-
PDF
- Split View
-
Views
-
Cite
Cite
Gaetano Pinnacchio, Gaetano Antonio Lanza, Alessandra Stazi, Giulia Careri, Ilaria Coviello, Roberto Mollo, Filippo Crea, Determinants of heart rate turbulence in individuals without apparent heart disease and in patients with stable coronary artery disease, EP Europace, Volume 17, Issue 12, December 2015, Pages 1855–1861, https://doi.org/10.1093/europace/euu338
Close - Share Icon Share
Abstract
To assess the characteristics and determinants of heart rate turbulence (HRT) in individuals without any apparent heart disease and in patients with coronary artery disease (CAD).
Heart rate turbulence parameters, turbulence onset (TO), and turbulence slope (TS) were calculated on 24 h electrocardiogram recordings in 209 individuals without any heart disease (group 1) and in 157 CAD patients (group 2). In group 1, only age independently predicted abnormal TO (≥0%) [odds ratio (OR), 1.05; P<0.001], while predictors of abnormal TS (≤2.5 ms/RR) were age (OR, 0.85; P < 0.001) and hypertension (OR, 0.19; P = 0.028). In group 2 patients, only age independently predicted TO (OR, 1.03; P = 0.038), while age (OR, 0.90; P = 0.001) and left ventricular ejection fraction (LVEF; OR, 1.07; P = 0.008) predicted TS. Heart rate turbulence values were different in groups 1 and 2. Turbulence onset was (mean, standard deviation) −1.80 ± 2.24 vs. −0.73 ± 1.61%, respectively (P < 0.001), whereas TS was (median, interquartile interval) 5.83 (3.25–10.55) vs. 2.93 (1.73–5.81) ms/RR, respectively (P < 0.001). Coronary artery disease group, however, did not predict abnormal HRT parameters in multivariable analyses, both in the whole population and when comparing two subgroups matched for age and gender. Age and (for TS) LVEF, indeed, were the only independent predictors of abnormal HRT.
Age is a major HRT determinant both in subjects without any apparent heart disease and in stable CAD patients. Hypertension and LVEF contribute independently to HRT in these two groups, respectively. Coronary artery disease group was not by itself associated with abnormal HRT parameters in multivariable analyses.
Our data show that, in subjects without any apparent cardiac disease, only age and, in part, hypertension are significant independent predictors of heart rate turbulence (HRT).
Heart rate turbulence is largely independent of cardiovascular risk factors, as well as medical therapy, in apparently healthy individuals.
Age remains a major modulator of HRT in coronary artery disease (CAD) patients, in whom left ventricular ejection function also assumes a major role.
Although HRT showed significantly different values in CAD patients compared with apparently healthy subjects, stable CAD is by itself only a weak independent predictor of HRT.
Introduction
Heart rate turbulence (HRT) represents a physiologic biphasic chronotropic response of sinus rhythm to a premature ventricular complex (PVC). This response is believed to be mainly mediated by the cardiac autonomic nervous system, and consists of an early heart rate acceleration, consistent with an immediate vagal withdrawal, and a late heart rate deceleration, reflecting a subsequent restoration of vagal activity.1–5 The two components of HRT are measured as turbulence onset (TO) and turbulence slope (TS), respectively.1
Several studies have shown that HRT impairment reflects cardiac autonomic dysfunction, in particular impaired vagal-mediated baroreflex sensitivity.6,7 Moreover, several studies have shown that impaired HRT predicts an increased risk of fatal events and ventricular tachyarrhythmias in patients with acute myocardial infarction (MI)8–12 or congestive heart failure.13,14
Despite its prognostic value, to date only few studies have tried to define the physiological characteristics of HRT in apparently healthy individuals, i.e. without any evidence of cardiac disease.15–18 Specifically, how clinical features, such as cardiovascular risk factors (CVRFs), left ventricular (LV) function, and drug therapy, affect HRT parameters in people without cardiac disease has poorly been investigated.
Thus, in this study, we aimed to define characteristics and determinants of HRT parameters in a large cohort of consecutive unselected subjects without any apparent heart disease undergoing routine 24 h Holter ECG recording. At the same time, we assessed whether factors influencing HRT were different in patients with documented coronary artery disease (CAD).
Methods
Population
From September 2009 to May 2010, we prospectively studied consecutive subjects without any overt heart disease referred to our centre to undergo 24 h ambulatory Holter ECG recording.
Subjects with a cardiac rhythm other than sinus, presence of pacemaker, a number <5 PVCs suitable for HRT analysis, evidence of diabetes, and/or significant systemic disease (e.g. neoplasia, acute or chronic inflammatory disease, respiratory, hepatic, or renal failure) were excluded.
Each subject underwent an interview with a physician, who collected detailed information about clinical history with the aid of a standardized questionnaire. Data acquired included age, gender, CVRFs, reason for Holter ECG recording, history of cardiovascular disease, and drug therapy.
Hypertension was defined as referred blood pressure ≥140/90 mmHg or assumption of anti-hypertensive drugs; hypercholesterolaemia was defined as referred blood cholesterol levels >200 mg/dL or assumption of anti-cholesterolemic drugs; smoking was defined as any usual cigarette smoking in the last 6 months; obesity was defined as a body mass index (derived from weight and height) ≥30 kg/m2; familial history for CAD was defined as evidence of CAD in first- or second-degree relatives, male <55 years and female <65 years.
Clinical examination, ECG at rest, and bi-dimensional echocardiography were also obtained in all subjects to rule out any structural cardiac diseases, including LV hypertrophy. Left ventricular ejection fraction (LVEF) was calculated on echocardiographic four-chamber and two-chamber views using the modified Simpson's method. Patients with a suspect of typical angina pectoris were excluded. Subjects with atypical chest pain underwent maximal exercise stress test and were included only when the test was normal.
A consecutive group of patients with a documented history of CAD referred to undergo Holter ECG during the same period was also studied. Coronary artery disease was diagnosed in case of a documented history of previous MI, percutaneous and/or surgical coronary intervention or documented coronary stenosis (>50%) in at least one major coronary artery at angiography. Only stable CAD patients were included. Accordingly, patients with a history of new onset (de novo) angina, recent destabilization of previously stable angina, or an acute coronary syndrome in the last 6 months were excluded. In addition, we excluded patients with any other cardiac and systemic disease.
The study was approved by the Institutional Ethical Committee and written informed consent was obtained from each individual to participate in the study.
Heart rate turbulence
All subjects underwent 24 h Holter ECG monitoring using three-channel tape recorders (Oxford Medilog FD5, Abingdon, UK). The recordings were analysed by two expert cardiologists using the system Oxford Excel 3.0.
