-
PDF
- Split View
-
Views
-
Cite
Cite
Rachit M. Shah, Dhavalkumar Patel, Janos Molnar, Kenneth A. Ellenbogen, Jayanthi N. Koneru, Cardiac-resynchronization therapy in patients with systolic heart failure and QRS interval ≤130 ms: insights from a meta-analysis, EP Europace, Volume 17, Issue 2, February 2015, Pages 267–273, https://doi.org/10.1093/europace/euu214
Close - Share Icon Share
Cardiac-resynchronization therapy (CRT) reduces morbidity and mortality in patients with chronic systolic heart failure (SHF) and a wide QRS complex. It is unclear whether the same benefit extends to patients with QRS duration (QRSd) <130 ms.
Our aim was to perform a meta-analysis of all randomized controlled trial (RCTs) and to evaluate the effect of implantable CRT defibrillator(CRTD) on all-cause mortality, HF mortality, and HF hospitalization in patients with QRSd <130 ms. We performed a systematic literature search to identify all RCTs, comparing CRTD therapy with implantable cardiac defibrillator (ICD) therapy in patients with SHF (ejection fraction <35%) and QRS ≤130 ms, published in Pubmed, Medline, EMBASE, Cochrane library, and Google scholar from June 1980 through June 2013. The search terms included CRT, QRS duration, narrow QRS, clinical trial, RCT, biventricular pacing, heart failure, systolic dysfunction, dyssynchrony, left ventricular remodelling, readmission, mortality, survival, and various combinations of these terms. We studied the trends of overall mortality, SHF mortality, and hospitalizations due to SHF between the two groups. Heterogeneity of the studies was analysed by Q statistic. A fixed-effect model was used to compute the relative risk (RR) of mortality due to SHF, while a random-effects model was used to compare hospitalization due to SHF. Out of a total of 12 100 citations, four RCTs comparing CRTD vs. ICD therapy in patients with SHF and QRS ≤130 ms fulfilled the inclusion criteria. The median follow-up was 12 months and the cumulative number of patients was 1177. Relative Risk for all-cause mortality in patients treated with CRTD was 1.66 with a 95% CI of 1.096–2.515 (P = 0.017) while for SHF mortality was 1.29 with 95% CI of 0.68–2.45 (P = 0.42). Relative risk for HF hospitalization in patients treated with CRTD was 0.94 with 95% CI of 0.50–1.74 (P = 0.84) in comparison to the ICD group.
Cardiac-resynchronization therapy defibrillator has no impact on SHF mortality and SHF hospitalization in patients with systolic HF with QRS duration ≤130 ms and is associated with higher all-cause mortality in comparison with ICD therapy.
In patients with systolic heart failure and QRSd <130 ms, when CRTD is compared to ICD therapy:
Cardiac-resynchronization therapy defibrillator has no impact on SHF mortality.
Cardiac-resynchronization therapy defibrillator has no impact on hospitalization rate in patients.
Cardiac-resynchronization therapy defibrillator is associated with higher all-cause mortality.
Introduction
Heart failure is the modern epidemic and a common cause of morbidity and mortality.1 The salutary effects of cardiac-resynchronization therapy (CRT) have been iteratively demonstrated in well-designed clinical studies that involved patients with systolic heart failure (SHF, ejection fraction <35%) and a prolonged QRS duration (QRSd).1–4 However, many patients with systolic failure have QRS durations <130 ms and nearly half of these patients demonstrate echocardiographic evidence of left ventricular mechanical dyssynchrony.5,6 Data from a few single center observational studies, have suggested that CRT could be beneficial in patients with QRS duration <130 ms.7–11 However, the results of subsequent, larger randomized controlled trials (RCTs) are controversial12–15 and suggest possible deleterious effects of CRT when used in patients with SHF and QRSd<130 ms. Hence, we conducted this meta-analysis of RCTs to assess the impact of CRT-D therapy on clinical endpoints: SHF mortality, all-cause mortality and HF hospitalization in comparison to ICD therapy alone in patients SHF and QRSd <130 ms.
