the reconnections more frequently occurred on the side of PVs where simultaneous PVP elimination had not been achieved at the initial CA. Simultaneous disappearance of superior and inferior PVPs in both right and left PVs independently reduced the risk of AF recurrence after the initial CA by 26%.
The simultaneous disappearance of superior and inferior PVPs in both right and left PVs is associated with less frequent PV–left atrium reconnection and may yield a better clinical outcome after the initial CA.
We studied 1149 consecutive patients after catheter ablation (CA) for symptomatic paroxysmal atrial fibrillation (PAF) and examined whether disappearance patterns of superior and inferior pulmonary vein potentials (PVPs) in left and right sides affect the outcome of CA for PAF. We found that:
During an initial CA, simultaneous disappearance of superior and inferior PVPs in both right and left PVs was observed in 464 (40.4%) patients.
These patients showed significantly higher AF-recurrence-free rate after the initial CA than the others (P = 0.004).
The incidence of PV–left atrium electrical reconnection was significantly lower in these patients compared with the others in the second (P = 0.004) and third (P = 0.03) CAs.
Multivariate analysis revealed that simultaneous disappearance of superior and inferior PVPs in both right and left PVs (HR, 0.74; P = 0.004), duration of AF history [hazard ratio (HR), 1.04; P < 0.0001], structural heart disease (HR, 1.42; P = 0.01), and left atrial dimension at end-systole (HR, 1.49 per 10 mm increase; P = 0.0004) were significantly associated with AF-recurrence after the initial CA.
Introduction
Catheter ablation (CA) targeting pulmonary veins (PVs) is a standard treatment for paroxysmal atrial fibrillation (PAF).1–3 Although multiple CA sessions are often required to achieve an optimal outcome, a lesser number of CA sessions with a better outcome would be more beneficial for patients. When extensive PV isolation (EPVI) is performed,4–6 superior and inferior PV potentials (PVPs) disappear simultaneously or individually. However, the relation between the disappearance pattern of superior and inferior PVPs in the left and right PVs and the outcome after the initial CA session for PAF has not been fully discussed. In the present study, we examined whether the simultaneous disappearance of superior and inferior PVPs is associated with a better outcome of CA for PAF.
Methods
Study population
This study included 1149 consecutive drug-refractory PAF patients (mean age, 61 ± 10 years; male, n = 874) who were referred to our institution for their first initial CA between 2005 and 2009. Atrial fibrillation (AF) was defined as paroxysmal when it terminated spontaneously and lasted for <7 days.7 All patients provided written informed consent, and our institutional review board approved the study protocol.
Electrophysiological study
Anti-arrhythmic drugs (AADs) were discontinued for >7 days (amiodarone was discontinued for >1 month) before the ablation; all patients underwent effective anticoagulation for >1 month. A 7 Fr, 20- or 14-pole, two-site mapping catheter (Irvine Biomedical) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion.
Catheter ablation technique
The strategy used for EPVI has been previously described.4–6 Briefly, after a transseptal puncture, pulmonary venography and contrast oesophagography were performed to determine the anatomical relationships of the PV ostia, left atrium (LA), and oesophagus. An activated clotting time of 250–350 s was maintained with a continuous infusion of heparin during the procedure. Two circular mapping catheters were placed in the superior and inferior PVs, and the left and right ipsilateral PVs were circumferentially and extensively ablated using a point-by-point technique. The LA posterior wall was anatomically ablated along a vertical and horizontal line, at a distance of 1–3 cm from the left- or right-side ostia of the PVs. Additionally, the distal edges of the anterior aspect of the PVs with early PV potentials or continuous PV and LA potentials were targeted for ablation (Figure 1). Isolation of the left PVs was performed during distal coronary sinus pacing and isolation of the right PVs was performed during sinus rhythm (SR). Radiofrequency current was delivered through an 8 mm tip ablation catheter (Japan Lifeline, Inc.) using the temperature control mode, with a target temperature of 55°C (maximum power of 35 W on the LA posterior wall; 40 W at the anterior aspect of the PVs). The oesophageal temperature was measured during the application.4 When the ipsilateral PVPs persisted despite the formation of an ipsilateral circular lesion, the gap on the line was first targeted for ablation. Thereafter, the earliest PV potential inside the encircling lesion was targeted as close to the encircling lesion as possible, without ablating the carina itself. The PVPs on one side—assessed using Lasso catheters that were placed on both the superior and inferior PVs—disappeared simultaneously or separately, as shown in Figure 2. Patients who exhibited simultaneous disappearance of superior and inferior PVPs in both the right and left PVs were assigned to Group S, whereas the remaining patients were assigned to Group Non-S. The endpoint of the procedure was a complete PVI, which was defined as the disappearance of all PVPs recorded by the double Lasso catheters (entrance block) and loss of capture of the LA by circumferential pacing from the Lasso catheters placed at the PV ostium (exit block). After completing the EPVI, adenosine triphosphate (20–40 mg) was injected to unmask any dormant conductions. If these conductions were present, radiofrequency applications were added until all dormant conductions disappeared.8 Thereafter, a cavotricuspid isthmus line was created with a bidirectional conduction block as the endpoint.9 Isoproterenol (5–20 μg/min) was injected intravenously before completing the procedure. If sustained or non-sustained AFs were reproducibly initiated from non-PV foci, they were ablated focally.10 When non-PV foci were located in the superior vena cava (SVC), the SVC was isolated electrically.11,12 Additional linear ablations (left atrial roof line, left atrial bottom line, and mitral isthmus line) were performed only when AFs from undetermined origins or macro-reentrant atrial tachycardia occurred spontaneously,6 with a bidirectional conduction block as the endpoint.13,14 At the end of the procedure, the endpoints of EPVI, SVC isolation, and linear ablations were re-confirmed and re-ablated in the case of reconnection. During repeat procedures, the reconnected electrical gaps between the LA and PVs were first ablated, and the same induction and ablation procedure mentioned above was performed.