To calculate HRT parameters, RR intervals were exported as text files and analysed using a dedicated and validated software system.17 Heart rate turbulence was obtained from sequences of sinus RR intervals related to isolated PVCs that fulfilled the following criteria: (i) index PVC embedded into at least five preceding and 20 succeeding normal RR intervals; (ii) cycle length of all considered RR intervals >300 ms but <2000 ms; (iii) beat-to-beat differences <200 ms; and (iv) differences <20% from the average of five preceding intervals.1,5 Accordingly, a single tachogram for HRT analysis consisted of a sequence of five RR intervals preceding and 20 RR intervals succeeding a PVC. Interpolated PVCs were excluded.
Heart rate turbulence was assessed using the two standard parameters TO and TS. Turbulence onset, expressed as a percentage, was calculated using the formula [(RR1 + RR2) − (RR−2 + RR−1)]/(RR−2 + RR−1)×100, where RR1 and RR2 were the first and the second sinus RR intervals after the PVC and RR−1 and RR−2 were the first and the second sinus RR intervals preceding the PVC.
Turbulence slope, expressed in ms/RR, was obtained as the maximal positive slope of all slopes of a series of regression lines obtained from all sequences (n = 16) of five consecutive RR intervals included between the 1st and 20th RR interval after the compensatory pause that followed the PVC.
Turbulence onset was calculated as the average of all TOs measured in the 24 h on individual tachograms of index PVCs, while TS was calculated on the averaged tachogram obtained averaging the RR intervals of individual tachograms preceding and following the index PVCs in the 24 h.
Although ‘normal’ HRT values have not been defined yet, normal TO is expected to be negative, whereas normal TS is expected to be positive. Previous studies showed that TO ≥ 0% and TS ≤ 2.5 ms/RR were significantly associated with a worse prognosis in cardiac patients.1,8–11 Accordingly, we used these dichotomized values as cut-offs to consider HRT parameters as abnormal.
A composite HRT variable was also obtained by combining TO and TS values, which results in three mutually exclusive categories: (i) TO ≤ 0% and TS ≥ 2.5 ms/RR interval (i.e. both parameters normal, category 0); (ii) either TO ≥ 0% or TS ≤ 2.5 ms/RR interval (i.e. only one parameter abnormal, category 1); and (iii) TO ≥ 0% and TS ≤ 2.5 ms/RR interval (i.e. both parameters abnormal, category 2).
Statistics
Statistical analyses were done by SPSS 20.0 (SPSS, Inc.). Turbulence onset showed a Gaussian distribution, while TS showed a skewed distribution, as assessed by Kolmogorov–Smirnov test. Accordingly, between-group values of TO and TS were compared using unpaired t-test and Mann–Whitney test, respectively. Proportions were compared using χ2 test. Pearson and Spearman rank-correlation tests, respectively, were used to assess the relation between HRT parameters with other variables.
Univariate and multivariable linear regression analysis was performed to identify variables predictive of HRT parameters, considered as continuous variables. Univariate and multivariable logistic regression was applied to identify variables associated with abnormal TO and TS values, as well as HRT category 1 or category 2, compared with category 0. All variables with a P-value≤ 0.1 on univariate analyses were included as independent covariates in the multivariable models.
Furthermore, we also assessed differences in HRT parameters by performing comparisons between two subgroups of 65 subjects of groups 1 and 2, which were matched for age and gender.
Data are reported as mean ± SD for normally distributed variables and as median (with interquartile interval) for variables with skewed distribution. Differences were considered significant for P < 0.05.
Results
General findings
During the period of the study, 519 subjects without any apparent cardiac disease were examined, 310 of whom were excluded due to an insufficient number of PVCs (n = 287) or a history of diabetes (n = 23). Thus, 209 individuals (age 61 ± 16 years, males 49%) satisfied the inclusion criteria and formed the group without any apparent cardiac disease (group 1).
In the same period, 392 patients with heart disease were screened. Overall, 235 patients were excluded, due to an insufficient number of PVCs (n = 155) or evidence of a non-ischaemic disease of the heart (n = 80). Thus, 157 patients (age 69 ± 10 years, males 78%) met the inclusion criteria for the study and constituted the group of stable CAD patients (group 2).
The main clinical characteristics of the two study groups are summarized in Table 1. Compared with group 2 patients, subjects of group 1 were younger, less frequently men, had a lower prevalence of CVRFs, and had a higher LVEF. Furthermore, they also had a lower number of PVCs.
| Variable . | Group 1 (n = 209) . | Group 2 (n = 157) . | P-value . |
|---|---|---|---|
| Age (years) | 61 ± 16 | 69 ± 10 | <0.001 |
| Men (%) | 49 | 78 | <0.001 |
| Holter data | |||
| PVCs (Nr/24 h) | 1111 ± 1942 | 1760 ± 3445 | 0.02 |
| Processed PVCs (Nr/24 h) | 149 ± 186 | 170 ± 202 | 0.32 |
| Mean heart rate (b.p.m.) | 70 ± 10 | 67 ± 9 | 0.005 |
| NSVT (%) | 1 | 19 | <0.001 |
| CVRFs | |||
| Family history of CVD (%) | 32 | 54 | <0.001 |
| Hypertension (%) | 54 | 80 | <0.001 |
| Dyslipidemia (%) | 38 | 65 | <0.001 |
| Smoking (%) | 17 | 13 | 0.382 |
| Drug therapy | |||
| β-Blockers (%) | 32 | 77 | <0.001 |
| ACE-inhibitors (%) | 23 | 56 | <0.001 |
| Statins (%) | 16 | 77 | <0.001 |
| Diuretics (%) | 20 | 41 | <0.001 |
| Reason for Holter recording | |||
| Follow-up (%) | 35 | 60 | |
| Palpitations (%) | 32 | 12 | |
| Chest pain (%) | 12 | 13 | <0.001 |
| Syncope (%) | 14 | 10 | |
| Other (%) | 7 | 5 | |
| LVEF (%) | 62 ± 5 | 51 ± 12 | <0.001 |
| Variable . | Group 1 (n = 209) . | Group 2 (n = 157) . | P-value . |
|---|---|---|---|
| Age (years) | 61 ± 16 | 69 ± 10 | <0.001 |
| Men (%) | 49 | 78 | <0.001 |
| Holter data | |||
| PVCs (Nr/24 h) | 1111 ± 1942 | 1760 ± 3445 | 0.02 |
| Processed PVCs (Nr/24 h) | 149 ± 186 | 170 ± 202 | 0.32 |
| Mean heart rate (b.p.m.) | 70 ± 10 | 67 ± 9 | 0.005 |
| NSVT (%) | 1 | 19 | <0.001 |
| CVRFs | |||
| Family history of CVD (%) | 32 | 54 | <0.001 |
| Hypertension (%) | 54 | 80 | <0.001 |
| Dyslipidemia (%) | 38 | 65 | <0.001 |
| Smoking (%) | 17 | 13 | 0.382 |
| Drug therapy | |||
| β-Blockers (%) | 32 | 77 | <0.001 |
| ACE-inhibitors (%) | 23 | 56 | <0.001 |
| Statins (%) | 16 | 77 | <0.001 |
| Diuretics (%) | 20 | 41 | <0.001 |
| Reason for Holter recording | |||
| Follow-up (%) | 35 | 60 | |
| Palpitations (%) | 32 | 12 | |
| Chest pain (%) | 12 | 13 | <0.001 |
| Syncope (%) | 14 | 10 | |
| Other (%) | 7 | 5 | |
| LVEF (%) | 62 ± 5 | 51 ± 12 | <0.001 |
PVCs, premature ventricular complexes; NSVT, non-sustained ventricular tachycardia; CVRFs, cardiovascular risk factors; CVD, cardiovascular disease; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction.
