-
PDF
- Split View
-
Views
-
Cite
Cite
Fiorenzo Gaita, Davide Sardi, Alberto Battaglia, Cristina Gallo, Elisabetta Toso, Arianna Michielon, Domenico Caponi, Lucia Garberoglio, Davide Castagno, Marco Scaglione, Incidence of cerebral thromboembolic events during long-term follow-up in patients treated with transcatheter ablation for atrial fibrillation, EP Europace, Volume 16, Issue 7, July 2014, Pages 980–986, https://doi.org/10.1093/europace/eut406
Close - Share Icon Share
Abstract
Net clinical benefit of long-term oral anticoagulation therapy (OAT) continuation after successful atrial fibrillation (AF) ablation is still controversial. To evaluate long-term thromboembolic (TE) and haemorrhagic events incidence according to OAT strategy used after AF transcatheter ablation.
Three months after AF ablation, OAT was discontinued in patients with CHADS2 ≤ 1 if no recurrences were documented, while OAT was maintained in patients with CHADS2 ≥ 2 regardless of AF recurrences. CHA2DS2VASc and HAS-BLED scores have been retrospectively evaluated. Seven hundred and sixty-six patients were followed for a median of 60.5 months. Six (6/267 = 2.2%) and five (5/499 = 1%) TE events occurred in the ON and the OFF-OAT patients, respectively (P = 0.145), all in concomitance with the AF recurrence. CHADS2 and CHA2DS2VASc ≥ 2 were associated with high TE incidence (P = 0.047 and P = 0.020). Among patients with a CHADS2 score of 0 or 1, a CHA2DS2VASc score ≥ 2 was predictive of TE events (P = 0.014). Overall, the incidence of the TE events in patients with CHA2DS2VASc ≥ 2 was 0.6 per 100 patient-years whereas seven haemorrhagic events occurred, all of them in the ON-OAT patients (7/267 = 2.6%).
Patients with AF undergoing transcatheter ablation have a lower incidence of TE events as compared with the general AF population, regardless of OAT maintenance. The unpredictable risk of AF recurrence, mandate the routine use of the CHADS2, CHA2DS2VASc, and HAS-BLED scores to guide clinical decision regarding OAT management in this peculiar setting of patients. The potential protective role of rhythm control strategy in the TE events needs to be confirmed by future large randomized trials.
The lower thromboembolic (TE) event incidence in post-ablation atrial fibrillation (AF) patients speculating the potential protective role of sinus rhythm maintenance.
The CHA2DS2VASc score had additional predictive value stratifying the TE events risk in the AF patients compared with CHADS2.
CHADS2, CHA2DS2VASc, and HAS-BLED should guide clinician choice about oral anticoagulation therapy strategy for each patient.
Introduction
Atrial fibrillation (AF) represents the most common supraventricular tachycardia, and it is associated with an increased risk of cerebral and systemic thromboembolic (TE) events.1,2 Atrial fibrillation transcatheter ablation has emerged as a promising therapeutic strategy for patients experiencing symptomatic AF recurrences despite antiarrhythmic drug therapy.3 Whether oral anticoagulation therapy (OAT) should be withheld after an apparently successful AF transcatheter ablation is still uncertain. The ESC/AHA guidelines1,4 recommend anticoagulation for a minimum of 3 months following transcatheter AF ablation and, after this period, decision about maintenance of the OAT should be based on individual TE risk assessment and the instrumental parameters (e.g. electrocardiographic and echocardiographic). A controversial point is whether post-transcatheter ablation OAT should be maintained in patients at high TE risk.5 Owing to the unpredictable risk of AF recurrences, even after AF transcatheter ablation, our policy is to propose OAT maintenance in high TE risk patients. Whether the maintenance of the sinus rhythm by means of a successful AF transcatheter ablation exerts a protective role on the TE events needs verification. The aim of our large observational single-centre study was to evaluate the incidence of the TE/haemorrhagic events according to the OAT strategy used during a long-term follow-up in a population of patients with AF who underwent transcatheter ablation.
Methods
Consecutive patients referred to our centre between 2001 and 2009 for AF transcatheter ablation were included in the AF Registry of our institution and in this observational study. All the patients gave written informed consent for participation in the study, which was approved by the institutional ethic committee and was performed according to the principles of the Declaration of Helsinki. Patients with hypertrophic cardiomyopathy, valvular heart disease, and prosthetic heart valves were excluded. Individual TE risk profile was stratified by using the CHADS2 score. Hence, we divided the study population into two groups: patients with low–moderate TE risk (CHADS2 between 0 and 1) and patients with high TE risk (CHADS2 score ≥ 2). Furthermore, the individual TE risk profile was also retrospectively evaluated by means of the CHA2DS2VASc scoring system and the haemorrhagic risk profile was evaluated with the HAS-BLED score.
Transcatheter ablation strategy
The procedural details have been reported elsewhere.6 In brief, each procedure was guided by a three-dimensional reconstruction of the left atrium and the pulmonary vein (PV) ostia with the use of electroanatomical mapping systems, CARTO (Biosense Webster) or Navx (St Jude Medical) and it was performed under conscious sedation. Paroxysmal AF patients underwent antral PV isolation (PVI) and, in case of redo procedure, linear lesions were performed. Redo procedures were proposed for patients with symptomatic recurrences considering patient's preference with the aim of reducing AF-related symptoms and improving the quality of life. In patients with persistent AF, a line connecting the superior PVs and a line from the left inferior PV to the mitralic annulus linear lesions (‘7’ scheme) were performed in addition to the PVI. If this approach was not sufficient to restore the sinus rhythm, since 2004, complex fragmented atrial electrograms were pursued and ablated. Radiofrequency was applied with the use of an open irrigated-tip catheter and delivered with an energy between 20 and 40 W, according to the localization. Antral PVI was confirmed by an exit block on the mapping catheter.