Methods
We conducted this meta-analysis in accordance with the Quality of Reporting of Meta-analysis statement and the Consolidated Standards of Reporting Trials group recommendation.16
Literature search
We performed a computerized search to identify all RCTs, comparing CRTD therapy vs. ICD therapy in patients with SHF (ejection fraction <35%) and QRS complex duration of <130 ms, published in Pubmed, Medline, EMBASE, Cochrane library, and Google scholar from June 1980 through June 2013. References of retrieved studies were also checked to identify additional studies. The search terms included CRT, QRS duration, narrow QRS, biventricular pacing, heart failure, systolic dysfunction, dyssynchrony, left ventricular remodeling, readmission, mortality, survival, clinical trial, RCT, and various combinations of these terms.
Study selection
All titles and abstracts obtained from the initial computerized search were reviewed by two authors to determine eligibility for inclusion of the study in meta-analysis. Studies were selected for further review if they met the following inclusion criteria: (1) RCT design, (ii) Studies that included patients with QRSd <130 ms and compared outcomes between patients with CRTD therapy vs. ICD therapy alone, (iii) studies that reported clinical outcomes relevant to our meta-analysis: all-cause mortality, deaths due to SHF, and HF-related hospitalizations.
Validity assessment and data abstraction
We used the criteria developed by US preventive services task force to determine the internal validity of studies included in this meta-analysis.17 Based on these criteria, two authors rated these studies in three categories: ‘good’, ‘fair’, and ‘poor’. Disagreement on collected data was resolved by the third investigator. We extracted the following data from each RCT selected for meta-analysis: characteristics of the study (author, year, duration, and sample size, inclusion and exclusion criteria, randomization, follow-up); demographic data (age and sex), clinical characteristics (diabetes, hypertension, MI, history of revascularization, NYHA class, ischaemic cardiomyopathy), baseline laboratory data, duration of QRS (ms) and left ventricular ejection fraction (LVEF). Two authors independently extracted data and assessed studies' outcomes. The main clinical outcomes in our meta-analysis were all cause mortality; deaths due to SHF, and hospitalization due to SHF.
Statistical analysis
We used Comprehensive Meta-analysis Software (Version 2, BioStat, Englewood) for our analysis. Continuous variables are reported in mean ± standard deviation, whereas categorical variables are reported as number (percentages). Cochran Q statistics and I2 was computed to quantify heterogeneity. Random effects model was used when statistically significant heterogeneity was present among studies; otherwise fixed-effect model was used. Relative risk (RR) and 95% confidence interval were calculated to demonstrate the overall effect of CRT on dichotomous outcomes such as all-cause mortality, SHF-related deaths, and hospitalizations. A two-sided α error of <0.05 was considered to be statistically significant (P < 0.05).
Results
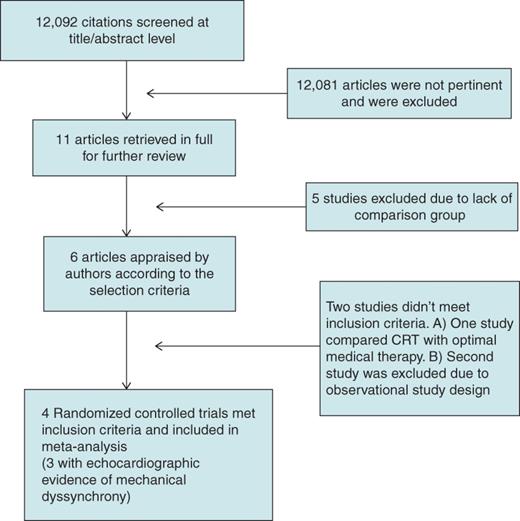
Table 1 shows the basic characteristics of studies included in the meta-analysis, while Table 2 details the patient characteristics from these studies. Three studies included patients with significant mechanical dyssynchrony on 2-D echocardiogram,12,14,15 while LESSER EARTH study included patients with narrow QRS complex irrespective of the evidence of mechanical dyssynchrony.13Table 3 details the definition of mechanical dyssynchrony used in each study. Three studies randomized patients after successful implantation of CRT-D in all patients to those who have active CRT programmed and those who did not.12–14 Muto et al.15 randomized heart failure patients into CRT-D and DDD-ICD group. Heart failure therapy was optimized for all patients by the treating physician. The majority of the included studies had a follow-up period of 12 months.