Extensive pulmonary vein isolation with the double-Lasso technique. Two circular mapping catheters were placed in the superior and inferior PVs, and the left and right ipsilateral PVs were ablated circumferentially and extensively by using the point-by-point technique under fluoroscopic and electrophysiological guidance. Under distal coronary sinus pacing, the LA posterior wall was ablated along a vertical line from the top of the LSPV (1), followed by a horizontal line (2). Thereafter, the anterior aspect of the left PVs was ablated from the roof of the LSPV to the bottom of the LIPV, targeting early PVPs or continuous PV and LA potentials (3). Right PVs were isolated in the same manner under SR (1′–3′). If the PVPs persisted despite the formation of a circular lesion, the gap on the line was first targeted for ablation; thereafter, the earliest PVP inside the encircling lesion was targeted as close to the encircling lesion as possible. ABL, ablation catheter; AP, anterior–posterior; LA, left atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; PVP, pulmonary vein potentials; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
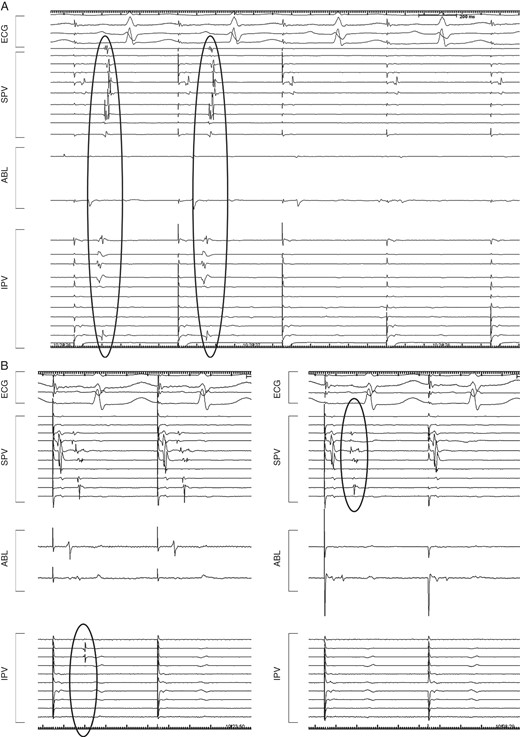
Intracardiac tracings exhibiting different disappearance patterns of the PVPs during PV isolation. (A) All PVPs disappeared simultaneously in both the superior and inferior PVs during EPVI. (B) The inferior and superior PVPs disappeared separately during EPVI. ABL, ablation catheter; ECG, electrocardiogram; EPVI, extensive pulmonary vein isolation; IPV, inferior pulmonary vein; PV, pulmonary vein; PVPs, pulmonar vein potentials; SPV, superior pulmonary vein.
Follow-up
Anti-arrhythmic drugs were not prescribed after the procedure. All patients were prospectively followed up at 2, 6, 10, 14, 24, 36, and 48 weeks after the procedure; 12-lead electrocardiograms were obtained at each visit and 24 h Holter monitoring was performed every 3 months. Thereafter, patients were followed-up every 1–3 months at our institution, or by a general physician. All patients were requested to measure their pulse at least twice per day. Patients with arrhythmias who were identified based on the findings of pulse taking and/or those with any symptoms underwent a 1-month event recorder monitoring. Anticoagulation was usually discontinued after 3–6 months in AF recurrence-free patients without risk factors of thromboembolism. Successful ablation was defined as the absence of any atrial tachyarrhythmias for more than 30 s, in the absence of AADs, after a blanking period of 1 month. Repeat ablation was recommended for patients with atrial tachyarrhythmias after this time point.
Statistical analysis
The data are expressed as means ± standard deviations for continuous variables, and frequencies and percentages for categorical variables. To compare the two groups, χ2 analysis or Fisher's exact test was used for categorical variables and an unpaired t-test or Wilcoxon analysis was used for continuous variables. All variables with a P-value of <0.20 in univariate analysis were included in a multivariate Cox proportional analysis, and HRs and 95% confidence intervals (CIs) were calculated. The follow-up period was calculated from the date of the procedure to that of AF recurrence or censoring. The event-free rates were calculated using the Kaplan–Meier analysis; log-rank statistics were used for group comparisons. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
Among the 1149 patients, 464 (40.4%) were assigned to Group S. Among the remaining 685 (59.6%) patients that were assigned to Group Non-S, the simultaneous disappearance of superior and inferior PVPs was achieved only in the left ipsilateral PVs in 235 (20.4%) patients, only in the right ipsilateral PVs in 162 (14.1%) patients, and was not achieved in either of the ipsilateral PVs in the remaining 288 (25.1%) patients. The baseline characteristics were similar between these two groups, except for the higher female prevalence in Group S (27.8 vs. 21.3%, P = 0.01), as shown in Table 1.