Anticoagulation strategy
All the patients underwent transoesophageal echocardiography, prior to the AF transcatheter ablation, to rule out the presence of atrial thrombi. After the transcatheter AF ablation, unfractionated heparin was administered for the first 24 h (aiming for an activated partial thromboplastin time between 60 and 80 s). Subsequently, oral anticoagulation was started together with low-molecular-weight heparin at an anticoagulant dose that was discontinued when the international normalized ratio (INR) reached the target (between 2 and 3). In all the cases, OAT was continued for at least 3 months after the procedure (considered as the blanking period). If no AF recurrences were detected during 3 months after the procedure, OAT was discontinued in patients with CHADS2 ≤ 1, except in a minority of cases in which OAT was maintained because of the referring physician's advice or the comorbidities. We considered as a recurrence the presence of a sustained AF/atrial flutter lasting >30 s either symptomatically or documented by means of an electrocardiogram (ECG) or 24 h Holter monitoring. Patients with a CHADS2 score ≤ 1 with late AF recurrences restarted the OAT and a redo procedure was proposed, with the attempt of discontinuing OAT after 3 months from the redo procedure. In patients with a CHADS2 score ≥ 2, regardless of the AF recurrences, OAT was maintained, except in case of the patient's refusal or a very high haemorrhagic risk. After the OAT discontinuation, acetylsalicylic acid (ASA) 75–325 mg/die was initiated if not contraindicated. The enrolment period lasts until 2009 when novel oral anticoagulants were not commercially available yet. As a consequence, none of the patients enroled were assuming these drugs.
Follow-up management
Follow-up data have been collected during ambulatory visits, by means of 24 h/7 days Holter monitorings (performed at 1, 3, 6 months, and then yearly) or by telephone follow-up and in a little percentage of patients (2.0%) by continuous ECG monitoring through implantable devices interrogations. Cerebral thromboembolic event was defined as a neurological deficit, transient or permanent, with evidence of infarction at cerebral imaging.7 Major haemorrhagic event occurring during the follow-up was defined, according to the latest ISTH definition of major bleeding, as a fatal bleeding, and/or symptomatic bleeding in a critical area or organ (such as intracranial or gastrointestinal), and/or bleeding leading to transfusion of two or more units of whole blood or red cells.8 In case of transient or permanent neurological deficit or haemorrhagic events reported by the patients, they were invited to a clinical ambulatory visit at our institution or they were asked to send us all the relevant documentation. All the patients with neurological deficit during the follow-up underwent a brain imaging during the acute phase and in the post-acute phase with a specialist visit by the neurologist.
Statistical analysis
Categorical variables are reported as count and percentages, while continuous variables are reported as median and interquartile range (IQR). Correlations between the two groups were tested in the cross-tabulations tables by means of the Pearson χ2 or Fisher's exact test and by one-way analysis of variance, respectively, for the categorical and the continuous variables. The performance of the CHADS2 and the CHA2DS2VASc scores in predicting the TE events was tested by means of receiver operating characteristic (ROC) curves and calculation of the area under the curve (AUC). A two-sided P value <0.05 was considered statistically significant; all the analyses were performed with SPSS 16.0 (SPSS Inc.).
Results
Seven hundred and sixty-six patients (612 males 79.9%, mean age 57 ± 11 years) were followed up for a median of 60.5 months (IQR 41.0–83.0). The clinical characteristics of the study population are presented in Table 1. The ON-OAT group consisted of 267 (35%) patients that were older with a higher prevalence of persistent AF and structural heart disease compared with the OFF-OAT group. The OFF-OAT group consisted of 499 (65%) patients who discontinued the OAT 3 months after the AF transcatheter ablation and among them 269 (54%) started the ASA therapy. Stratification of the patients according to the individual TE risk profile and OAT continuation or discontinuation are shown in Table 2. Fifteen (2.0%) out of the 766 patients had a loop recorder implanted and transtelephonic monitoring was performed every month with recording of all the symptoms reported by the patients.