| Study . | Study design . | Patient selection . | LV dyssynchrony . | Randomization . | Scheduled follow-up . | Primary outcomes . | Duration . | Internal validity . |
|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | Randomized double blinded, multicentre | LVEF ≤35%, QRSd <120 ms, NYHA class III | Not assessed | CRTD implanted and randomized to CRT programme active and inactive | 6 months 12 months | Submaximal exercise duration | October 2003–January 2011. | Good |
| Beshai et al.12 (RethinQ ) | Randomized double blinded, multicentre | LVEF <35%, NYHA Class III, QRSd ≤ 130 ms | Present | CRTD implanted and randomized 1 : 1 with CRTD programme on and off | 6 months | Change in peak oxygen consumption | August 2005–January 2007 | Good |
| Ruschitzka et al.14 (The echoCRT study) | Randomized double blinded, multicentre | LVEF ≤ 35%, NYHA Class III or IV, QRSd ≤ 130ms | Present | CRTD implanted and then randomized 1 : 1 with CRT on and off | 1 month, 3 month, then every 3 months until trial terminated | Combination of death and HF hospitalization | August 2008–March 2013 | Good |
| Muto et al.15 (The NARROW-CRT study) | Randomized single blind, multicentre | LVEF <35%, NYHA Class II and III, QRSd ≤120 ms | Present | 1 : 1 implantation of CRTD vs. DDD-ICD(dual-chamber ICD) | 6 months and then 12 months | HF clinical composite score | January 2008–May 2010 | Good |
| Study . | Study design . | Patient selection . | LV dyssynchrony . | Randomization . | Scheduled follow-up . | Primary outcomes . | Duration . | Internal validity . |
|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | Randomized double blinded, multicentre | LVEF ≤35%, QRSd <120 ms, NYHA class III | Not assessed | CRTD implanted and randomized to CRT programme active and inactive | 6 months 12 months | Submaximal exercise duration | October 2003–January 2011. | Good |
| Beshai et al.12 (RethinQ ) | Randomized double blinded, multicentre | LVEF <35%, NYHA Class III, QRSd ≤ 130 ms | Present | CRTD implanted and randomized 1 : 1 with CRTD programme on and off | 6 months | Change in peak oxygen consumption | August 2005–January 2007 | Good |
| Ruschitzka et al.14 (The echoCRT study) | Randomized double blinded, multicentre | LVEF ≤ 35%, NYHA Class III or IV, QRSd ≤ 130ms | Present | CRTD implanted and then randomized 1 : 1 with CRT on and off | 1 month, 3 month, then every 3 months until trial terminated | Combination of death and HF hospitalization | August 2008–March 2013 | Good |
| Muto et al.15 (The NARROW-CRT study) | Randomized single blind, multicentre | LVEF <35%, NYHA Class II and III, QRSd ≤120 ms | Present | 1 : 1 implantation of CRTD vs. DDD-ICD(dual-chamber ICD) | 6 months and then 12 months | HF clinical composite score | January 2008–May 2010 | Good |
CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; QRSd, QRS duration.
