-
PDF
- Split View
-
Views
-
Cite
Cite
Konstantinos C. Koskinas, Nikolaos Fragakis, Demosthenes Katritsis, Vassileios Skeberis, Vassileios Vassilikos, Ranolazine enhances the efficacy of amiodarone for conversion of recent-onset atrial fibrillation, EP Europace, Volume 16, Issue 7, July 2014, Pages 973–979, https://doi.org/10.1093/europace/eut407
Close - Share Icon Share
Abstract
Amiodarone is used commonly for pharmacological cardioversion of atrial fibrillation (AF), but it is limited by moderate efficacy and delayed action. Ranolazine and amiodarone are markedly synergistic in suppressing experimental AF in vitro, yet the clinical efficacy of ranolazine combined with amiodarone for AF conversion has only undergone minimal investigation. This prospective, single-blinded, randomized study compared the safety and efficacy of ranolazine added to amiodarone vs. amiodarone alone for conversion of recent-onset AF.
We enroled 121 patients (64 ± 10 years, 45% male) with recent-onset (<48 h duration) AF who were eligible for pharmacological cardioversion. Patients received either 24 h amiodarone infusion (loading dose 5 mg/kg followed by maintenance dose of 50 mg/h; n = 60), or amiodarone infusion at the same dosage plus a single oral dose of ranolazine 1500 mg (n = 61). Patients in the amiodarone plus ranolazine group compared with the amiodarone-only group showed significantly higher conversion rates at 24 h (87 vs. 70%, respectively; P = 0.024) and at 12 h (52 vs. 32%; P = 0.021), and shorter time to conversion (10.2 ± 3.3 vs. 13.3 ± 4.1 h; P = 0.001). Subgroup analysis identified higher 24 h conversion in patients with left atrial (LA) diameter >46 mm who received the combination treatment vs. amiodarone alone (81 vs. 54%; P = 0.02), whereas the efficacy of the two interventions did not differ among patients with LA diameter ≤46 mm (P = 0.77). There was modest QT prolongation in both the groups, no serious adverse reactions, and no pro-arrhythmic events.
Addition of ranolazine to amiodarone was safe and well tolerated in this study, and it demonstrated efficacy superior to amiodarone alone for conversion of recent-onset AF. These findings may have clinical implications by offering a simple therapeutic manoeuvre to enhance amiodarone's effectiveness for conversion of AF.
In patients who receive amiodarone for conversion of recent-onset atrial fibrillation (AF), the addition of ranolazine has a dual beneficial effect by significantly increasing the conversion rate and substantially accelerating cardioversion.
The beneficial additive effect of ranolazine is more prominent in patients with left atrial enlargement—a challenging group of patients who are less likely to convert to sinus rhythm with amiodarone alone.
The synergistic clinical effect of amiodarone and ranolazine demonstrated in the present study may have clinical implications by offering a simple, safe, and well-tolerated therapeutic manoeuvre to enhance amiodarone's effectiveness for conversion of AF.
Introduction
Restoration of sinus rhythm (SR) is a mainstay in the management of patients with atrial fibrillation (AF). Amiodarone is currently recommended for pharmacological conversion of recent-onset AF particularly in patients with depressed left ventricular function or ischaemia.1–3 While amiodarone combines important advantages including the lack of significant pro-arrhythmia and its safe use in patients with structural heart disease,1,4 the delayed onset of action is an important drawback of amiodarone compared with other, more effective drugs in this context.
Experimental investigations indicate that ranolazine can effectively suppress AF via atrial-selective inhibition of the late sodium current (INa).5–9 Consistently, recent studies have translated those pre-clinical insights into clinical practice by demonstrating the potential of ranolazine for prevention of AF across different clinical settings.10–14 In addition to the AF-suppressing effect of ranolazine alone, ranolazine and amiodarone are markedly synergistic in preventing the development and facilitating the termination of AF in experimental models ex vivo.15–17 To date, the clinical effect of ranolazine when added to amiodarone for conversion of AF has only undergone minimal investigation.
We previously conducted a small pilot study indicating that the addition of ranolazine may improve amiodarone's efficacy for conversion of AF; that proof-of-concept study was nonetheless limited by the small sample size, and it only found a statistically non-significant trend for treatment benefit of the ranolazine plus amiodarone combination.18 The purpose of the present study was to demonstrate the superior efficacy and accelerated action of amiodarone plus ranolazine compared with amiodarone alone for conversion of recent-onset AF. Because left atrium (LA) size critically influences the pharmacological conversion of AF with amiodarone and other antiarrhythmic drugs,4,19,20 a secondary goal assessed the effectiveness of the amiodarone plus ranolazine combination in relation to LA size as determined by echocardiography.
Methods
Study design and patient selection
This was a single-centre, single-blinded, prospective, randomized study. The study protocol was approved by the Institutional Ethics Committee, and all of the study participants provided informed consent. Eligible patients were adults with symptomatic AF of recent onset (<48 h duration), who were suitable for pharmacological cardioversion and were taking adequate anticoagulation therapy as recommended by current guidelines.1,2 Symptomatic AF was defined as at least one AF-related symptom including palpitations, irregular pulse, fatigue, shortness of breath, and chest discomfort. Exclusion criteria were contra-indications for ranolazine administration21 (use of strong inhibitors of CYP3A or inducers of CYP3A, clinically significant hepatic impairment), cardiogenic shock, acute coronary syndrome, pulmonary embolism, atrial flutter, symptomatic bradycardia, history of sick sinus syndrome or atrioventricular block, severe valvular disease, hypertrophic obstructive cardiomyopathy, QTc > 440 ms, pacemaker, thyroid disorders, end-stage renal disease, uncorrected electrolyte imbalance, previous exposure to ranolazine, and use of Classes I or III antiarrhythmic drugs within 24 h before study entrance.