| Variable . | Group 1 (n = 209) . | Group 2 (n = 157) . | P-value . |
|---|---|---|---|
| Age (years) | 61 ± 16 | 69 ± 10 | <0.001 |
| Men (%) | 49 | 78 | <0.001 |
| Holter data | |||
| PVCs (Nr/24 h) | 1111 ± 1942 | 1760 ± 3445 | 0.02 |
| Processed PVCs (Nr/24 h) | 149 ± 186 | 170 ± 202 | 0.32 |
| Mean heart rate (b.p.m.) | 70 ± 10 | 67 ± 9 | 0.005 |
| NSVT (%) | 1 | 19 | <0.001 |
| CVRFs | |||
| Family history of CVD (%) | 32 | 54 | <0.001 |
| Hypertension (%) | 54 | 80 | <0.001 |
| Dyslipidemia (%) | 38 | 65 | <0.001 |
| Smoking (%) | 17 | 13 | 0.382 |
| Drug therapy | |||
| β-Blockers (%) | 32 | 77 | <0.001 |
| ACE-inhibitors (%) | 23 | 56 | <0.001 |
| Statins (%) | 16 | 77 | <0.001 |
| Diuretics (%) | 20 | 41 | <0.001 |
| Reason for Holter recording | |||
| Follow-up (%) | 35 | 60 | |
| Palpitations (%) | 32 | 12 | |
| Chest pain (%) | 12 | 13 | <0.001 |
| Syncope (%) | 14 | 10 | |
| Other (%) | 7 | 5 | |
| LVEF (%) | 62 ± 5 | 51 ± 12 | <0.001 |
| Variable . | Group 1 (n = 209) . | Group 2 (n = 157) . | P-value . |
|---|---|---|---|
| Age (years) | 61 ± 16 | 69 ± 10 | <0.001 |
| Men (%) | 49 | 78 | <0.001 |
| Holter data | |||
| PVCs (Nr/24 h) | 1111 ± 1942 | 1760 ± 3445 | 0.02 |
| Processed PVCs (Nr/24 h) | 149 ± 186 | 170 ± 202 | 0.32 |
| Mean heart rate (b.p.m.) | 70 ± 10 | 67 ± 9 | 0.005 |
| NSVT (%) | 1 | 19 | <0.001 |
| CVRFs | |||
| Family history of CVD (%) | 32 | 54 | <0.001 |
| Hypertension (%) | 54 | 80 | <0.001 |
| Dyslipidemia (%) | 38 | 65 | <0.001 |
| Smoking (%) | 17 | 13 | 0.382 |
| Drug therapy | |||
| β-Blockers (%) | 32 | 77 | <0.001 |
| ACE-inhibitors (%) | 23 | 56 | <0.001 |
| Statins (%) | 16 | 77 | <0.001 |
| Diuretics (%) | 20 | 41 | <0.001 |
| Reason for Holter recording | |||
| Follow-up (%) | 35 | 60 | |
| Palpitations (%) | 32 | 12 | |
| Chest pain (%) | 12 | 13 | <0.001 |
| Syncope (%) | 14 | 10 | |
| Other (%) | 7 | 5 | |
| LVEF (%) | 62 ± 5 | 51 ± 12 | <0.001 |
PVCs, premature ventricular complexes; NSVT, non-sustained ventricular tachycardia; CVRFs, cardiovascular risk factors; CVD, cardiovascular disease; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction.
Heart rate turbulence in subjects without cardiac disease
Premature ventricular complexes suitable for HRT assessment in group 1 subjects were 149 ± 186. Turbulence onset, in this group, was −1.80 ± 2.24%, while TS was 5.83 (3.25–10.55) ms/RR.
At univariate analysis, a highly significant correlation was found between age and both HRT parameters (TO: r = 0.38, P < 0.001; TS: r = −0.63, P < 0.001; Figure 1). Furthermore, higher TO values and lower TS values were observed in subjects with, compared with those without, hypertension or dyslipidemia; female gender was also associated with lower TS values, compared with males (Table 2).