Clinical characteristics of the study population at the time of the AF transcatheter ablation
| . | Total population n = 766 patients (%) . | ON-OAT group n = 267 patients (%) . | OFF-OAT group n = 499 patients (%) . | P value . |
|---|---|---|---|---|
| Male sex | 612 (79.9) | 192 (71.9) | 420 (84.2) | <0.01 |
| Age, years ± SD | 57 ± 11 | 61 ± 10 | 55 ± 11 | 0.021 |
| Paroxysmal AF | 326 (42.6) | 91 (34.1) | 235 (47.1) | <0.01 |
| Persistent AF | 440 (57.4) | 176 (65.9) | 264 (52.9) | <0.01 |
| Structural heart disease | 69 (9.0) | 33 (12.4) | 36 (7.2) | 0.026 |
| Congestive heart failure | 28 (3.7) | 17 (6.4) | 11 (2.2) | |
| Hypertension | 362 (47.3) | 161 (60.3) | 201 (40.3) | |
| Age ≥ 65 years | 216 (28.2) | 117 (43.8) | 99 (19.8) | |
| Age ≥ 75 years | 23 (3.0) | 13 (4.9) | 10 (2.0) | |
| Diabetes | 32 (4.2) | 15 (5.6) | 17 (3.4) | |
| Previous stroke/TIA | 69 (9.0) | 53 (19.9) | 16 (3.2) | |
| Female sex | 154 (20.1) | 75 (28.1) | 79 (15.8) | |
| Vascular disease | 74 (9.7) | 35 (13.1) | 39 (7.8) | |
| Liver and/or kidney disease | 3 (0.4) | 2 (0.7) | 1 (0.2) | |
| Bleeding | 2 (0.3) | 2 (0.7) | 0 (0.0) | |
| Drugs and/or alcohol | 90 (11.7) | 20 (7.4) | 70 (14.0) | |
| Labile INR | 122 (15.9) | 122 (45.7) | – | |
| CHADS2 score | <0.01 | |||
| ≤1 | 646 (84.3) | 188 (70.4) | 458 (91.8) | |
| ≥2 | 120 (15.7) | 79 (29.6) | 41 (8.2) | |
| ≥3 | 43 (5.6) | 34 (12.7) | 9 (1.8) | |
| CHA2DS2VASc score | <0.01 | |||
| ≤1 | 478 (62.4) | 101 (37.8) | 377 (75.6) | |
| ≥2 | 288 (37.6) | 166 (62.2) | 122 (24.5) | |
| ≥3 | 127 (16.6) | 87 (32.6) | 40 (8.0) | |
| HASBLED score | <0.01 | |||
| ≤1 | 605 (79.0) | 159 (59.6) | 446 (89.4) | |
| ≥2 | 161 (21.0) | 108 (40.4) | 53 (10.6) | |
| . | Total population n = 766 patients (%) . | ON-OAT group n = 267 patients (%) . | OFF-OAT group n = 499 patients (%) . | P value . |
|---|---|---|---|---|
| Male sex | 612 (79.9) | 192 (71.9) | 420 (84.2) | <0.01 |
| Age, years ± SD | 57 ± 11 | 61 ± 10 | 55 ± 11 | 0.021 |
| Paroxysmal AF | 326 (42.6) | 91 (34.1) | 235 (47.1) | <0.01 |
| Persistent AF | 440 (57.4) | 176 (65.9) | 264 (52.9) | <0.01 |
| Structural heart disease | 69 (9.0) | 33 (12.4) | 36 (7.2) | 0.026 |
| Congestive heart failure | 28 (3.7) | 17 (6.4) | 11 (2.2) | |
| Hypertension | 362 (47.3) | 161 (60.3) | 201 (40.3) | |
| Age ≥ 65 years | 216 (28.2) | 117 (43.8) | 99 (19.8) | |
| Age ≥ 75 years | 23 (3.0) | 13 (4.9) | 10 (2.0) | |
| Diabetes | 32 (4.2) | 15 (5.6) | 17 (3.4) | |
| Previous stroke/TIA | 69 (9.0) | 53 (19.9) | 16 (3.2) | |
| Female sex | 154 (20.1) | 75 (28.1) | 79 (15.8) | |
| Vascular disease | 74 (9.7) | 35 (13.1) | 39 (7.8) | |
| Liver and/or kidney disease | 3 (0.4) | 2 (0.7) | 1 (0.2) | |
| Bleeding | 2 (0.3) | 2 (0.7) | 0 (0.0) | |
| Drugs and/or alcohol | 90 (11.7) | 20 (7.4) | 70 (14.0) | |
| Labile INR | 122 (15.9) | 122 (45.7) | – | |
| CHADS2 score | <0.01 | |||
| ≤1 | 646 (84.3) | 188 (70.4) | 458 (91.8) | |
| ≥2 | 120 (15.7) | 79 (29.6) | 41 (8.2) | |
| ≥3 | 43 (5.6) | 34 (12.7) | 9 (1.8) | |
| CHA2DS2VASc score | <0.01 | |||
| ≤1 | 478 (62.4) | 101 (37.8) | 377 (75.6) | |
| ≥2 | 288 (37.6) | 166 (62.2) | 122 (24.5) | |
| ≥3 | 127 (16.6) | 87 (32.6) | 40 (8.0) | |
| HASBLED score | <0.01 | |||
| ≤1 | 605 (79.0) | 159 (59.6) | 446 (89.4) | |
| ≥2 | 161 (21.0) | 108 (40.4) | 53 (10.6) | |
AF, atrial fibrillation; OAT, oral anticoagulation therapy; TIA, transient ischaemic attack; INR, international normalized ratio.