| Study . | Study design . | Patient selection . | LV dyssynchrony . | Randomization . | Scheduled follow-up . | Primary outcomes . | Duration . | Internal validity . |
|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | Randomized double blinded, multicentre | LVEF ≤35%, QRSd <120 ms, NYHA class III | Not assessed | CRTD implanted and randomized to CRT programme active and inactive | 6 months 12 months | Submaximal exercise duration | October 2003–January 2011. | Good |
| Beshai et al.12 (RethinQ ) | Randomized double blinded, multicentre | LVEF <35%, NYHA Class III, QRSd ≤ 130 ms | Present | CRTD implanted and randomized 1 : 1 with CRTD programme on and off | 6 months | Change in peak oxygen consumption | August 2005–January 2007 | Good |
| Ruschitzka et al.14 (The echoCRT study) | Randomized double blinded, multicentre | LVEF ≤ 35%, NYHA Class III or IV, QRSd ≤ 130ms | Present | CRTD implanted and then randomized 1 : 1 with CRT on and off | 1 month, 3 month, then every 3 months until trial terminated | Combination of death and HF hospitalization | August 2008–March 2013 | Good |
| Muto et al.15 (The NARROW-CRT study) | Randomized single blind, multicentre | LVEF <35%, NYHA Class II and III, QRSd ≤120 ms | Present | 1 : 1 implantation of CRTD vs. DDD-ICD(dual-chamber ICD) | 6 months and then 12 months | HF clinical composite score | January 2008–May 2010 | Good |
| Study . | Study design . | Patient selection . | LV dyssynchrony . | Randomization . | Scheduled follow-up . | Primary outcomes . | Duration . | Internal validity . |
|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | Randomized double blinded, multicentre | LVEF ≤35%, QRSd <120 ms, NYHA class III | Not assessed | CRTD implanted and randomized to CRT programme active and inactive | 6 months 12 months | Submaximal exercise duration | October 2003–January 2011. | Good |
| Beshai et al.12 (RethinQ ) | Randomized double blinded, multicentre | LVEF <35%, NYHA Class III, QRSd ≤ 130 ms | Present | CRTD implanted and randomized 1 : 1 with CRTD programme on and off | 6 months | Change in peak oxygen consumption | August 2005–January 2007 | Good |
| Ruschitzka et al.14 (The echoCRT study) | Randomized double blinded, multicentre | LVEF ≤ 35%, NYHA Class III or IV, QRSd ≤ 130ms | Present | CRTD implanted and then randomized 1 : 1 with CRT on and off | 1 month, 3 month, then every 3 months until trial terminated | Combination of death and HF hospitalization | August 2008–March 2013 | Good |
| Muto et al.15 (The NARROW-CRT study) | Randomized single blind, multicentre | LVEF <35%, NYHA Class II and III, QRSd ≤120 ms | Present | 1 : 1 implantation of CRTD vs. DDD-ICD(dual-chamber ICD) | 6 months and then 12 months | HF clinical composite score | January 2008–May 2010 | Good |
CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; QRSd, QRS duration.
| Study . | Group . | No. of patients . | Sex (male) No. of patients (%) . | Age (years) (mean ± SD) . | QRSd (ms) (mean ± SD) . | NYHA class No. of patients (%) . | Ischaemic CMP No. of patients (%) . | LVEF (%) (mean ± SD) . | Intraventricular mechanical delay (mean ± SD) . |