Clinical predictors of TO and TS in group 1 subjects at univariate linear regression analysis
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.05 (1.034–1.071) | <0.001 | 0.83 (0.80–86) | <0.001 |
| Male gender | 0.82 (0.44–1.51) | 0.52 | 6.47 (1.54–27) | 0.011 |
| Mean heart rate | 0.99 (0.96–1.03) | 0.77 | 0.94 (0.88–1.01) | 0.068 |
| CVRFs | ||||
| Hypertension | 2.78 (1.52–5.05) | 0.001 | 0.01 (0.01–0.04) | <0.001 |
| Dyslipidemia | 2.14 (1.15–3.99) | 0.017 | 0.12 (0.03–0.50) | 0.004 |
| Smoke | 0.57 (0.25–1.27) | 0.169 | 5.39 (0.79–36) | 0.084 |
| Obesity | 1.03 (0.66–1.59) | 0.882 | 0.52 (0.19–1.45) | 0.213 |
| Therapy | ||||
| β-Blockers | 1.34 (0.69–2.58) | 0.381 | 0.19 (0.04–0.92) | 0.040 |
| Ca2+-antagonists | 1.86 (0.82–4.22) | 0.135 | 0.05 (0.01–0.30) | 0.002 |
| ACE-inhibitors | 1.87 (0.91–3.88) | 0.088 | 0.04 (0.01–0.22) | <0.001 |
| Statins | 1.73 (0.76–3.91) | 0.190 | 0.04 (0.01–0.24) | 0.001 |
| Diuretics | 2.10 (0.99–4.47) | 0.052 | 0.06 (0.01–0.37) | 0.002 |
| LVEF | 0.96 (0.89–1.03) | 0.238 | 1.13 (0.94–1.35) | 0.194 |
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.05 (1.034–1.071) | <0.001 | 0.83 (0.80–86) | <0.001 |
| Male gender | 0.82 (0.44–1.51) | 0.52 | 6.47 (1.54–27) | 0.011 |
| Mean heart rate | 0.99 (0.96–1.03) | 0.77 | 0.94 (0.88–1.01) | 0.068 |
| CVRFs | ||||
| Hypertension | 2.78 (1.52–5.05) | 0.001 | 0.01 (0.01–0.04) | <0.001 |
| Dyslipidemia | 2.14 (1.15–3.99) | 0.017 | 0.12 (0.03–0.50) | 0.004 |
| Smoke | 0.57 (0.25–1.27) | 0.169 | 5.39 (0.79–36) | 0.084 |
| Obesity | 1.03 (0.66–1.59) | 0.882 | 0.52 (0.19–1.45) | 0.213 |
| Therapy | ||||
| β-Blockers | 1.34 (0.69–2.58) | 0.381 | 0.19 (0.04–0.92) | 0.040 |
| Ca2+-antagonists | 1.86 (0.82–4.22) | 0.135 | 0.05 (0.01–0.30) | 0.002 |
| ACE-inhibitors | 1.87 (0.91–3.88) | 0.088 | 0.04 (0.01–0.22) | <0.001 |
| Statins | 1.73 (0.76–3.91) | 0.190 | 0.04 (0.01–0.24) | 0.001 |
| Diuretics | 2.10 (0.99–4.47) | 0.052 | 0.06 (0.01–0.37) | 0.002 |
| LVEF | 0.96 (0.89–1.03) | 0.238 | 1.13 (0.94–1.35) | 0.194 |
CVRFs, cardiovascular risk factors; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction.
Clinical predictors of TO and TS in group 1 subjects at univariate linear regression analysis
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.05 (1.034–1.071) | <0.001 | 0.83 (0.80–86) | <0.001 |
| Male gender | 0.82 (0.44–1.51) | 0.52 | 6.47 (1.54–27) | 0.011 |
| Mean heart rate | 0.99 (0.96–1.03) | 0.77 | 0.94 (0.88–1.01) | 0.068 |
| CVRFs | ||||
| Hypertension | 2.78 (1.52–5.05) | 0.001 | 0.01 (0.01–0.04) | <0.001 |
| Dyslipidemia | 2.14 (1.15–3.99) | 0.017 | 0.12 (0.03–0.50) | 0.004 |
| Smoke | 0.57 (0.25–1.27) | 0.169 | 5.39 (0.79–36) | 0.084 |
| Obesity | 1.03 (0.66–1.59) | 0.882 | 0.52 (0.19–1.45) | 0.213 |
| Therapy | ||||
| β-Blockers | 1.34 (0.69–2.58) | 0.381 | 0.19 (0.04–0.92) | 0.040 |
| Ca2+-antagonists | 1.86 (0.82–4.22) | 0.135 | 0.05 (0.01–0.30) | 0.002 |
| ACE-inhibitors | 1.87 (0.91–3.88) | 0.088 | 0.04 (0.01–0.22) | <0.001 |
| Statins | 1.73 (0.76–3.91) | 0.190 | 0.04 (0.01–0.24) | 0.001 |
| Diuretics | 2.10 (0.99–4.47) | 0.052 | 0.06 (0.01–0.37) | 0.002 |
| LVEF | 0.96 (0.89–1.03) | 0.238 | 1.13 (0.94–1.35) | 0.194 |
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.05 (1.034–1.071) | <0.001 | 0.83 (0.80–86) | <0.001 |
| Male gender | 0.82 (0.44–1.51) | 0.52 | 6.47 (1.54–27) | 0.011 |
| Mean heart rate | 0.99 (0.96–1.03) | 0.77 | 0.94 (0.88–1.01) | 0.068 |
| CVRFs | ||||
| Hypertension | 2.78 (1.52–5.05) | 0.001 | 0.01 (0.01–0.04) | <0.001 |
| Dyslipidemia | 2.14 (1.15–3.99) | 0.017 | 0.12 (0.03–0.50) | 0.004 |
| Smoke | 0.57 (0.25–1.27) | 0.169 | 5.39 (0.79–36) | 0.084 |
| Obesity | 1.03 (0.66–1.59) | 0.882 | 0.52 (0.19–1.45) | 0.213 |
| Therapy | ||||
| β-Blockers | 1.34 (0.69–2.58) | 0.381 | 0.19 (0.04–0.92) | 0.040 |
| Ca2+-antagonists | 1.86 (0.82–4.22) | 0.135 | 0.05 (0.01–0.30) | 0.002 |
| ACE-inhibitors | 1.87 (0.91–3.88) | 0.088 | 0.04 (0.01–0.22) | <0.001 |
| Statins | 1.73 (0.76–3.91) | 0.190 | 0.04 (0.01–0.24) | 0.001 |
| Diuretics | 2.10 (0.99–4.47) | 0.052 | 0.06 (0.01–0.37) | 0.002 |
| LVEF | 0.96 (0.89–1.03) | 0.238 | 1.13 (0.94–1.35) | 0.194 |
CVRFs, cardiovascular risk factors; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction.
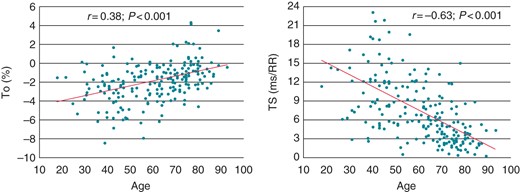
Correlation of age with TO (left) and TS (right) in individuals without any apparent heart disease.
At multivariable analysis, however, the only independent predictor of TO was age (OR, 1.05; CI: 1.02–1.07; P < 0.001), and the only independent predictors of TS were age (OR, 0.85; CI: 0.81–0.88; P < 0.001) and hypertension (OR, 0.19; CI: 0.04–0.83; P = 0.028).
Heart rate turbulence in coronary artery disease patients
In group 2 patients, the number of PVCs suitable of HRT analysis was 170 ± 202. Turbulence onset in this group was −0.73 ± 1.61%, while TS was 2.93 (1.73–5.81) ms/RR.