| . | Total (N = 1149) . | Group S (N = 464, 40.4%) . | Group Non-S (N = 685, 59.6%) . | P-value . |
|---|---|---|---|---|
| Patient age, year | 61 ± 10 | 61 ± 10.3 | 61 ± 10 | 0.69 |
| Age ≥ 75 years, n (%) | 73 (6.4) | 33 (7.1) | 28 (7.1) | 0.21 |
| Gender, female, n (%) | 275 (23.9) | 129 (27.8) | 146 (21.3) | 0.01 |
| BMI, kg/m2 | 23.5 ± 2.9 | 23.5 ± 2.8 | 23.4 ± 3.0 | 0.63 |
| Duration of AF history, year | 5.05 ± 5.46 | 5.05 ± 5.55 | 5.06 ± 5.39 | 0.98 |
| Structural heart disease, n (%) | 205 (17.8) | 75 (16.2) | 130 (18.9) | 0.24 |
| Hypertension, n (%) | 522 (45.4) | 208 (44.8) | 314 (45.8) | 0.74 |
| Diabetes, n (%) | 124 (10.8) | 42 (9.1) | 82 (11.9) | 0.12 |
| Congestive heart failure, n (%) | 75 (6.5) | 31 (6.7) | 44 (6.4) | 0.86 |
| Stroke, n (%) | 91 (7.9) | 38 (8.2) | 53 (7.7) | 0.78 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 0.9 ± 1.0 | 0.8 |
| Echocardiography | ||||
| LAD, mm | 37.8 ± 5.1 | 37.6 ± 4.9 | 37.9 ± 5.2 | 0.43 |
| LVEF, % | 66.4 ± 7.0 | 66.7 ± 6.8 | 71.6 ± 27.4 | 0.24 |
| . | Total (N = 1149) . | Group S (N = 464, 40.4%) . | Group Non-S (N = 685, 59.6%) . | P-value . |
|---|---|---|---|---|
| Patient age, year | 61 ± 10 | 61 ± 10.3 | 61 ± 10 | 0.69 |
| Age ≥ 75 years, n (%) | 73 (6.4) | 33 (7.1) | 28 (7.1) | 0.21 |
| Gender, female, n (%) | 275 (23.9) | 129 (27.8) | 146 (21.3) | 0.01 |
| BMI, kg/m2 | 23.5 ± 2.9 | 23.5 ± 2.8 | 23.4 ± 3.0 | 0.63 |
| Duration of AF history, year | 5.05 ± 5.46 | 5.05 ± 5.55 | 5.06 ± 5.39 | 0.98 |
| Structural heart disease, n (%) | 205 (17.8) | 75 (16.2) | 130 (18.9) | 0.24 |
| Hypertension, n (%) | 522 (45.4) | 208 (44.8) | 314 (45.8) | 0.74 |
| Diabetes, n (%) | 124 (10.8) | 42 (9.1) | 82 (11.9) | 0.12 |
| Congestive heart failure, n (%) | 75 (6.5) | 31 (6.7) | 44 (6.4) | 0.86 |
| Stroke, n (%) | 91 (7.9) | 38 (8.2) | 53 (7.7) | 0.78 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 0.9 ± 1.0 | 0.8 |
| Echocardiography | ||||
| LAD, mm | 37.8 ± 5.1 | 37.6 ± 4.9 | 37.9 ± 5.2 | 0.43 |
| LVEF, % | 66.4 ± 7.0 | 66.7 ± 6.8 | 71.6 ± 27.4 | 0.24 |
AF, atrial fibrillation; BMI, body mass index; LAD, left atrial dimension at end-systole; LVEF, left ventricular ejection fraction.
| . | Total (N = 1149) . | Group S (N = 464, 40.4%) . | Group Non-S (N = 685, 59.6%) . | P-value . |
|---|---|---|---|---|
| Patient age, year | 61 ± 10 | 61 ± 10.3 | 61 ± 10 | 0.69 |
| Age ≥ 75 years, n (%) | 73 (6.4) | 33 (7.1) | 28 (7.1) | 0.21 |
| Gender, female, n (%) | 275 (23.9) | 129 (27.8) | 146 (21.3) | 0.01 |
| BMI, kg/m2 | 23.5 ± 2.9 | 23.5 ± 2.8 | 23.4 ± 3.0 | 0.63 |
| Duration of AF history, year | 5.05 ± 5.46 | 5.05 ± 5.55 | 5.06 ± 5.39 | 0.98 |
| Structural heart disease, n (%) | 205 (17.8) | 75 (16.2) | 130 (18.9) | 0.24 |
| Hypertension, n (%) | 522 (45.4) | 208 (44.8) | 314 (45.8) | 0.74 |
| Diabetes, n (%) | 124 (10.8) | 42 (9.1) | 82 (11.9) | 0.12 |
| Congestive heart failure, n (%) | 75 (6.5) | 31 (6.7) | 44 (6.4) | 0.86 |
| Stroke, n (%) | 91 (7.9) | 38 (8.2) | 53 (7.7) | 0.78 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 0.9 ± 1.0 | 0.8 |
| Echocardiography | ||||
| LAD, mm | 37.8 ± 5.1 | 37.6 ± 4.9 | 37.9 ± 5.2 | 0.43 |
| LVEF, % | 66.4 ± 7.0 | 66.7 ± 6.8 | 71.6 ± 27.4 | 0.24 |
| . | Total (N = 1149) . | Group S (N = 464, 40.4%) . | Group Non-S (N = 685, 59.6%) . | P-value . |
|---|---|---|---|---|
| Patient age, year | 61 ± 10 | 61 ± 10.3 | 61 ± 10 | 0.69 |
| Age ≥ 75 years, n (%) | 73 (6.4) | 33 (7.1) | 28 (7.1) | 0.21 |
| Gender, female, n (%) | 275 (23.9) | 129 (27.8) | 146 (21.3) | 0.01 |
| BMI, kg/m2 | 23.5 ± 2.9 | 23.5 ± 2.8 | 23.4 ± 3.0 | 0.63 |
| Duration of AF history, year | 5.05 ± 5.46 | 5.05 ± 5.55 | 5.06 ± 5.39 | 0.98 |
| Structural heart disease, n (%) | 205 (17.8) | 75 (16.2) | 130 (18.9) | 0.24 |
| Hypertension, n (%) | 522 (45.4) | 208 (44.8) | 314 (45.8) | 0.74 |
| Diabetes, n (%) | 124 (10.8) | 42 (9.1) | 82 (11.9) | 0.12 |
| Congestive heart failure, n (%) | 75 (6.5) | 31 (6.7) | 44 (6.4) | 0.86 |
| Stroke, n (%) | 91 (7.9) | 38 (8.2) | 53 (7.7) | 0.78 |
| CHADS2 score | 0.8 ± 1.0 | 0.8 ± 1.0 | 0.9 ± 1.0 | 0.8 |
| Echocardiography | ||||
| LAD, mm | 37.8 ± 5.1 | 37.6 ± 4.9 | 37.9 ± 5.2 | 0.43 |
| LVEF, % | 66.4 ± 7.0 | 66.7 ± 6.8 | 71.6 ± 27.4 | 0.24 |
AF, atrial fibrillation; BMI, body mass index; LAD, left atrial dimension at end-systole; LVEF, left ventricular ejection fraction.