|---|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | CRT on | 44 | 28 (64%) | 62 ± 10 | 105 ± 10 | III–IV 17 (39.5%) | 32 (73%) | 29 ± 9 | NA |
| CRT off | 41 | 32 (78%) | 60 ± 12 | 104 ± 9 | III–IV 12 (29.3%) | 27 (66%) | 31 ± 8 | NA | |
| Beshai et al.12 (RethinQ ) | CRT- D on | 87 | 62 (71%) | 60 ± 12 | 107 ± 12 | III—87 (100%) | 47 (54%) | 25 ± 5 | 9 ± 28 |
| CRT- D off | 85 | 49 (58%) | 58 ± 14 | 106 ± 13 | III—84 (99%) | 43 (51%) | 26 ± 6 | 8 ± 31 | |
| Ruschitzka et al.14 (The echoCRT study) | CRT- D on | 404 | 294 (73%) | 58 ± 13 | 106 ± 13 | II—7(1.7%), III—385 (95.3%), IV—10 (2.5%) | 218 (54%) | 27 ± 6 | NA |
| CRT- D off | 405 | 291 (72%) | 58 ± 13 | 106 ± 12 | II—12 (3%), III — 374 (92.3%), IV 16 (4%) | 214 (53%) | 27 ± 5 | NA | |
| Muto et al.15 (The NARROW-CRT study) | CRT | 60 | 53 (88%) | 65 ± 9 | 107 ± 14 | II—23 (38%) III—37 (62%) | 60 (100%) | 28 ± 5 | 79 ± 19 |
| D-ICD | 60 | 50 (83%) | 68 ± 9 | 104 ± 14 | II—25 (42%) III—35 (58%) | 60 (100%) | 29 ± 5 | 81 ± 21 |
| Study . | Group . | No. of patients . | Sex (male) No. of patients (%) . | Age (years) (mean ± SD) . | QRSd (ms) (mean ± SD) . | NYHA class No. of patients (%) . | Ischaemic CMP No. of patients (%) . | LVEF (%) (mean ± SD) . | Intraventricular mechanical delay (mean ± SD) . |
|---|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | CRT on | 44 | 28 (64%) | 62 ± 10 | 105 ± 10 | III–IV 17 (39.5%) | 32 (73%) | 29 ± 9 | NA |
| CRT off | 41 | 32 (78%) | 60 ± 12 | 104 ± 9 | III–IV 12 (29.3%) | 27 (66%) | 31 ± 8 | NA | |
| Beshai et al.12 (RethinQ ) | CRT- D on | 87 | 62 (71%) | 60 ± 12 | 107 ± 12 | III—87 (100%) | 47 (54%) | 25 ± 5 | 9 ± 28 |
| CRT- D off | 85 | 49 (58%) | 58 ± 14 | 106 ± 13 | III—84 (99%) | 43 (51%) | 26 ± 6 | 8 ± 31 | |
| Ruschitzka et al.14 (The echoCRT study) | CRT- D on | 404 | 294 (73%) | 58 ± 13 | 106 ± 13 | II—7(1.7%), III—385 (95.3%), IV—10 (2.5%) | 218 (54%) | 27 ± 6 | NA |
| CRT- D off | 405 | 291 (72%) | 58 ± 13 | 106 ± 12 | II—12 (3%), III — 374 (92.3%), IV 16 (4%) | 214 (53%) | 27 ± 5 | NA | |
| Muto et al.15 (The NARROW-CRT study) | CRT | 60 | 53 (88%) | 65 ± 9 | 107 ± 14 | II—23 (38%) III—37 (62%) | 60 (100%) | 28 ± 5 | 79 ± 19 |
| D-ICD | 60 | 50 (83%) | 68 ± 9 | 104 ± 14 | II—25 (42%) III—35 (58%) | 60 (100%) | 29 ± 5 | 81 ± 21 |
CMP, cardiomyopathy; CRTD, cardiac-resynchronization therapy defibrillator; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; QRSd, QRS duration; SD, standard deviation.
| Study . | Group . | No. of patients . | Sex (male) No. of patients (%) . | Age (years) (mean ± SD) . | QRSd (ms) (mean ± SD) . | NYHA class No. of patients (%) . | Ischaemic CMP No. of patients (%) . | LVEF (%) (mean ± SD) . | Intraventricular mechanical delay (mean ± SD) . |
|---|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | CRT on | 44 | 28 (64%) | 62 ± 10 | 105 ± 10 | III–IV 17 (39.5%) | 32 (73%) | 29 ± 9 | NA |
| CRT off | 41 | 32 (78%) | 60 ± 12 | 104 ± 9 | III–IV 12 (29.3%) | 27 (66%) | 31 ± 8 | NA | |
| Beshai et al.12 (RethinQ ) | CRT- D on | 87 | 62 (71%) | 60 ± 12 | 107 ± 12 | III—87 (100%) | 47 (54%) | 25 ± 5 | 9 ± 28 |