At univariate analysis, a significant, although modest, correlation was found on both HRT parameters with age (r = 0.24, P = 0.002 and r = −0.34, P < 0.001, for TO and TS, respectively; Figure 2), LVEF (r = −0.23, P = 0.012 and r = 0.43, P < 0.001, for TO and TS, respectively; Figure 3), and, for TS only, heart rate (r = −0.24, P = 0.01). Furthermore, higher TO values were observed in patients with a history of previous coronary artery by-pass surgery (P = 0.036) and in those treated with diuretic or antiarrhythmic drugs. Lower TS values were instead found in females and in patients with diabetes or hypertension (Table 3).
Clinical predictors of TO and TS in group 2 patients at univariate linear regression analysis
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.06) | 0.002 | 0.85 (0.81–0.90) | <0.001 |
| Male gender | 0.64 (0.35–1.18) | 0.15 | 5.12 (1.15–22.8) | 0.03 |
| Mean heart rate | 1.01 (0.98–1.04) | 0.35 | 0.87 (0.82–0.92) | 0.01 |
| CVRFs | ||||
| Diabetes | 1.54 (0.91–2.59) | 0.10 | 0.15 (0.04–0.52) | 0.003 |
| Hypertension | 1.48 (0.79–2.77) | 0.22 | 0.06 (0.02–0.27) | <0.001 |
| Dyslipidemia | 0.79 (0.46–1.35) | 0.39 | 1.34 (0.36–4.99) | 0.65 |
| Family history of CVD | 0.87 (0.52–1.45) | 0.59 | 0.94 (0.27–3.29) | 0.92 |
| Smoke | 0.96 (0.45–2.02) | 0.91 | 0.69 (0.11–4.29) | 0.68 |
| Obesity | 0.94 (0.67–1.31) | 0.70 | 1.43 (0.62–3.27) | 0.39 |
| Therapy | ||||
| β-Blockers | 1.02 (0.55–1.86) | 0.96 | 3.30 (0.76–14.3) | 0.11 |
| Ca2+-antagonists | 1.63 (0.88–3.01) | 0.11 | 0.18 (0.04–0.81) | 0.02 |
| ACE-inhibitors | 0.76 (0.46–1.26) | 0.29 | 1.96 (0.56–6.86) | 0.29 |
| ARA | 1.74 (0.97–3.13) | 0.06 | 0.21 (0.05–0.86) | 0.03 |
| Statins | 0.75 (0.41–1.37) | 0.35 | 1.96 (0.45–8.63) | 0.37 |
| Anti-platelets | 1.39 (0.37–5.23) | 0.62 | 3.91 (0.15–99) | 0.41 |
| Digoxin | 6.78 (0.71–64) | 0.09 | 0.07 (0.01–19) | 0.35 |
| Nitrates | 1.33 (0.65–2.73) | 0.43 | 0.40 (0.07–2.32) | 0.30 |
| Antiarrhythmics | 2.62 (1.22–5.64) | 0.014 | 0.20 (0.03–1.34) | 0.09 |
| Diuretics | 1.87 (1.12–3.04) | 0.016 | 0.17 (0.05–0.59) | 0.006 |
| LVEF | 0.97 (0.95–0.99) | 0.012 | 1.10 (1.04–1.16) | 0.001 |
| Previous AMI | 0.80 (0.43–1.50) | 0.48 | 1.52 (0.32–7.13) | 0.59 |
| Previous PCI | 0.68 (0.41–1.13) | 0.14 | 1.22 (0.34–4.33) | 0.75 |
| Previous CABG | 1.75 (1.04–2.95) | 0.036 | 0.35 (0.10–1.28) | 0.11 |
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.06) | 0.002 | 0.85 (0.81–0.90) | <0.001 |
| Male gender | 0.64 (0.35–1.18) | 0.15 | 5.12 (1.15–22.8) | 0.03 |
| Mean heart rate | 1.01 (0.98–1.04) | 0.35 | 0.87 (0.82–0.92) | 0.01 |
| CVRFs | ||||
| Diabetes | 1.54 (0.91–2.59) | 0.10 | 0.15 (0.04–0.52) | 0.003 |
| Hypertension | 1.48 (0.79–2.77) | 0.22 | 0.06 (0.02–0.27) | <0.001 |
| Dyslipidemia | 0.79 (0.46–1.35) | 0.39 | 1.34 (0.36–4.99) | 0.65 |
| Family history of CVD | 0.87 (0.52–1.45) | 0.59 | 0.94 (0.27–3.29) | 0.92 |
| Smoke | 0.96 (0.45–2.02) | 0.91 | 0.69 (0.11–4.29) | 0.68 |
| Obesity | 0.94 (0.67–1.31) | 0.70 | 1.43 (0.62–3.27) | 0.39 |
| Therapy | ||||
| β-Blockers | 1.02 (0.55–1.86) | 0.96 | 3.30 (0.76–14.3) | 0.11 |
| Ca2+-antagonists | 1.63 (0.88–3.01) | 0.11 | 0.18 (0.04–0.81) | 0.02 |
| ACE-inhibitors | 0.76 (0.46–1.26) | 0.29 | 1.96 (0.56–6.86) | 0.29 |
| ARA | 1.74 (0.97–3.13) | 0.06 | 0.21 (0.05–0.86) | 0.03 |
| Statins | 0.75 (0.41–1.37) | 0.35 | 1.96 (0.45–8.63) | 0.37 |
| Anti-platelets | 1.39 (0.37–5.23) | 0.62 | 3.91 (0.15–99) | 0.41 |
| Digoxin | 6.78 (0.71–64) | 0.09 | 0.07 (0.01–19) | 0.35 |
| Nitrates | 1.33 (0.65–2.73) | 0.43 | 0.40 (0.07–2.32) | 0.30 |
| Antiarrhythmics | 2.62 (1.22–5.64) | 0.014 | 0.20 (0.03–1.34) | 0.09 |
| Diuretics | 1.87 (1.12–3.04) | 0.016 | 0.17 (0.05–0.59) | 0.006 |
| LVEF | 0.97 (0.95–0.99) | 0.012 | 1.10 (1.04–1.16) | 0.001 |
| Previous AMI | 0.80 (0.43–1.50) | 0.48 | 1.52 (0.32–7.13) | 0.59 |
| Previous PCI | 0.68 (0.41–1.13) | 0.14 | 1.22 (0.34–4.33) | 0.75 |
| Previous CABG | 1.75 (1.04–2.95) | 0.036 | 0.35 (0.10–1.28) | 0.11 |
CVRFs, cardiovascular risk factors; CVD, cardiovascular disease; ACE, angiotensin converting enzyme; ARA, aldosterone receptor antagonists; LVEF, left ventricular ejection fraction; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft.