Details of the procedures
The EPVI endpoint was achieved in all patients at the initial CA session. The mean procedure time was 179 ± 42 min (168 ± 41 min in Group S vs. 184 ± 42 min in Group Non-S; P < 0.0001), and the mean fluoroscopy time was 81 ± 34 min (71 ± 27 min in Group S vs. 85 ± 35 min in Group Non-S; P < 0.0001). Table 2 shows the details of multiple CA sessions. The incidences of SVC isolation and/or focal ablation and linear ablation were similar between Groups S and Non-S throughout the CA sessions. However, the incidence of PV–LA electrical reconnection was significantly lower in Group S than in Group Non-S: 65.6% in Group S vs. 82.1% in Group Non-S (P = 0.004) in the second CA session and 8.3% in Group S vs. 50% in Group Non-S (P = 0.03) in the third CA session. Furthermore, as indicated in Figure 3, among 272 patients who received the second CA, the PV–LA electrical reconnection was documented in the right PVs in 151 (55.5%) patients and in the left PVs in 127 (46.7%) patients; the reconnected gap was located frequently in the vicinity of the PV carina. At the third CA session, among 33 patients, the PV–LA electrical reconnection was documented in the right PVs in seven (21.2%) patients and in the left PVs in four (12.1%) patients.
| First CA . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| PVI | 1149 (100) | 464 (100) | 685 (100) | – |
| SVCI and/or focal ablation | 215 (18.7) | 83 (17.9) | 137 (20.0) | 0.4 |
| Linear ablation | 8 (0.7) | 3 (0.7) | 5 (0.7) | 0.99 |
| AF recurrence | 383 (33.3) | 136 (29.3) | 247 (36.1) | 0.02 |
| Second CA | (N = 272) | (N = 93) | (N = 179) | P-value |
| PVI | 208 (76.5) | 61 (65.6) | 147 (82.1) | 0.004 |
| SVCI and/or focal ablation | 134 (49.6) | 48 (51.6) | 86 (48.6) | 0.70 |
| Linear ablation | 31 (11.4) | 12 (12.9) | 19 (10.6) | 0.57 |
| AF recurrence | 89 (32.7) | 35 (37.6) | 54 (30.2) | 0.22 |
| Third CA | (N = 33) | (N = 12) | (N = 21) | P-value |
| PVI | 11 (33.3) | 1 (8.3) | 10 (47.6) | 0.03 |
| SVCI and/or focal ablation | 20 (60.6) | 8 (66.7) | 12 (57.1) | 0.72 |
| Linear ablation | 14 (42.4) | 6 (50.0) | 8 (38.1) | 0.72 |
| AF recurrence | 12 (36.4) | 5 (41.7) | 7 (33.3) | 0.72 |
| First CA . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| PVI | 1149 (100) | 464 (100) | 685 (100) | – |
| SVCI and/or focal ablation | 215 (18.7) | 83 (17.9) | 137 (20.0) | 0.4 |
| Linear ablation | 8 (0.7) | 3 (0.7) | 5 (0.7) | 0.99 |
| AF recurrence | 383 (33.3) | 136 (29.3) | 247 (36.1) | 0.02 |
| Second CA | (N = 272) | (N = 93) | (N = 179) | P-value |
| PVI | 208 (76.5) | 61 (65.6) | 147 (82.1) | 0.004 |
| SVCI and/or focal ablation | 134 (49.6) | 48 (51.6) | 86 (48.6) | 0.70 |
| Linear ablation | 31 (11.4) | 12 (12.9) | 19 (10.6) | 0.57 |
| AF recurrence | 89 (32.7) | 35 (37.6) | 54 (30.2) | 0.22 |
| Third CA | (N = 33) | (N = 12) | (N = 21) | P-value |
| PVI | 11 (33.3) | 1 (8.3) | 10 (47.6) | 0.03 |
| SVCI and/or focal ablation | 20 (60.6) | 8 (66.7) | 12 (57.1) | 0.72 |
| Linear ablation | 14 (42.4) | 6 (50.0) | 8 (38.1) | 0.72 |
| AF recurrence | 12 (36.4) | 5 (41.7) | 7 (33.3) | 0.72 |
Data presented in the table are n (%). Among 12 patients who had a failed third CA, only two patients in Group Non-S underwent the fourth CA: both had no PV recurrence and received only linear ablations.
AF, atrial fibrillation; CA, catheter ablation; PVI, pulmonary vein isolation; SVCI, superior vena cava isolation.