| CRT- D off | 85 | 49 (58%) | 58 ± 14 | 106 ± 13 | III—84 (99%) | 43 (51%) | 26 ± 6 | 8 ± 31 | |
| Ruschitzka et al.14 (The echoCRT study) | CRT- D on | 404 | 294 (73%) | 58 ± 13 | 106 ± 13 | II—7(1.7%), III—385 (95.3%), IV—10 (2.5%) | 218 (54%) | 27 ± 6 | NA |
| CRT- D off | 405 | 291 (72%) | 58 ± 13 | 106 ± 12 | II—12 (3%), III — 374 (92.3%), IV 16 (4%) | 214 (53%) | 27 ± 5 | NA | |
| Muto et al.15 (The NARROW-CRT study) | CRT | 60 | 53 (88%) | 65 ± 9 | 107 ± 14 | II—23 (38%) III—37 (62%) | 60 (100%) | 28 ± 5 | 79 ± 19 |
| D-ICD | 60 | 50 (83%) | 68 ± 9 | 104 ± 14 | II—25 (42%) III—35 (58%) | 60 (100%) | 29 ± 5 | 81 ± 21 |
| Study . | Group . | No. of patients . | Sex (male) No. of patients (%) . | Age (years) (mean ± SD) . | QRSd (ms) (mean ± SD) . | NYHA class No. of patients (%) . | Ischaemic CMP No. of patients (%) . | LVEF (%) (mean ± SD) . | Intraventricular mechanical delay (mean ± SD) . |
|---|---|---|---|---|---|---|---|---|---|
| Thibault et al.13 (LESSER-EARTH) | CRT on | 44 | 28 (64%) | 62 ± 10 | 105 ± 10 | III–IV 17 (39.5%) | 32 (73%) | 29 ± 9 | NA |
| CRT off | 41 | 32 (78%) | 60 ± 12 | 104 ± 9 | III–IV 12 (29.3%) | 27 (66%) | 31 ± 8 | NA | |
| Beshai et al.12 (RethinQ ) | CRT- D on | 87 | 62 (71%) | 60 ± 12 | 107 ± 12 | III—87 (100%) | 47 (54%) | 25 ± 5 | 9 ± 28 |
| CRT- D off | 85 | 49 (58%) | 58 ± 14 | 106 ± 13 | III—84 (99%) | 43 (51%) | 26 ± 6 | 8 ± 31 | |
| Ruschitzka et al.14 (The echoCRT study) | CRT- D on | 404 | 294 (73%) | 58 ± 13 | 106 ± 13 | II—7(1.7%), III—385 (95.3%), IV—10 (2.5%) | 218 (54%) | 27 ± 6 | NA |
| CRT- D off | 405 | 291 (72%) | 58 ± 13 | 106 ± 12 | II—12 (3%), III — 374 (92.3%), IV 16 (4%) | 214 (53%) | 27 ± 5 | NA | |
| Muto et al.15 (The NARROW-CRT study) | CRT | 60 | 53 (88%) | 65 ± 9 | 107 ± 14 | II—23 (38%) III—37 (62%) | 60 (100%) | 28 ± 5 | 79 ± 19 |
| D-ICD | 60 | 50 (83%) | 68 ± 9 | 104 ± 14 | II—25 (42%) III—35 (58%) | 60 (100%) | 29 ± 5 | 81 ± 21 |
CMP, cardiomyopathy; CRTD, cardiac-resynchronization therapy defibrillator; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; QRSd, QRS duration; SD, standard deviation.
| Study . | Definition for Mechanical Dyssynchrony . |
|---|---|
| Beshai et al.12 (RethinQ) | Septal-to-posterior wall delay in peak velocity of an interval ≥130 ms and opposing wall delay between the anteroseptal-to-posterior wall and the septal-to-lateral wall of ≥65 ms |
| Ruschitzka et al.14 (The echoCRT study) | Opposing-wall delay in the peak systolic velocity of ≥80 ms or delay in the anteroseptal-to-posterior wall of ≥130 ms |
| Muto et al.15 (The NARROW-CRT study) | Difference of peak systolic velocity between the septal and lateral delays of ≥60 ms |
| Study . | Definition for Mechanical Dyssynchrony . |
|---|---|
| Beshai et al.12 (RethinQ) | Septal-to-posterior wall delay in peak velocity of an interval ≥130 ms and opposing wall delay between the anteroseptal-to-posterior wall and the septal-to-lateral wall of ≥65 ms |
| Ruschitzka et al.14 (The echoCRT study) | Opposing-wall delay in the peak systolic velocity of ≥80 ms or delay in the anteroseptal-to-posterior wall of ≥130 ms |