Clinical characteristics of the study population at the time of the AF transcatheter ablation
| . | Total population n = 766 patients (%) . | ON-OAT group n = 267 patients (%) . | OFF-OAT group n = 499 patients (%) . | P value . |
|---|---|---|---|---|
| Male sex | 612 (79.9) | 192 (71.9) | 420 (84.2) | <0.01 |
| Age, years ± SD | 57 ± 11 | 61 ± 10 | 55 ± 11 | 0.021 |
| Paroxysmal AF | 326 (42.6) | 91 (34.1) | 235 (47.1) | <0.01 |
| Persistent AF | 440 (57.4) | 176 (65.9) | 264 (52.9) | <0.01 |
| Structural heart disease | 69 (9.0) | 33 (12.4) | 36 (7.2) | 0.026 |
| Congestive heart failure | 28 (3.7) | 17 (6.4) | 11 (2.2) | |
| Hypertension | 362 (47.3) | 161 (60.3) | 201 (40.3) | |
| Age ≥ 65 years | 216 (28.2) | 117 (43.8) | 99 (19.8) | |
| Age ≥ 75 years | 23 (3.0) | 13 (4.9) | 10 (2.0) | |
| Diabetes | 32 (4.2) | 15 (5.6) | 17 (3.4) | |
| Previous stroke/TIA | 69 (9.0) | 53 (19.9) | 16 (3.2) | |
| Female sex | 154 (20.1) | 75 (28.1) | 79 (15.8) | |
| Vascular disease | 74 (9.7) | 35 (13.1) | 39 (7.8) | |
| Liver and/or kidney disease | 3 (0.4) | 2 (0.7) | 1 (0.2) | |
| Bleeding | 2 (0.3) | 2 (0.7) | 0 (0.0) | |
| Drugs and/or alcohol | 90 (11.7) | 20 (7.4) | 70 (14.0) | |
| Labile INR | 122 (15.9) | 122 (45.7) | – | |
| CHADS2 score | <0.01 | |||
| ≤1 | 646 (84.3) | 188 (70.4) | 458 (91.8) | |
| ≥2 | 120 (15.7) | 79 (29.6) | 41 (8.2) | |
| ≥3 | 43 (5.6) | 34 (12.7) | 9 (1.8) | |
| CHA2DS2VASc score | <0.01 | |||
| ≤1 | 478 (62.4) | 101 (37.8) | 377 (75.6) | |
| ≥2 | 288 (37.6) | 166 (62.2) | 122 (24.5) | |
| ≥3 | 127 (16.6) | 87 (32.6) | 40 (8.0) | |
| HASBLED score | <0.01 | |||
| ≤1 | 605 (79.0) | 159 (59.6) | 446 (89.4) | |
| ≥2 | 161 (21.0) | 108 (40.4) | 53 (10.6) | |
| . | Total population n = 766 patients (%) . | ON-OAT group n = 267 patients (%) . | OFF-OAT group n = 499 patients (%) . | P value . |
|---|---|---|---|---|
| Male sex | 612 (79.9) | 192 (71.9) | 420 (84.2) | <0.01 |
| Age, years ± SD | 57 ± 11 | 61 ± 10 | 55 ± 11 | 0.021 |
| Paroxysmal AF | 326 (42.6) | 91 (34.1) | 235 (47.1) | <0.01 |
| Persistent AF | 440 (57.4) | 176 (65.9) | 264 (52.9) | <0.01 |
| Structural heart disease | 69 (9.0) | 33 (12.4) | 36 (7.2) | 0.026 |
| Congestive heart failure | 28 (3.7) | 17 (6.4) | 11 (2.2) | |
| Hypertension | 362 (47.3) | 161 (60.3) | 201 (40.3) | |
| Age ≥ 65 years | 216 (28.2) | 117 (43.8) | 99 (19.8) | |
| Age ≥ 75 years | 23 (3.0) | 13 (4.9) | 10 (2.0) | |
| Diabetes | 32 (4.2) | 15 (5.6) | 17 (3.4) | |
| Previous stroke/TIA | 69 (9.0) | 53 (19.9) | 16 (3.2) | |
| Female sex | 154 (20.1) | 75 (28.1) | 79 (15.8) | |
| Vascular disease | 74 (9.7) | 35 (13.1) | 39 (7.8) | |
| Liver and/or kidney disease | 3 (0.4) | 2 (0.7) | 1 (0.2) | |
| Bleeding | 2 (0.3) | 2 (0.7) | 0 (0.0) | |
| Drugs and/or alcohol | 90 (11.7) | 20 (7.4) | 70 (14.0) | |
| Labile INR | 122 (15.9) | 122 (45.7) | – | |
| CHADS2 score | <0.01 | |||
| ≤1 | 646 (84.3) | 188 (70.4) | 458 (91.8) | |
| ≥2 | 120 (15.7) | 79 (29.6) | 41 (8.2) | |
| ≥3 | 43 (5.6) | 34 (12.7) | 9 (1.8) | |
| CHA2DS2VASc score | <0.01 | |||
| ≤1 | 478 (62.4) | 101 (37.8) | 377 (75.6) | |
| ≥2 | 288 (37.6) | 166 (62.2) | 122 (24.5) | |
| ≥3 | 127 (16.6) | 87 (32.6) | 40 (8.0) | |
| HASBLED score | <0.01 | |||
| ≤1 | 605 (79.0) | 159 (59.6) | 446 (89.4) | |
| ≥2 | 161 (21.0) | 108 (40.4) | 53 (10.6) | |
AF, atrial fibrillation; OAT, oral anticoagulation therapy; TIA, transient ischaemic attack; INR, international normalized ratio.
Thromboembolic and haemorrhagic events according to the CHA2DS2VASc score at the end of follow-up
| . | CHA2DS2VASc ≤ 1 . | CHA2DS2VASc ≥ 2 . | ||
|---|---|---|---|---|
| Patients, n (%) | 465/766 (60.7) | 301/766 (39.3) | ||
| OFF OAT | ON OAT | OFF OAT | ON OAT | |
| 368/465 (79.1) | 97/465 (20.9) | 131/301 (43.5) | 170/301 (56.5) | |
| TE events, n (%) | 1/368 (0.3) | 1/97 (1.0) | 4/131 (3.1) | 5/170 (2.9) |
| Haemorrhagic events, n (%) | 0/368 (0%) | 3/97 (3.1%) | 0/131 (0%) | 4/170 (2.3%) |
| . | CHA2DS2VASc ≤ 1 . | CHA2DS2VASc ≥ 2 . | ||
|---|---|---|---|---|
| Patients, n (%) | 465/766 (60.7) | 301/766 (39.3) | ||
| OFF OAT | ON OAT | OFF OAT | ON OAT | |
| 368/465 (79.1) | 97/465 (20.9) | 131/301 (43.5) | 170/301 (56.5) | |
| TE events, n (%) | 1/368 (0.3) | 1/97 (1.0) | 4/131 (3.1) | 5/170 (2.9) |
| Haemorrhagic events, n (%) | 0/368 (0%) | 3/97 (3.1%) | 0/131 (0%) | 4/170 (2.3%) |
OAT, oral anticoagulation therapy; TE, thromboembolic.