| First CA . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| PVI | 1149 (100) | 464 (100) | 685 (100) | – |
| SVCI and/or focal ablation | 215 (18.7) | 83 (17.9) | 137 (20.0) | 0.4 |
| Linear ablation | 8 (0.7) | 3 (0.7) | 5 (0.7) | 0.99 |
| AF recurrence | 383 (33.3) | 136 (29.3) | 247 (36.1) | 0.02 |
| Second CA | (N = 272) | (N = 93) | (N = 179) | P-value |
| PVI | 208 (76.5) | 61 (65.6) | 147 (82.1) | 0.004 |
| SVCI and/or focal ablation | 134 (49.6) | 48 (51.6) | 86 (48.6) | 0.70 |
| Linear ablation | 31 (11.4) | 12 (12.9) | 19 (10.6) | 0.57 |
| AF recurrence | 89 (32.7) | 35 (37.6) | 54 (30.2) | 0.22 |
| Third CA | (N = 33) | (N = 12) | (N = 21) | P-value |
| PVI | 11 (33.3) | 1 (8.3) | 10 (47.6) | 0.03 |
| SVCI and/or focal ablation | 20 (60.6) | 8 (66.7) | 12 (57.1) | 0.72 |
| Linear ablation | 14 (42.4) | 6 (50.0) | 8 (38.1) | 0.72 |
| AF recurrence | 12 (36.4) | 5 (41.7) | 7 (33.3) | 0.72 |
| First CA . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| PVI | 1149 (100) | 464 (100) | 685 (100) | – |
| SVCI and/or focal ablation | 215 (18.7) | 83 (17.9) | 137 (20.0) | 0.4 |
| Linear ablation | 8 (0.7) | 3 (0.7) | 5 (0.7) | 0.99 |
| AF recurrence | 383 (33.3) | 136 (29.3) | 247 (36.1) | 0.02 |
| Second CA | (N = 272) | (N = 93) | (N = 179) | P-value |
| PVI | 208 (76.5) | 61 (65.6) | 147 (82.1) | 0.004 |
| SVCI and/or focal ablation | 134 (49.6) | 48 (51.6) | 86 (48.6) | 0.70 |
| Linear ablation | 31 (11.4) | 12 (12.9) | 19 (10.6) | 0.57 |
| AF recurrence | 89 (32.7) | 35 (37.6) | 54 (30.2) | 0.22 |
| Third CA | (N = 33) | (N = 12) | (N = 21) | P-value |
| PVI | 11 (33.3) | 1 (8.3) | 10 (47.6) | 0.03 |
| SVCI and/or focal ablation | 20 (60.6) | 8 (66.7) | 12 (57.1) | 0.72 |
| Linear ablation | 14 (42.4) | 6 (50.0) | 8 (38.1) | 0.72 |
| AF recurrence | 12 (36.4) | 5 (41.7) | 7 (33.3) | 0.72 |
Data presented in the table are n (%). Among 12 patients who had a failed third CA, only two patients in Group Non-S underwent the fourth CA: both had no PV recurrence and received only linear ablations.
AF, atrial fibrillation; CA, catheter ablation; PVI, pulmonary vein isolation; SVCI, superior vena cava isolation.
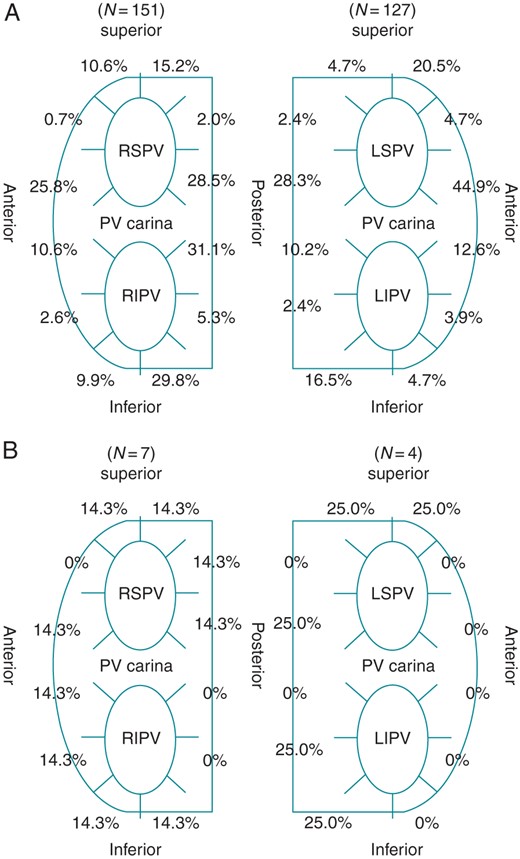
The location of the reconnected gaps between the LA and PVs at the second (A) and third (B) CA sessions.
Relation between the disappearance pattern of superior and inferior pulmonary vein potentials at the initial catheter ablation session and the pulmonary vein reconnection in subsequent procedures
During the second CA session, PV–LA reconnection on the right PVs occurred less frequently in those that showed simultaneous elimination of the right ipsilateral PVPs at the initial CA session [61/137 (48.2%)] compared with those that did not show this finding [85/135 (63.0%)] (P = 0.01; Figure 4A). Moreover, during the second CA session, the PV–LA reconnection on the left PVs occurred less frequently in those that showed simultaneous elimination of the left ipsilateral PVPs at the initial CA session [66/160 (41.3%)] compared with those that did not show this finding [61/112 (54.5%); P = 0.03]. These tendencies were also maintained at the third CA session (Figure 4B). Although the difference between the two groups did not reach statistical significance, complications tended to occur more frequently among patients in Group Non-S (Table 3).
| . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| Cardiac tamponade/effusion | 21 (1.8) | 5 (1.1) | 16 (2.3) | 0.18 |
| TIA/CI | 7 (0.6) | 2 (0.4) | 5 (0.7) | 0.71 |
| Myocardial infarction | 1 (0.1) | 0 (0) | 1 (0.1) | 0.99 |
| DVT/PE | 3 (0.3) | 1 (0.2) | 2 (0.3) | 0.99 |
| Phrenic nerve injury (reversible) | 8[7] (0.7) | 3[2] (0.6) | 5[5] (0.7) | 0.99 |
| Vagal nerve injury (reversible) | 5[4] (0.4) | 0 (0.0) | 5[4] (0.7) | 0.09 |
| Pneumothorax | 2 (0.2) | 1 (0.2) | 1 (0.1) | 0.99 |
| Vascular injury | 3 (0.2) | 2 (0.4) | 1 (0.1) | 0.57 |
| Total | 50 (4.4) | 14 (3.0) | 36 (5.3) | 0.08 |
| . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| Cardiac tamponade/effusion | 21 (1.8) | 5 (1.1) | 16 (2.3) | 0.18 |
| TIA/CI | 7 (0.6) | 2 (0.4) | 5 (0.7) | 0.71 |
| Myocardial infarction | 1 (0.1) | 0 (0) | 1 (0.1) | 0.99 |
| DVT/PE | 3 (0.3) | 1 (0.2) | 2 (0.3) | 0.99 |
| Phrenic nerve injury (reversible) | 8[7] (0.7) | 3[2] (0.6) | 5[5] (0.7) | 0.99 |
| Vagal nerve injury (reversible) | 5[4] (0.4) | 0 (0.0) | 5[4] (0.7) | 0.09 |
| Pneumothorax | 2 (0.2) | 1 (0.2) | 1 (0.1) | 0.99 |
| Vascular injury | 3 (0.2) | 2 (0.4) | 1 (0.1) | 0.57 |
| Total | 50 (4.4) | 14 (3.0) | 36 (5.3) | 0.08 |
Data presented in the table are n (%).