Clinical predictors of TO and TS in group 2 patients at univariate linear regression analysis
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.06) | 0.002 | 0.85 (0.81–0.90) | <0.001 |
| Male gender | 0.64 (0.35–1.18) | 0.15 | 5.12 (1.15–22.8) | 0.03 |
| Mean heart rate | 1.01 (0.98–1.04) | 0.35 | 0.87 (0.82–0.92) | 0.01 |
| CVRFs | ||||
| Diabetes | 1.54 (0.91–2.59) | 0.10 | 0.15 (0.04–0.52) | 0.003 |
| Hypertension | 1.48 (0.79–2.77) | 0.22 | 0.06 (0.02–0.27) | <0.001 |
| Dyslipidemia | 0.79 (0.46–1.35) | 0.39 | 1.34 (0.36–4.99) | 0.65 |
| Family history of CVD | 0.87 (0.52–1.45) | 0.59 | 0.94 (0.27–3.29) | 0.92 |
| Smoke | 0.96 (0.45–2.02) | 0.91 | 0.69 (0.11–4.29) | 0.68 |
| Obesity | 0.94 (0.67–1.31) | 0.70 | 1.43 (0.62–3.27) | 0.39 |
| Therapy | ||||
| β-Blockers | 1.02 (0.55–1.86) | 0.96 | 3.30 (0.76–14.3) | 0.11 |
| Ca2+-antagonists | 1.63 (0.88–3.01) | 0.11 | 0.18 (0.04–0.81) | 0.02 |
| ACE-inhibitors | 0.76 (0.46–1.26) | 0.29 | 1.96 (0.56–6.86) | 0.29 |
| ARA | 1.74 (0.97–3.13) | 0.06 | 0.21 (0.05–0.86) | 0.03 |
| Statins | 0.75 (0.41–1.37) | 0.35 | 1.96 (0.45–8.63) | 0.37 |
| Anti-platelets | 1.39 (0.37–5.23) | 0.62 | 3.91 (0.15–99) | 0.41 |
| Digoxin | 6.78 (0.71–64) | 0.09 | 0.07 (0.01–19) | 0.35 |
| Nitrates | 1.33 (0.65–2.73) | 0.43 | 0.40 (0.07–2.32) | 0.30 |
| Antiarrhythmics | 2.62 (1.22–5.64) | 0.014 | 0.20 (0.03–1.34) | 0.09 |
| Diuretics | 1.87 (1.12–3.04) | 0.016 | 0.17 (0.05–0.59) | 0.006 |
| LVEF | 0.97 (0.95–0.99) | 0.012 | 1.10 (1.04–1.16) | 0.001 |
| Previous AMI | 0.80 (0.43–1.50) | 0.48 | 1.52 (0.32–7.13) | 0.59 |
| Previous PCI | 0.68 (0.41–1.13) | 0.14 | 1.22 (0.34–4.33) | 0.75 |
| Previous CABG | 1.75 (1.04–2.95) | 0.036 | 0.35 (0.10–1.28) | 0.11 |
| . | TO . | P-value . | TS . | P-value . |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.06) | 0.002 | 0.85 (0.81–0.90) | <0.001 |
| Male gender | 0.64 (0.35–1.18) | 0.15 | 5.12 (1.15–22.8) | 0.03 |
| Mean heart rate | 1.01 (0.98–1.04) | 0.35 | 0.87 (0.82–0.92) | 0.01 |
| CVRFs | ||||
| Diabetes | 1.54 (0.91–2.59) | 0.10 | 0.15 (0.04–0.52) | 0.003 |
| Hypertension | 1.48 (0.79–2.77) | 0.22 | 0.06 (0.02–0.27) | <0.001 |
| Dyslipidemia | 0.79 (0.46–1.35) | 0.39 | 1.34 (0.36–4.99) | 0.65 |
| Family history of CVD | 0.87 (0.52–1.45) | 0.59 | 0.94 (0.27–3.29) | 0.92 |
| Smoke | 0.96 (0.45–2.02) | 0.91 | 0.69 (0.11–4.29) | 0.68 |
| Obesity | 0.94 (0.67–1.31) | 0.70 | 1.43 (0.62–3.27) | 0.39 |
| Therapy | ||||
| β-Blockers | 1.02 (0.55–1.86) | 0.96 | 3.30 (0.76–14.3) | 0.11 |
| Ca2+-antagonists | 1.63 (0.88–3.01) | 0.11 | 0.18 (0.04–0.81) | 0.02 |
| ACE-inhibitors | 0.76 (0.46–1.26) | 0.29 | 1.96 (0.56–6.86) | 0.29 |
| ARA | 1.74 (0.97–3.13) | 0.06 | 0.21 (0.05–0.86) | 0.03 |
| Statins | 0.75 (0.41–1.37) | 0.35 | 1.96 (0.45–8.63) | 0.37 |
| Anti-platelets | 1.39 (0.37–5.23) | 0.62 | 3.91 (0.15–99) | 0.41 |
| Digoxin | 6.78 (0.71–64) | 0.09 | 0.07 (0.01–19) | 0.35 |
| Nitrates | 1.33 (0.65–2.73) | 0.43 | 0.40 (0.07–2.32) | 0.30 |
| Antiarrhythmics | 2.62 (1.22–5.64) | 0.014 | 0.20 (0.03–1.34) | 0.09 |
| Diuretics | 1.87 (1.12–3.04) | 0.016 | 0.17 (0.05–0.59) | 0.006 |
| LVEF | 0.97 (0.95–0.99) | 0.012 | 1.10 (1.04–1.16) | 0.001 |
| Previous AMI | 0.80 (0.43–1.50) | 0.48 | 1.52 (0.32–7.13) | 0.59 |
| Previous PCI | 0.68 (0.41–1.13) | 0.14 | 1.22 (0.34–4.33) | 0.75 |
| Previous CABG | 1.75 (1.04–2.95) | 0.036 | 0.35 (0.10–1.28) | 0.11 |
CVRFs, cardiovascular risk factors; CVD, cardiovascular disease; ACE, angiotensin converting enzyme; ARA, aldosterone receptor antagonists; LVEF, left ventricular ejection fraction; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft.