| Muto et al.15 (The NARROW-CRT study) | Difference of peak systolic velocity between the septal and lateral delays of ≥60 ms |
| Study . | Definition for Mechanical Dyssynchrony . |
|---|---|
| Beshai et al.12 (RethinQ) | Septal-to-posterior wall delay in peak velocity of an interval ≥130 ms and opposing wall delay between the anteroseptal-to-posterior wall and the septal-to-lateral wall of ≥65 ms |
| Ruschitzka et al.14 (The echoCRT study) | Opposing-wall delay in the peak systolic velocity of ≥80 ms or delay in the anteroseptal-to-posterior wall of ≥130 ms |
| Muto et al.15 (The NARROW-CRT study) | Difference of peak systolic velocity between the septal and lateral delays of ≥60 ms |
| Study . | Definition for Mechanical Dyssynchrony . |
|---|---|
| Beshai et al.12 (RethinQ) | Septal-to-posterior wall delay in peak velocity of an interval ≥130 ms and opposing wall delay between the anteroseptal-to-posterior wall and the septal-to-lateral wall of ≥65 ms |
| Ruschitzka et al.14 (The echoCRT study) | Opposing-wall delay in the peak systolic velocity of ≥80 ms or delay in the anteroseptal-to-posterior wall of ≥130 ms |
| Muto et al.15 (The NARROW-CRT study) | Difference of peak systolic velocity between the septal and lateral delays of ≥60 ms |
A total of 1186 patients with SHF and a narrow QRS complex (<130 ms) are included in our analysis, of which 595 were treated with CRT and 591 did not. Echo CRT study contributes to the majority of patients (n = 809) in our meta-analysis.14Table 2 shows baseline characteristics of patients included in meta-analysis. There was no significant difference in age, sex, and NYHA heart failure class. The frequency of patients with ischaemic cardiomyopathy, diabetes, hypertension, previous MI, and revascularization procedures was similar between the two groups in each study. All studies included patients with LVEF ≤35% and baseline ejection fraction (EF) was similar between the two groups. QRS duration was also similar between the two groups.
Outcomes
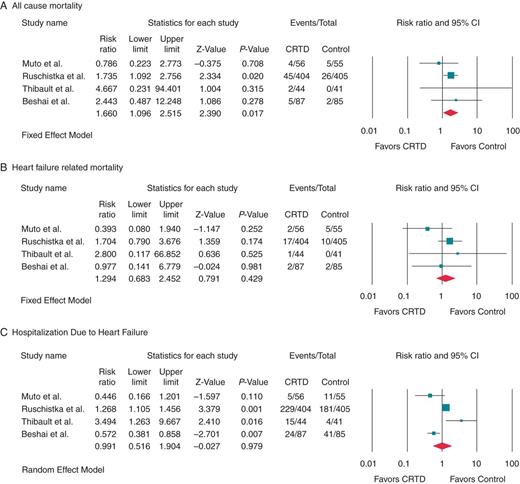
Clinical outcomes among heart failure patients with EF ≤35% and QRS interval ≤130 ms or less who received CRTD compared with those who had ICD therapy (control) alone.
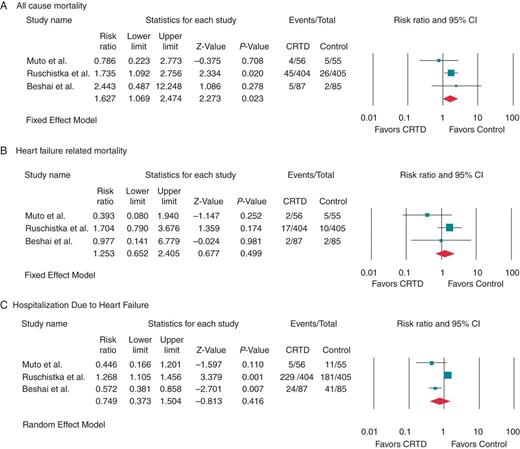
Clinical outcomes among heart failure patients with EF ≤35% and echocardiographic evidence of mechanical dyssynchrony with QRSd<130 ms who received CRTD therapy compared with those who had ICD therapy (control) alone.
Discussion
This systematic review and meta-analysis is, to the best of our knowledge, the first to compare the effects of CRT-D therapy on all-cause mortality, HF mortality, and HF hospitalizations in patients with SHF and QRS <130 ms to stand-alone ICD therapy. The results of our analysis suggest that (i) CRT-D does not reduce HF mortality or HF hospitalizations in symptomatic SHF patients with QRS duration ≤130 ms in comparison with ICD alone, (ii) CRT-D is associated with higher all-cause mortality in comparison with ICD therapy in this patient population.