CA, catheter ablation; CI, cerebral infarction; DVT, deep vein thrombosis; PE, pulmonary embolism; PV, pulmonary vein; TIA, transient ischaemic attacks.
| . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| Cardiac tamponade/effusion | 21 (1.8) | 5 (1.1) | 16 (2.3) | 0.18 |
| TIA/CI | 7 (0.6) | 2 (0.4) | 5 (0.7) | 0.71 |
| Myocardial infarction | 1 (0.1) | 0 (0) | 1 (0.1) | 0.99 |
| DVT/PE | 3 (0.3) | 1 (0.2) | 2 (0.3) | 0.99 |
| Phrenic nerve injury (reversible) | 8[7] (0.7) | 3[2] (0.6) | 5[5] (0.7) | 0.99 |
| Vagal nerve injury (reversible) | 5[4] (0.4) | 0 (0.0) | 5[4] (0.7) | 0.09 |
| Pneumothorax | 2 (0.2) | 1 (0.2) | 1 (0.1) | 0.99 |
| Vascular injury | 3 (0.2) | 2 (0.4) | 1 (0.1) | 0.57 |
| Total | 50 (4.4) | 14 (3.0) | 36 (5.3) | 0.08 |
| . | Total (N = 1149) . | Group S (N = 464) . | Group Non-S (N = 685) . | P-value . |
|---|---|---|---|---|
| Cardiac tamponade/effusion | 21 (1.8) | 5 (1.1) | 16 (2.3) | 0.18 |
| TIA/CI | 7 (0.6) | 2 (0.4) | 5 (0.7) | 0.71 |
| Myocardial infarction | 1 (0.1) | 0 (0) | 1 (0.1) | 0.99 |
| DVT/PE | 3 (0.3) | 1 (0.2) | 2 (0.3) | 0.99 |
| Phrenic nerve injury (reversible) | 8[7] (0.7) | 3[2] (0.6) | 5[5] (0.7) | 0.99 |
| Vagal nerve injury (reversible) | 5[4] (0.4) | 0 (0.0) | 5[4] (0.7) | 0.09 |
| Pneumothorax | 2 (0.2) | 1 (0.2) | 1 (0.1) | 0.99 |
| Vascular injury | 3 (0.2) | 2 (0.4) | 1 (0.1) | 0.57 |
| Total | 50 (4.4) | 14 (3.0) | 36 (5.3) | 0.08 |
Data presented in the table are n (%).
CA, catheter ablation; CI, cerebral infarction; DVT, deep vein thrombosis; PE, pulmonary embolism; PV, pulmonary vein; TIA, transient ischaemic attacks.
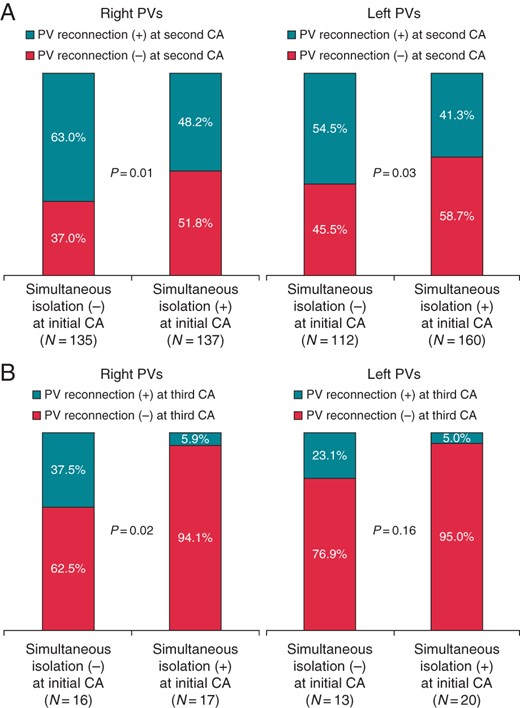
The relation between the disappearance pattern of the superior and inferior PVPs in the right and left PVs at the initial CA session and the PV reconnection at the second (A) and third (B) CA sessions. PV, pulmonary vein; PVP, pulmonary-vein potentials; CA, catheter ablation.
Clinical outcomes
The overall incidence of AF recurrence among the 1149 patients after the first, second, and third CA sessions was 389 (33.9%), 206 (17.9%), and 185 (16.1%), respectively, as shown in Figure 5A. Atrial fibrillation recurred in 136/464 (29.3%) patients in Groups S and 247/685 (36.1%) patients in Group Non-S. Atrial fibrillation recurrence-free survival rates at 1, 3, and 5 years after the initial CA session in Group S were 78.9, 71.9, and 68.1%, respectively, which were higher than those in Group Non-S (P = 0.004) (Figure 5B). However, ultimately, during a mean follow-up period of 38.0 ± 21.8 months, the AF recurrence-free rate was similar in both groups after the final CA session (P = 0.48), as shown in Figure 5C, regardless of the fact that the procedures were less frequently performed in Group S than in Group Non-S (1.2 ± 0.5 vs. 1.3 ± 0.5; P = 0.03).