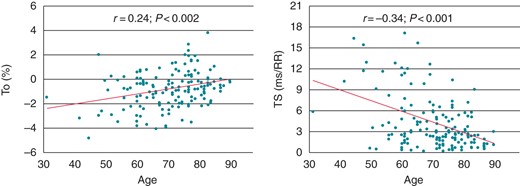
Correlation of age with TO (left) and TS (right) in patients with stable coronary artery disease.
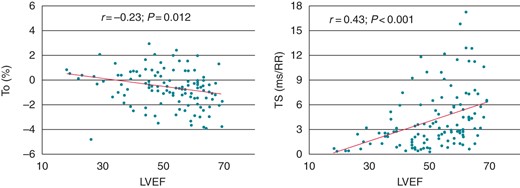
Correlation of LVEF with TO (left) and TS (right) in patients with stable coronary artery disease.
At multivariable analysis, the only independent predictor of TO was age (OR, 1.03; CI: 1.002–1.06; P = 0.038), whereas independent predictors of TS were age (OR, 0.90; CI: 0.84–0.95; P = 0.001) and LVEF (OR, 1.07; CI: 1.02–1.13; P = 0.008).
Between-group comparisons
Values of both HRT parameters were significantly different in group 1 and group 2 patients, being worse in the latter (P < 0.001 for both TO and TS).
An abnormal TO (>0%) was found in 26 group 1 subjects (12.4%) and 42 group 2 patients (26.8%; P = 0.001), whereas an abnormal TS (<2.5 ms/RR) was found in 31 group 1 subjects (14.8%) and 64 group 2 patients (40.8%; P = 0.001), respectively. Furthermore, HRT categories 0, 1, and 2 were detected in 160 (76.6%), 41 (19.6%), and 8 (3.8%) subjects of group 1, but in 78 (49.7%), 52 (33.1%), and 27 (17.2%) patients of group 2, respectively (P < 0.001). All these findings remained unchanged when only patients with frequent PVCs (≥10/h) were included in the analyses (data not shown).
In multivariable linear regression analysis, presence of CAD (group 2) showed an independent association with TO (OR, 2.23; P = 0.02), but not with TS (OR, 0.40; P = 0.18). Moreover, in multivariable logistic regression analysis, the variable ‘group’ did not show a significant association with both ‘abnormal’ TO and TS values and with HRT category 1 or 2, whereas age (P<0.001) and LVEF (P < 0.001) remained the only independent predictor of HRT parameters.
Comparison of matched groups
Two samples of 65 subjects of the two groups could be matched for age and gender. As shown in Table 4, the two groups were also comparable with regard to CVRFs, whereas HRT parameters were significantly different between the two groups.
| Variable . | Group 1 (n = 65) . | Group 2 (n = 65) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 6 | 67 ± 9 | 1 |
| Men (%) | 50 | 50 | 1 |
| Holter data | |||
| PVCs (Nr/24 h) | 1120 ± 1969 | 1246 ± 1816 | 0.70 |
| Processed PVCs (Nr/24 h) | 150 ± 156 | 178 ± 218 | 0.43 |
| Mean heart rate (b.p.m.) | 70 ± 9 | 69 ± 9 | 0.40 |
| NSVT (%) | 1 | 16 | 0.004 |
| CVRFs | |||
| Diabetes (%) | 0 | 0 | 1 |
| Hypertension (%) | 69 | 77 | 0.42 |
| Dyslipidemia (%) | 58 | 72 | 0.13 |
| Smoking (%) | 19 | 19 | 1 |
| Drug therapy | |||
| β-Blockers (%) | 42 | 76 | <0.001 |
| ACE-inhibitors (%) | 35 | 48 | 0.15 |
| Statins (%) | 28 | 77 | <0.001 |
| Diuretics (%) | 41 | 50 | 0.37 |
| LVEF (%) | 61 ± 6 | 50 ± 14 | <0.001 |
| HRT parameters | |||
| TO (%) | −1.24 ± 2.2 | –0.53 ± 1.2 | 0.03 |
| TS (ms/RR) | 5.08 (2.95–7.30) | 2.18 (1.19–4.64) | <0.001 |
| Variable . | Group 1 (n = 65) . | Group 2 (n = 65) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 6 | 67 ± 9 | 1 |
| Men (%) | 50 | 50 | 1 |
| Holter data | |||
| PVCs (Nr/24 h) | 1120 ± 1969 | 1246 ± 1816 | 0.70 |
| Processed PVCs (Nr/24 h) | 150 ± 156 | 178 ± 218 | 0.43 |
| Mean heart rate (b.p.m.) | 70 ± 9 | 69 ± 9 | 0.40 |
| NSVT (%) | 1 | 16 | 0.004 |
| CVRFs | |||
| Diabetes (%) | 0 | 0 | 1 |
| Hypertension (%) | 69 | 77 | 0.42 |
| Dyslipidemia (%) | 58 | 72 | 0.13 |
| Smoking (%) | 19 | 19 | 1 |
| Drug therapy | |||
| β-Blockers (%) | 42 | 76 | <0.001 |
| ACE-inhibitors (%) | 35 | 48 | 0.15 |
| Statins (%) | 28 | 77 | <0.001 |
| Diuretics (%) | 41 | 50 | 0.37 |
| LVEF (%) | 61 ± 6 | 50 ± 14 | <0.001 |
| HRT parameters | |||
| TO (%) | −1.24 ± 2.2 | –0.53 ± 1.2 | 0.03 |
| TS (ms/RR) | 5.08 (2.95–7.30) | 2.18 (1.19–4.64) | <0.001 |
PVCs, premature ventricular complexes; NSVT, non-sustained ventricular tachycardia; CVRFs, cardiovascular risk factors; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction; HRT, heart rate turbulence; TO, turbulence onset; TS, turbulence slope.