Mentioned CHA2DS2VASc scores are the mean values.
Thromboembolic and haemorrhagic events according to the CHA2DS2VASc score at the end of follow-up
| . | CHA2DS2VASc ≤ 1 . | CHA2DS2VASc ≥ 2 . | ||
|---|---|---|---|---|
| Patients, n (%) | 465/766 (60.7) | 301/766 (39.3) | ||
| OFF OAT | ON OAT | OFF OAT | ON OAT | |
| 368/465 (79.1) | 97/465 (20.9) | 131/301 (43.5) | 170/301 (56.5) | |
| TE events, n (%) | 1/368 (0.3) | 1/97 (1.0) | 4/131 (3.1) | 5/170 (2.9) |
| Haemorrhagic events, n (%) | 0/368 (0%) | 3/97 (3.1%) | 0/131 (0%) | 4/170 (2.3%) |
| . | CHA2DS2VASc ≤ 1 . | CHA2DS2VASc ≥ 2 . | ||
|---|---|---|---|---|
| Patients, n (%) | 465/766 (60.7) | 301/766 (39.3) | ||
| OFF OAT | ON OAT | OFF OAT | ON OAT | |
| 368/465 (79.1) | 97/465 (20.9) | 131/301 (43.5) | 170/301 (56.5) | |
| TE events, n (%) | 1/368 (0.3) | 1/97 (1.0) | 4/131 (3.1) | 5/170 (2.9) |
| Haemorrhagic events, n (%) | 0/368 (0%) | 3/97 (3.1%) | 0/131 (0%) | 4/170 (2.3%) |
OAT, oral anticoagulation therapy; TE, thromboembolic.
Mentioned CHA2DS2VASc scores are the mean values.
Cerebral thromboembolic events and haemorrhagic events
Thromboembolic events occurred in 6 out of the 267 ON-OAT patients (2.2%) and in 5 out of the 499 OFF-OAT patients (1.0%, P = 0.145 for the comparison with the ON-OAT group). The annual ischaemic stroke rate was 0.43 per 100 patient-years [95% confidence interval (CI) 0.40–0.46] in the ON-OAT patients and 0.20 per 100 patient-years (95% CI 0.18–0.22) in the OFF-OAT patients (P = 0.150 for the comparison with the ON-OAT group).
Overall, the median time elapsed between the AF ablation procedure and the occurrence of a TE event was 24 months (IQR 13–60). All the patients experienced AF recurrence at the time of the neurological event and in 30% of the cases AF was completely asymptomatic with the incidental documentation. Seven haemorrhagic events were reported in the ON-OAT group (7/267 = 2.6% P = 0.001). The median time elapsed between the procedure and the haemorrhagic event was 10 months (IQR 6.0–41.5). Details regarding the cerebral thromboembolic and major haemorrhagic events are reported in Table 3.
| Patient no. . | Age (years) . | Time from ablation (months) . | If not on OAT, months from suspension . | CHADS2 score . | CHA2DS2 VASc score . | HASBLED score . | Rhythm . | OAT/ASA . | INR . | Permanent injury or deceased . |
|---|---|---|---|---|---|---|---|---|---|---|
| Thromboembolic events | ||||||||||
| 1 | 66 | 14 | – | 1 | 4 | 2 | AF | OAT | LMWH switching | Aphasia |
| 2 | 56 | 12 | – | 0 | 1 | 0 | AF | OAT | LMWH switching | None |
| 3 | 73 | 6 | – | 3 | 4 | 3 | AF | OAT | <2 (Labile INR) | None |
| 4 | 77 | 19 | – | 5 | 7 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 5 | 66 | 88 | – | 3 | 4 | 2 | AF | OAT | In range | None |
| 6 | 80 | 65 | – | 3 | 4 | 2 | AF | OAT | In range | Haemorrhagic transformation |
| 7 | 71 | 109 | 103 | 1 | 2 | 1 | AF | ASA | – | None |
| 8 | 68 | 7 | 1 | 0 | 1 | 1 | AF | None | – | Deceased (cerebral TE) |
| 9 | 70 | 46 | 40 | 1 | 2 | 2 | AF | ASA | – | Dysarthria |
| 10 | 71 | 55 | 49 | 1 | 2 | 2 | AF | ASA | – | Apraxia |
| 11 | 63 | 24 | 18 | 2 | 2 | 1 | AF | ASA | – | None |
| Haemorrhagic events | ||||||||||
| 6 | 80 | 66 | – | 5 | 6 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 12 | 70 | 10 | – | 1 | 3 | 2 | SR | OAT | >3 | Hemiparesis |
| 13 | 58 | 3 | – | 0 | 0 | 2 | SR | OAT | In range | None (neurosurgery) |
| 14 | 63 | 90 | – | 1 | 1 | 1 | SR | OAT | In range | None (GE bleeding) |