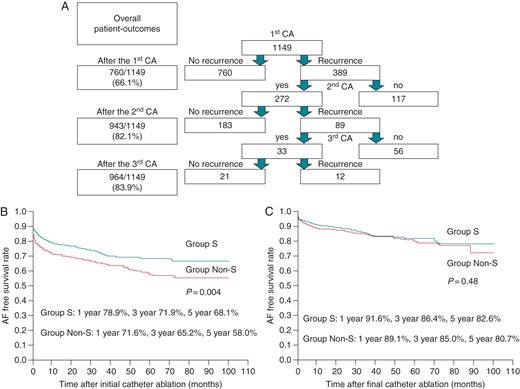
A ladder diagram of the CA sessions and outcomes (A), and the AF recurrence-free rate after the initial (B) and final CA session (C). The SR maintenance rate after the initial CA session in Group S was significantly higher than in Group Non-S (P = 0.004) during a mean follow-up period of 32.1 ± 24.2 months. In contrast, the SR maintenance rate after the final CA was similar between Group S and Group Non-S during a mean follow-up period of 38.0 ± 21.8 months; however, Group Non-S required significantly more frequent CA sessions than Group S (1.3 ± 0.5 vs. 1.2 ± 0.5, P = 0.03). CA, catheter ablation.
Clinical predictors of atrial fibrillation recurrence after the initial catheter ablation session
As shown in Table 4, a multivariate analysis revealed that being in Group S (HR, 0.74; 95% CI, 0.59–0.91; P = 0.004), duration of AF history (HR, 1.04; 95% CI, 1.02–1.05; P < 0.0001), structural heart disease (HR, 1.42; 95% CI, 1.08–1.85; P = 0.01), and left atrial dimension at end-systole (LAD) (HR, 1.49 per 10 mm increase; 95% CI, 1.20–1.86; P = 0.0004) were significantly associated with AF recurrence after the initial CA session.
| . | Univariate . | Multivariate . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age, year | 0.86 | 0.25 | 0.66 | 0.33–1.34 |
| Sex, female | 0.11 | 0.1 | 1.22 | 0.96–1.54 |
| BMI, kg/m2 | 0.23 | 0.06 | 0.97 | 0.93–1.04 |
| Duration of AF history, year | 0.0001 | <0.0001 | 1.04 | 1.02–1.05 |
| Structural heart disease | 0.0007 | 0.01 | 1.42 | 1.08–1.85 |
| Hypertension | 0.81 | 0.36 | 0.79 | 0.49–1.32 |
| Diabetes | 0.47 | 0.72 | 0.9 | 0.53–1.58 |
| Congestive heart failure | 0.07 | 0.53 | 0.82 | 0.45–1.53 |
| Stroke | 0.48 | 0.75 | 0.85 | 0.33–2.31 |
| CHADS2 score | 0.29 | 0.55 | 1.15 | 0.72–1.75 |
| Group S | 0.004 | 0.004 | 0.74 | 0.59–0.91 |
| Echocardiography | ||||
| LAD per 10 mm increase | 0.0005 | 0.0004 | 1.49 | 1.20–1.86 |
| LVEF per 10% increase | 0.78 | 0.34 | 1.08 | 0.93–1.25 |
| . | Univariate . | Multivariate . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age, year | 0.86 | 0.25 | 0.66 | 0.33–1.34 |
| Sex, female | 0.11 | 0.1 | 1.22 | 0.96–1.54 |
| BMI, kg/m2 | 0.23 | 0.06 | 0.97 | 0.93–1.04 |
| Duration of AF history, year | 0.0001 | <0.0001 | 1.04 | 1.02–1.05 |
| Structural heart disease | 0.0007 | 0.01 | 1.42 | 1.08–1.85 |
| Hypertension | 0.81 | 0.36 | 0.79 | 0.49–1.32 |
| Diabetes | 0.47 | 0.72 | 0.9 | 0.53–1.58 |
| Congestive heart failure | 0.07 | 0.53 | 0.82 | 0.45–1.53 |
| Stroke | 0.48 | 0.75 | 0.85 | 0.33–2.31 |
| CHADS2 score | 0.29 | 0.55 | 1.15 | 0.72–1.75 |
| Group S | 0.004 | 0.004 | 0.74 | 0.59–0.91 |
| Echocardiography | ||||
| LAD per 10 mm increase | 0.0005 | 0.0004 | 1.49 | 1.20–1.86 |
| LVEF per 10% increase | 0.78 | 0.34 | 1.08 | 0.93–1.25 |
The other abbreviations are as in the footnote of Table 1.
CI, confidence interval; HR, hazard ratio.