Cardiac-resynchronization therapy has been shown to improve mortality and morbidity rates in patients with SHF with QRS duration >150 ms, but not in patients with QRS duration <150 ms in a meta-analysis of five clinical trials involving a total of 6501 patients.20 Subgroup analyses from two large clinical trials suggested that a QRS duration of <150 ms is a risk factor for poor response to CRT therapy.2,3 However, many patients with heart failure have QRS duration of <130 ms.5 Up to 50% of these patients show echocardiographic evidence of left ventricular mechanical dyssynchrony.5,6 Small-scale observational studies have reported improved outcomes with CRT in patients with narrow QRS.7–11 Such observations have led to frequent off label use of CRT in patients with QRS <130 ms.14
The understanding of mechanical dyssynchrony has led to enthusiasm for extending benefits of CRT to patients with a narrow QRS who demonstrate dyssynchrony. The mechanism for poor response to CRT-D therapy among patients with narrow QRS complex even when mechanical dyssynchrony is present is not understood. The limitations of current imaging modalities to accurately define mechanical dyssynchrony as well as the inability to embrace a universally adopted definition of dyssynchrony are obvious barriers, not to mention the inter and intra-observer variations in measuring dyssynchrony. The ESTEEM-CRT study18 evaluated the haemodynamic, clinical, and structural effects of CRT in patients with SHF, a narrow QRS, and mechanical dyssynchrony. In this study, LV dyssynchrony was evaluated by 13 different echo indices and there was wide disagreement in the prevalence of dyssynchrony (0–74%) in the study group, depending on the echo index chosen. Moreover, none of the echo dyssynchrony indices (baseline value or acute change with CRT) were able to predict chronic outcomes as measured by EF, peak oxygen consumption (VO2), left ventricular end systolic volume, left ventricular end diastolic volume, or quality of life. Forced biventricular pacing may in fact lead to worsening of heart failure in these patients.14 In the same study, continuous pacing at shorter AV delay (60% or less than intrinsic AV delay) caused reductions in LV dP/dtmax. Placement of an LV lead obviously adds to procedural duration, complexity and the risks. In the EchoCRT study, the rate of adverse events related to a CRT device implantation was significantly higher than the control group.14
Limitation
Our study has all the limitations inherent to a meta-analysis. Few studies were included and the EchoCRT study contributed the majority of patients. We studied only hard clinical endpoints and did not evaluate the ‘softer’ endpoints: improvement in symptoms, quality of life or change in echocardiographic parameters with CRT therapy. Studies included in our meta-analysis did not have tailored LV lead placement to derive optimal benefit from CRT which might affect overall results; however, this is a limitation that extends to most CRT studies.
In summary, our meta-analysis indicates that CRT-D does not reduce all-cause mortality, HF mortality, or HF readmissions in symptomatic SHF patients with QRS duration ≤130 ms. Instead, CRT-D therapy is associated with increased overall mortality in this patient population.
Clinical implications and future direction
This meta-analysis not only emphasizes the futility of CRT in patients with QRS duration ≤130 ms, but also points to potential adverse outcome of higher all-cause mortality. When we performed subgroup analysis of three clinical trials involving patients with LV mechanical dyssynchrony and QRSd <130 ms (Muto et al., Ruschitzka et al., and Beshai et al.), we found that there was a higher all-cause mortality in these patients as well compared with the control group. Until future research provides robust measures of dyssynchrony and clear insights into how to improve outcomes of CRT in heart failure patients with narrow QRS complex, its use should be strongly discouraged in this population.
Conflict of interest: J.N.K has received honoraria from Medtronik, Biotronik, St Jude Medical (all <$5000). This has no bearing on the manuscript. K.A.E is a consultant for Medtronik, Boston Science, Biotronik and has received honoraria for speaking.
References
- heart failure, systolic
- left ventricular remodeling
- wide qrs complex
- implantable defibrillators
- heart failure
- heterogeneity
- follow-up
- medline
- patient readmission
- morbidity
- mortality
- systolic dysfunction
- cardiac resynchronization therapy
- ejection fraction
- biventricular pacing devices
- defibrillators
- cochrane collaboration
- qrs complex duration
- embase