| 15 | 64 | 6 | – | 1 | 2 | 1 | AF | OAT | Not available | None (GE bleeding) |
| 16 | 64 | 17 | – | 1 | 1 | 0 | AF | OAT | >3 | None (neurosurgery) |
| 17 | 75 | 6 | – | 2 | 4 | 2 | AF | OAT | >3 | None (neurosurgery) |
| Patient no. . | Age (years) . | Time from ablation (months) . | If not on OAT, months from suspension . | CHADS2 score . | CHA2DS2 VASc score . | HASBLED score . | Rhythm . | OAT/ASA . | INR . | Permanent injury or deceased . |
|---|---|---|---|---|---|---|---|---|---|---|
| Thromboembolic events | ||||||||||
| 1 | 66 | 14 | – | 1 | 4 | 2 | AF | OAT | LMWH switching | Aphasia |
| 2 | 56 | 12 | – | 0 | 1 | 0 | AF | OAT | LMWH switching | None |
| 3 | 73 | 6 | – | 3 | 4 | 3 | AF | OAT | <2 (Labile INR) | None |
| 4 | 77 | 19 | – | 5 | 7 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 5 | 66 | 88 | – | 3 | 4 | 2 | AF | OAT | In range | None |
| 6 | 80 | 65 | – | 3 | 4 | 2 | AF | OAT | In range | Haemorrhagic transformation |
| 7 | 71 | 109 | 103 | 1 | 2 | 1 | AF | ASA | – | None |
| 8 | 68 | 7 | 1 | 0 | 1 | 1 | AF | None | – | Deceased (cerebral TE) |
| 9 | 70 | 46 | 40 | 1 | 2 | 2 | AF | ASA | – | Dysarthria |
| 10 | 71 | 55 | 49 | 1 | 2 | 2 | AF | ASA | – | Apraxia |
| 11 | 63 | 24 | 18 | 2 | 2 | 1 | AF | ASA | – | None |
| Haemorrhagic events | ||||||||||
| 6 | 80 | 66 | – | 5 | 6 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 12 | 70 | 10 | – | 1 | 3 | 2 | SR | OAT | >3 | Hemiparesis |
| 13 | 58 | 3 | – | 0 | 0 | 2 | SR | OAT | In range | None (neurosurgery) |
| 14 | 63 | 90 | – | 1 | 1 | 1 | SR | OAT | In range | None (GE bleeding) |
| 15 | 64 | 6 | – | 1 | 2 | 1 | AF | OAT | Not available | None (GE bleeding) |
| 16 | 64 | 17 | – | 1 | 1 | 0 | AF | OAT | >3 | None (neurosurgery) |
| 17 | 75 | 6 | – | 2 | 4 | 2 | AF | OAT | >3 | None (neurosurgery) |
ASA, acetylsalicylic acid; AF, atrial fibrillation; INR, international normalized ratio; OAT, oral anticoagulation therapy; SR, sinus rhythm; GE, gastroenteric; LMWH, low-molecular-weight heparin.
| Patient no. . | Age (years) . | Time from ablation (months) . | If not on OAT, months from suspension . | CHADS2 score . | CHA2DS2 VASc score . | HASBLED score . | Rhythm . | OAT/ASA . | INR . | Permanent injury or deceased . |
|---|---|---|---|---|---|---|---|---|---|---|
| Thromboembolic events | ||||||||||
| 1 | 66 | 14 | – | 1 | 4 | 2 | AF | OAT | LMWH switching | Aphasia |
| 2 | 56 | 12 | – | 0 | 1 | 0 | AF | OAT | LMWH switching | None |
| 3 | 73 | 6 | – | 3 | 4 | 3 | AF | OAT | <2 (Labile INR) | None |
| 4 | 77 | 19 | – | 5 | 7 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 5 | 66 | 88 | – | 3 | 4 | 2 | AF | OAT | In range | None |
| 6 | 80 | 65 | – | 3 | 4 | 2 | AF | OAT | In range | Haemorrhagic transformation |
| 7 | 71 | 109 | 103 | 1 | 2 | 1 | AF | ASA | – | None |
| 8 | 68 | 7 | 1 | 0 | 1 | 1 | AF | None | – | Deceased (cerebral TE) |
| 9 | 70 | 46 | 40 | 1 | 2 | 2 | AF | ASA | – | Dysarthria |
| 10 | 71 | 55 | 49 | 1 | 2 | 2 | AF | ASA | – | Apraxia |
| 11 | 63 | 24 | 18 | 2 | 2 | 1 | AF | ASA | – | None |
| Haemorrhagic events | ||||||||||
| 6 | 80 | 66 | – | 5 | 6 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 12 | 70 | 10 | – | 1 | 3 | 2 | SR | OAT | >3 | Hemiparesis |
| 13 | 58 | 3 | – | 0 | 0 | 2 | SR | OAT | In range | None (neurosurgery) |
| 14 | 63 | 90 | – | 1 | 1 | 1 | SR | OAT | In range | None (GE bleeding) |
| 15 | 64 | 6 | – | 1 | 2 | 1 | AF | OAT | Not available | None (GE bleeding) |