| Variable . | Group 1 (n = 65) . | Group 2 (n = 65) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 6 | 67 ± 9 | 1 |
| Men (%) | 50 | 50 | 1 |
| Holter data | |||
| PVCs (Nr/24 h) | 1120 ± 1969 | 1246 ± 1816 | 0.70 |
| Processed PVCs (Nr/24 h) | 150 ± 156 | 178 ± 218 | 0.43 |
| Mean heart rate (b.p.m.) | 70 ± 9 | 69 ± 9 | 0.40 |
| NSVT (%) | 1 | 16 | 0.004 |
| CVRFs | |||
| Diabetes (%) | 0 | 0 | 1 |
| Hypertension (%) | 69 | 77 | 0.42 |
| Dyslipidemia (%) | 58 | 72 | 0.13 |
| Smoking (%) | 19 | 19 | 1 |
| Drug therapy | |||
| β-Blockers (%) | 42 | 76 | <0.001 |
| ACE-inhibitors (%) | 35 | 48 | 0.15 |
| Statins (%) | 28 | 77 | <0.001 |
| Diuretics (%) | 41 | 50 | 0.37 |
| LVEF (%) | 61 ± 6 | 50 ± 14 | <0.001 |
| HRT parameters | |||
| TO (%) | −1.24 ± 2.2 | –0.53 ± 1.2 | 0.03 |
| TS (ms/RR) | 5.08 (2.95–7.30) | 2.18 (1.19–4.64) | <0.001 |
| Variable . | Group 1 (n = 65) . | Group 2 (n = 65) . | P-value . |
|---|---|---|---|
| Age (years) | 67 ± 6 | 67 ± 9 | 1 |
| Men (%) | 50 | 50 | 1 |
| Holter data | |||
| PVCs (Nr/24 h) | 1120 ± 1969 | 1246 ± 1816 | 0.70 |
| Processed PVCs (Nr/24 h) | 150 ± 156 | 178 ± 218 | 0.43 |
| Mean heart rate (b.p.m.) | 70 ± 9 | 69 ± 9 | 0.40 |
| NSVT (%) | 1 | 16 | 0.004 |
| CVRFs | |||
| Diabetes (%) | 0 | 0 | 1 |
| Hypertension (%) | 69 | 77 | 0.42 |
| Dyslipidemia (%) | 58 | 72 | 0.13 |
| Smoking (%) | 19 | 19 | 1 |
| Drug therapy | |||
| β-Blockers (%) | 42 | 76 | <0.001 |
| ACE-inhibitors (%) | 35 | 48 | 0.15 |
| Statins (%) | 28 | 77 | <0.001 |
| Diuretics (%) | 41 | 50 | 0.37 |
| LVEF (%) | 61 ± 6 | 50 ± 14 | <0.001 |
| HRT parameters | |||
| TO (%) | −1.24 ± 2.2 | –0.53 ± 1.2 | 0.03 |
| TS (ms/RR) | 5.08 (2.95–7.30) | 2.18 (1.19–4.64) | <0.001 |
PVCs, premature ventricular complexes; NSVT, non-sustained ventricular tachycardia; CVRFs, cardiovascular risk factors; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction; HRT, heart rate turbulence; TO, turbulence onset; TS, turbulence slope.
In multivariable linear regression analysis, CAD group showed an independent association with TO (OR, 2.43; P = 0.028), but not with TS (OR, 0.31; P = 0.22). However, age (P < 0.05) and LVEF (P < 0.05), but not CAD group, were independent predictors of abnormal TO and TS values and of HRT category 1 or 2 in these matched selected individuals.
Discussion
Two main findings emerge from our results. First, our data showed that, in subjects without any apparent cardiac disease, only age and, in part, hypertension significantly influence in an independent way HRT parameters. Second, age remains a major modulator of HRT in CAD patients, in whom LVEF also assumes a major role.
Heart rate turbulence is an autonomically mediated phenomenon with a close relationship to baroreflex mechanisms. Premature ventricular complexes, indeed, produce a fall in blood pressure, which results in vagal withdrawal and sympathetic activation, with an initial acceleration of sinus node activity and return of blood pressure to normal values; this in turn results in baroreceptor stimulation with vagal recruitment and sympathetic inhibition, thus leading to a later deceleration of sinus node activity.2
Increasing age has consistently been shown to be associated with a decrease in cardiac vagal modulation, using various measures of cardiac autonomic function, as basal heart rate and heart rate variability, which increase and decrease with age, respectively.19 Accordingly, some previous studies, in restricted populations of subjects, showed that age was a significant determinant of HRT values.15–17 These findings are confirmed in our study, in which age was the most powerful independent predictor of HRT in subjects without cardiac disease, with increasing age being associated with progressively higher TO and lower TS values, respectively.
Furthermore, our study is the first to show that HRT is largely independent of CVRFs, as well as medical therapy, in apparently healthy individuals. Until now, the relation between CVRFs and HRT parameters in healthy people had indeed poorly been investigated, with the few studies including only small samples of volunteers.15–18 Our study shows that only hypertension has an independent, although weak, association with abnormal HRT, in agreement with the well-known impairment of baroreflex sensitivity observed in hypertensive patients.20 Of note, hypertension independently influenced TS but not TO, suggesting that the abnormality related to hypertension resides in the reflex mechanism that follows an increased, rather than in the one that follows a decreased, stimulation of baroceptors.
A further main finding of our study is that an impaired LVEF is the only other major determinant of HRT in stable CAD patients. The latter result is not surprising, as a reduced LV function has consistently been found to be a major determinant of sympatho-vagal imbalance in several clinical settings, including acute and old MI and dilated cardiomyopathy,21,22 and a relation of HRT with LVEF was already shown in our previous study.17 Of note, the presence of a previous acute MI was instead by itself insufficient to significantly influence HRT values, suggesting that it may exert its influence on HRT only when it is large enough to impair LV function. Similarly, although HRT showed significantly different values in CAD compared with healthy subjects, CAD presence was only a weak predictor of HRT, being independently associated only with TO, when considered as a continuous variable, in multivariable analyses.
Of note, the poor relation of obstructive CAD by itself with HRT parameters was confirmed when comparing two subgroups of non-cardiac subjects and of CAD patients selected for being similar in age and gender. In the latter subgroup differences were again found in HRT parameters, but statistical significance was lost when corrected for LVEF, in agreement with the independent association of LVEF with HRT parameters in the whole sample of CAD patients.
Some limitations of our study should be acknowledged. First, we cannot be sure that all apparently ‘healthy’ individuals did not have any form of CAD, as most of them had some CVRF. However, the lack of any symptom and the evidence of normal ECG, echocardiography, and maximal exercise stress test makes unlikely that a sizeable proportion had significant CAD.
Second, several subjects were being treated with cardiologic drugs, in particular β-blockers, which might have influenced HRT. However, drugs did not emerge as correlated with HRT in multivariable analyses, suggesting that their influence is, if any, limited. Our finding is, in fact, in agreement with the lack of significant effect of beta-blockade on HRT parameters found in previous studies of apparently healthy individuals.4
Conclusions
In conclusion, our study shows that age is a major determinant of HRT values both in subjects without any apparent cardiac disease and in CAD patients. Only hypertension, among CVRFs, contributes significantly, although in a moderate way, to HRT in people without cardiac disease, whereas LVEF becomes a major predictor of HRT in CAD patients.
Conflict of interest: none declared.