| . | Univariate . | Multivariate . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age, year | 0.86 | 0.25 | 0.66 | 0.33–1.34 |
| Sex, female | 0.11 | 0.1 | 1.22 | 0.96–1.54 |
| BMI, kg/m2 | 0.23 | 0.06 | 0.97 | 0.93–1.04 |
| Duration of AF history, year | 0.0001 | <0.0001 | 1.04 | 1.02–1.05 |
| Structural heart disease | 0.0007 | 0.01 | 1.42 | 1.08–1.85 |
| Hypertension | 0.81 | 0.36 | 0.79 | 0.49–1.32 |
| Diabetes | 0.47 | 0.72 | 0.9 | 0.53–1.58 |
| Congestive heart failure | 0.07 | 0.53 | 0.82 | 0.45–1.53 |
| Stroke | 0.48 | 0.75 | 0.85 | 0.33–2.31 |
| CHADS2 score | 0.29 | 0.55 | 1.15 | 0.72–1.75 |
| Group S | 0.004 | 0.004 | 0.74 | 0.59–0.91 |
| Echocardiography | ||||
| LAD per 10 mm increase | 0.0005 | 0.0004 | 1.49 | 1.20–1.86 |
| LVEF per 10% increase | 0.78 | 0.34 | 1.08 | 0.93–1.25 |
| . | Univariate . | Multivariate . | HR . | 95% CI . |
|---|---|---|---|---|
| Patient age, year | 0.86 | 0.25 | 0.66 | 0.33–1.34 |
| Sex, female | 0.11 | 0.1 | 1.22 | 0.96–1.54 |
| BMI, kg/m2 | 0.23 | 0.06 | 0.97 | 0.93–1.04 |
| Duration of AF history, year | 0.0001 | <0.0001 | 1.04 | 1.02–1.05 |
| Structural heart disease | 0.0007 | 0.01 | 1.42 | 1.08–1.85 |
| Hypertension | 0.81 | 0.36 | 0.79 | 0.49–1.32 |
| Diabetes | 0.47 | 0.72 | 0.9 | 0.53–1.58 |
| Congestive heart failure | 0.07 | 0.53 | 0.82 | 0.45–1.53 |
| Stroke | 0.48 | 0.75 | 0.85 | 0.33–2.31 |
| CHADS2 score | 0.29 | 0.55 | 1.15 | 0.72–1.75 |
| Group S | 0.004 | 0.004 | 0.74 | 0.59–0.91 |
| Echocardiography | ||||
| LAD per 10 mm increase | 0.0005 | 0.0004 | 1.49 | 1.20–1.86 |
| LVEF per 10% increase | 0.78 | 0.34 | 1.08 | 0.93–1.25 |
The other abbreviations are as in the footnote of Table 1.
CI, confidence interval; HR, hazard ratio.
Discussion
Major findings
The findings of this study were as follows: (i) Group S was associated with a lower AF recurrence rate after the initial CA session; (ii) better outcomes were achieved after the repeat CA sessions regardless of the PVP disappearance pattern at the initial CA session; and (iii) an electrical reconnection between the LA and PVs occurred more frequently during the repeat procedure in the ipsilateral PVs where superior and inferior PVPs had not disappeared simultaneously during the initial CA session.
Impact of the simultaneous disappearance of superior and inferior pulmonary vein potentials in both the right and left pulmonary veins
In the present study, through multivariate analysis, we noted that the failure of the disappearance of superior and inferior PVPs in both the right and left PVs (Group Non-S) was significantly associated with AF recurrence, in addition to the previously proved risk factors of AF recurrence, such as longer duration of AF history15 and larger left atrial dimension.2,16 Furthermore, being in Group S independently reduced the risk of AF recurrence after the initial CA session by 26%, as shown in Table 4.
Mechanisms underlying the better outcome in Group S after the initial catheter ablation session
The mechanisms underlying the more favourable outcome after the initial CA session in Group S remain unknown. However, several findings in the present study have provided some clues: The incidence of PV–LA electrical reconnection during the repeat CA sessions was lower in Group S. Moreover, the PV–LA electrical reconnection tended to occur less frequently in the ipsilateral PVs where superior and inferior PVPs had simultaneously disappeared at the initial CA session. This finding suggests that the achievement of the simultaneous disappearance of superior and inferior PVPs may be related to the presence of completely continuous and transmural circle lesions without any dormant conductions, where electrostimulation is conducted through the anatomically unablated visual gaps as described by Miller et al.17 Additionally, the shorter procedure time in Group S implies a less frequent application along the circle lesions to eliminate PV potentials, resulting in less myocardial tissue oedema around the PV ostia; this may make the assessment of local potentials easier, and may contribute to the formation of complete transmural lesions with less dormant conduction, thereby reducing the risk of unnecessary damage to tissues that may lead to arrhythmogenicity. Furthermore, even if the isolation of four PVs was confirmed, an incomplete isolation of the LA antrum, including the PV carina, could occur more frequently in Group Non-S, and it may increase AF recurrence.18 Previous studies have reported the arrhythmogenicity around the PV carina, and the non-isolation of the PV carina may be related to the higher incidence of AF recurrence.19,20
Although our data suggest that the simultaneous disappearance of superior and inferior PVPs in both the right and left PVs might lead to better outcomes after the initial CA session, this does not occur at all times because the differences in the PVP disappearance pattern of superior and inferior PVs may be associated with a combination of differences in anatomy, including PV diameter, size and amount of the PV sleeves, carina characteristics, the overcrossing position of the superior PV towards the inferior PV, the position of the ablation catheter towards the PV (more proximal or more distal), contact force between ablation catheter and the tissue, and the encircling lesion drawn by the catheter. These anatomical drawbacks may be overcome by using electroanatomical mapping and/or measuring the contact force.
Clinical implications
The present study provided unique findings. The simultaneous disappearance of superior and inferior PVPs in both the right and left ipsilateral PVs at the initial CA session was associated with the need for less repeat CA sessions. However, even if simultaneous PVP disappearance in the right and left ipsilateral PVs is not achieved, repeat CA sessions may increase the success rate, possibly because of the complete isolation of the PV antrum, including both PVs and carina.
Limitations
First, this study was subject to the limitations inherent to a retrospective design. However, the clinical and demographic characteristics, ablation data, and follow-up outcomes were prospectively collected in a large sample, and the ablation technique and devices used during the procedure were not changed through the study period; these factors helped to offset such limitations. Second, an 8 mm tip non-irrigation catheter was used because of the unavailability of irrigation catheters in Japan during the study period. Third, the lack of electroanatomic mapping may have been a limitation to draw a complete isolation line. However, simple vertical and horizontal lines at the LA posterior wall and detailed and precise assessment of local electrical potentials at the anterior aspect under the guidance of fluoroscopy can minimize this disadvantage. Finally, even though 1-month event recording was performed in every patient who reported symptoms or abnormal findings on pulse checking, nocturnal or even short episodes of AF could have been missed in this protocol, and the actual recurrence rate of AF could have been higher.
Conclusions
The simultaneous disappearance of superior and inferior PVPs in both the right and left PVs was associated with less frequent PV–LA reconnection and could yield a better clinical outcome after the initial CA session.
Conflict of interest: none declared.