| 16 | 64 | 17 | – | 1 | 1 | 0 | AF | OAT | >3 | None (neurosurgery) |
| 17 | 75 | 6 | – | 2 | 4 | 2 | AF | OAT | >3 | None (neurosurgery) |
| Patient no. . | Age (years) . | Time from ablation (months) . | If not on OAT, months from suspension . | CHADS2 score . | CHA2DS2 VASc score . | HASBLED score . | Rhythm . | OAT/ASA . | INR . | Permanent injury or deceased . |
|---|---|---|---|---|---|---|---|---|---|---|
| Thromboembolic events | ||||||||||
| 1 | 66 | 14 | – | 1 | 4 | 2 | AF | OAT | LMWH switching | Aphasia |
| 2 | 56 | 12 | – | 0 | 1 | 0 | AF | OAT | LMWH switching | None |
| 3 | 73 | 6 | – | 3 | 4 | 3 | AF | OAT | <2 (Labile INR) | None |
| 4 | 77 | 19 | – | 5 | 7 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 5 | 66 | 88 | – | 3 | 4 | 2 | AF | OAT | In range | None |
| 6 | 80 | 65 | – | 3 | 4 | 2 | AF | OAT | In range | Haemorrhagic transformation |
| 7 | 71 | 109 | 103 | 1 | 2 | 1 | AF | ASA | – | None |
| 8 | 68 | 7 | 1 | 0 | 1 | 1 | AF | None | – | Deceased (cerebral TE) |
| 9 | 70 | 46 | 40 | 1 | 2 | 2 | AF | ASA | – | Dysarthria |
| 10 | 71 | 55 | 49 | 1 | 2 | 2 | AF | ASA | – | Apraxia |
| 11 | 63 | 24 | 18 | 2 | 2 | 1 | AF | ASA | – | None |
| Haemorrhagic events | ||||||||||
| 6 | 80 | 66 | – | 5 | 6 | 3 | AF | OAT | In range | Deceased (cerebral TE) |
| 12 | 70 | 10 | – | 1 | 3 | 2 | SR | OAT | >3 | Hemiparesis |
| 13 | 58 | 3 | – | 0 | 0 | 2 | SR | OAT | In range | None (neurosurgery) |
| 14 | 63 | 90 | – | 1 | 1 | 1 | SR | OAT | In range | None (GE bleeding) |
| 15 | 64 | 6 | – | 1 | 2 | 1 | AF | OAT | Not available | None (GE bleeding) |
| 16 | 64 | 17 | – | 1 | 1 | 0 | AF | OAT | >3 | None (neurosurgery) |
| 17 | 75 | 6 | – | 2 | 4 | 2 | AF | OAT | >3 | None (neurosurgery) |
ASA, acetylsalicylic acid; AF, atrial fibrillation; INR, international normalized ratio; OAT, oral anticoagulation therapy; SR, sinus rhythm; GE, gastroenteric; LMWH, low-molecular-weight heparin.
CHADS2 and CHA2DS2VASc scores as predictors of thromboembolic risk
When the study population was stratified by the CHADS2 score, a CHADS2 score ≥ 2 was significantly associated with the TE complications (P = 0.047). Similarly, when the individual TE risk was retrospectively calculated with the CHA2DS2VASc scoring scheme, a score ≥ 2 was significantly associated with a higher TE risk (P = 0.020). At the ROC curves analysis, both the CHADS2 and CHA2DS2VASc scores showed adequate TE events predictive value, with a numerical but not statistically significant superiority of the CHA2DS2VASc over the CHADS2, in terms of the AUC (AUC CHA2DS2VASc = 0.774, AUC CHADS2 = 0.712, P = 0.62) (Figures 1A and B). Especially among patients with a CHADS2 score of 0 or 1, the use of the CHA2DS2VASc score proved to be useful for TE risk restratification. Among these patients, a CHA2DS2VASc score ≥ 2 was associated with a higher event rate compared with those with a CHA2DS2VASc score ≤ 1 (P = 0.014) (Figure 2).
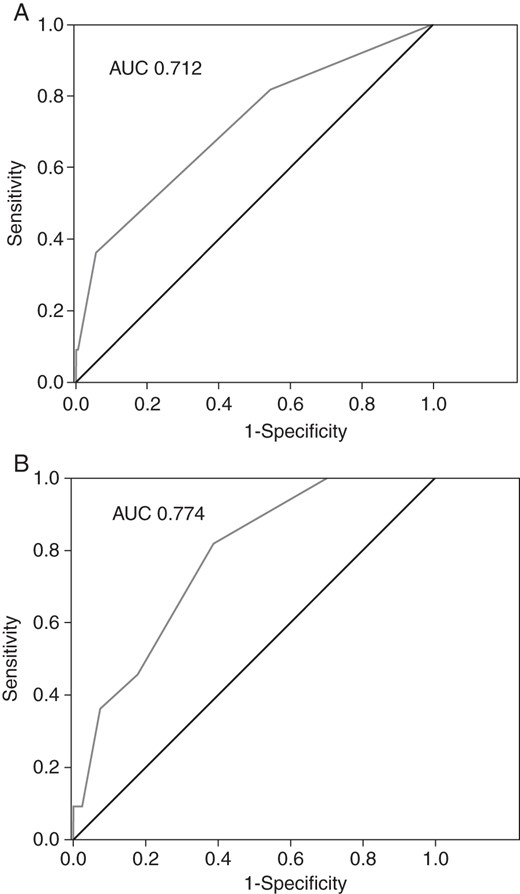
(A) Receiver operating characteristic curve for the CHADS2 score and the TE events prediction. (B) Receiver operating characteristic curve for the CHA2DS2VASc score and the TE events prediction.
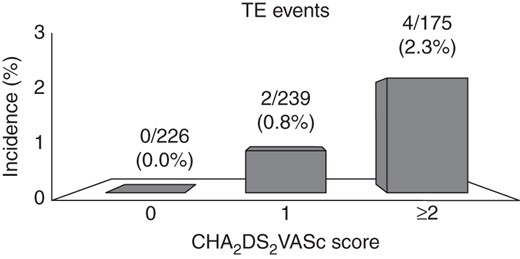
Thromboembolic (TE) events in patients with a CHADS2 score of 0 or 1 stratified by the CHA2DS2VASc score.
HAS-BLED score as the predictor of haemorrhagic risk
In our study population, a HAS-BLED score ≥ 2 was associated with a higher incidence of haemorrhagic events (P = 0.038).
Discussion
The main findings of our observational study were the overall low incidence of the thromboembolic/haemorrhagic events during long-term follow-up after the AF transcatheter ablation and the importance of the combined risk stratification by means of the CHADS2, CHA2DS2VASc, and HASBLED scores in this peculiar setting of patients. To the best of our knowledge, this is one of the longest post-AF transcatheter ablation follow-ups reported in the literature on this topic, with a median observation time of 60.5 months. Accordingly, the late onset of the adverse events together with the AF recurrences was included in our analysis. This notwithstanding, the overall annual TE events incidence in our study population was lower than reported in the literature for the general AF population9 even after the TE risk stratification by means of the CHA2DS2VASc score (0.6 vs. 1.71 per 100 patient-years in patients with CHA2DS2VASc ≥ 2 and 0.09 vs. 0.45 per 100 patient-years in patients with CHA2DS2VASc ≤ 1). These results are in agreement with those reported in a previous study by Reynolds et al.10 in which the annual stroke rate was significantly different between the ablated and non-ablated AF patients during a 3 years follow-up (1.6 per 100 vs. 2.7 per 100 patient-years). Interestingly, at the Emergency Department, admission for cerebral TE events ECG was done and in all the patients AF was documented.
These findings, although observational, may suggest a potential protective role of the TE events exerted by a rhythm control strategy, as already reported in the other studies,11,12 highlighting the need for strict arrhythmic recurrences monitoring by means of implantable devices (e.g. ‘Loop Recorder’) and the aggressive treatment of the AF relapses to achieve better outcomes. Nevertheless, these intriguing hypotheses still need to be confirmed in prospective randomized trials currently ongoing such as the Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial13 whose results are expected in a few years.
The surely protective role of OAT in the TE events did not reach statistical significance in our study population. One possible explanation may be the higher cardiovascular risk profile of the patients receiving oral anticoagulation (the ON-OAT group) as compared with those off anticoagulant (the OFF-OAT group) (e.g. patients receiving the OAT were older, with a higher prevalence of structural heart disease and persistent or long-term/persistent AF compared with patients off OAT) and the low incidence of adverse events. The great number of patients with increased TE risk (CHA2DS2VASc ≥ 2) were treated with OAT and this fact could have mitigated the TE events incidence in this subgroup of patients.
Considering the unpredictable risk of the AF recurrences,14–16 and the high prevalence of the subclinical presentations17 (30% in our study population), there is need for accurate predictors of long-term TE risk to guide the clinical decisions regarding OAT management.
The predictive value of the CHADS2 score has been previously validated in the general AF population.18 Our data expand these findings to the subset of AF patients undergoing transcatheter ablation highlighting an increased TE risk in those with a CHADS2 score ≥ 2. This is in contrast with the results reported by Themistoclakis et al.,5 failed to show a significant association between a high CHADS2 score and the TE risk. This discrepancy may be due to the shorter follow-up duration in their study compared with ours (mean of 26 vs. 63 months follow-up, respectively). Interestingly, in our study population, we documented the TE events also in the OFF-OAT patients with a CHADS2 score of 0 or 1, usually deemed to be at low TE risk. Similar findings have been recently reported by Chao et al.19 in an analogous clinical setting. The unpredictable incidence of the AF recurrences, even after the AF transcatheter ablation, guided us to propose OAT maintenance in the high TE risk patients leading to a low TE occurrence despite a not irrelevant number of haemorrhagic complications. Indeed, not surprisingly, major haemorrhagic events were recorded in the ON-OAT patients only, underlying the importance of balancing between the TE and the haemorrhagic risk in defining the OAT strategy among the AF patients. With this regard in our study, all the haemorrhagic events occurred in patients receiving OAT with a moderate/high bleeding risk (four out of seven events occurred in patients with a HAS-BLED score ≥ 2). As described before, OAT was maintained in these patients because of either the AF recurrences or a CHADS2 ≥ 2. This point underlines how critical the management of the high-risk patients may be, as the CHADS2 and the HAS-BLED scores very often rise in conjunction. With the appearance on the market of new oral anticoagulants, the risk of major bleeding is probably going to reduce:20,21 hopefully, this may imply a greater net clinical benefit derived from maintaining OAT in most of the patients with a high TE risk following AF transcatheter ablation.
Limitations
In interpreting our results, the following limitations should be considered. First, this study shares all the weaknesses of the non-randomized studies; however, the fact that all the procedures were performed in a single high-volume centre with a standardized AF transcatheter ablation approach may help mitigate these limitations. Secondly, our OAT prescription policy may have been influenced by the patients' compliance, whose entity is poorly predictable, and the effective time spent in the therapeutic INR range may have driven the decision regarding OAT discontinuation. Nevertheless, the CHADS2 and especially the CHA2DS2VASc scores proved to be effective in TE risk stratification in this ‘real world’ scenario showing an adequate predictive value. Thirdly, continuous monitoring systems were not available in all our patients; therefore, some asymptomatic AF relapses may have been unrecognized. Fourthly, the low incidence of adverse clinical events may have limited the statistical power of the study. Finally, although we adjusted for the demographic and the clinical characteristics imbalances in comparing our cohort with other general AF populations (i.e. only patients with a CHA2DS2VASc score ≥ 2 were considered), the possibility of residual confounding could not be excluded.
Conclusion
The overall incidence of the TE events in this cohort of patients with AF treated with transcatheter ablation was lower than in the general anticoagulated AF population. Considering the unpredictable nature of the TE events, CHADS2, CHA2DS2VASc, and HAS-BLED should be used to guide the clinical decision regarding OAT management in the peculiar setting of post-ablated patients. Whether rhythm control strategy by means of successful transcatheter AF ablation exerts a protective role on the TE events, as suggested by our observational findings, needs to be confirmed by future large randomized trials.
Conflict of interest: none declared.