Study interventions
Patients were randomized in a 1 : 1 ratio to either intravenous amiodarone (60 min loading dose of 5 mg/kg followed by maintenance infusion of 50 mg/h until conversion to SR, for a maximum of 24 h) or intravenous amiodarone at the same dosage and duration plus oral ranolazine 1500 mg given once at the time of randomization. We chose the 24 h duration for amiodarone infusion based on prior reports that tested intravenous amiodarone at similar doses for AF conversion.4,19,22 Patients remained under continuous electrocardiogram (ECG) monitoring in the coronary care unit (CCU) throughout the 24 h study period. Rate control with oral beta-blockers (excluding sotalol) was permitted at the treating physician's discretion. Amiodarone was discontinued if any of the following was observed: QTc > 550 ms; heart rate <40 b.p.m. or symptomatic bradycardia, systolic blood pressure <80 mmHg not responding to intravenous fluid administration, or intolerable side-effects. Direct-current electrical cardioversion was attempted at 24 h after the start of amiodarone infusion, if the patient was still in AF.
The QT interval was measured at baseline, at the time of AF conversion, and at 24 h, and was corrected for heart rate (QTc) using Bazett's formula. All patients underwent transthoracic echocardiographic (TTE) examination by an experienced operator blinded to treatment allocation and outcome. We assessed LA diastolic diameter, and LA area determined by LA cavity planimetry from the apical four-chamber view.23 We defined LA enlargement as diastolic LA diameter by TTE above the median of all patients' values.
Efficacy and safety analyses
The primary efficacy endpoint was the proportion of patients with conversion of AF to SR within 24 h of exposure to study treatment. Secondary endpoints were the conversion rate at 12 h,22 time to AF conversion, and the proportion of patients who maintained SR with no AF relapse at 24 h. Given the significant influence of LA size on the pharmacological conversion of AF with amiodarone or other drugs,4,19 and also given the experimentally documented synergistic AF-suppressing effect of amiodarone and ranolazine in the setting of atrial enlargement,17 a pre-specified subgroup analysis assessed the 24 h conversion rate and the time to conversion in relation to LA size as determined by TTE. All efficacy analyses are reported on an intention-to-treat basis.
Safety was continuously assessed in the CCU throughout the study period by monitoring blood pressure, vital signs, 12-lead ECG, and possible adverse reactions. Safety events were evaluated by independent investigators blinded to study intervention and outcome. Pro-arrhythmic events were defined as new onset of sustained ventricular tachycardia (VT), ventricular fibrillation, or torsades de pointes (TdP).
Statistical analyses
A sample size of 120 patients (n = 60 per group) was calculated to provide 80% power to detect a treatment effect of 23% at a significance level of 0.05, based on our prior pilot study.18 Statistical analyses were performed with SPSS 17.0 (SPSS Inc.). Continuous variables are summarized as mean ± standard deviation (SD) and were compared by unpaired samples t-test (for between-group comparison) or paired samples t-test (for within-group comparison). Categorical variables are presented as absolute numbers and percentages, and were compared by χ2 test. All reported P values were based on two-sided tests and were considered significant at the 0.05 level.
Results
From April 2012 to July 2013, a total of 121 consecutive patients eligible for pharmacological conversion of AF were enroled. Patients were allocated to amiodarone (n = 60) or amiodarone plus ranolazine treatment (n = 61). The two groups were well balanced regarding demographic, clinical, and echocardiographic characteristics, as summarized in Table 1.
| Variable . | Amiodarone (n = 60) . | Amiodarone plus ranolazine (n = 61) . | P value . |
|---|---|---|---|
| Age (years) | 64 ± 9 | 66 ± 11 | 0.56 |
| Men | 29 (48%) | 25 (41%) | 0.41 |
| Number of previous AF episodes | |||
| 0–3 | 37 (62%) | 41 (67%) | 0.54 |
| >3 | 23 (38%) | 20 (33%) | 0.54 |
| Duration of current AF episode (h) | 19 ± 7 | 17 ± 6 | 0.33 |
| Arterial hypertension | 49 (82%) | 44 (72%) | 0.21 |
| Structural heart disease* | 26 (43%) | 30 (49%) | 0.51 |
| Ischemic heart disease | 18 (30%) | 21 (34%) | 0.60 |
| Prior myocardial infarction | 9 (15%) | 14 (23%) | 0.26 |
| Valvular heart disease | 8 (13%) | 6 (10%) | 0.55 |
| Left atrium diameter (cm) | 4.6 ± 0.6 | 4.9 ± 0.8 | 0.44 |
| Left ventricular ejection fraction (%) | 54 ± 10 | 58 ± 7 | 0.32 |
| Left ventricular ejection fraction <50% | 12 (20%) | 15 (25%) | 0.54 |
| Medications | |||
| Beta-blockers | 47 (78%) | 44 (72%) | 0.43 |
| Calcium-channel blockers | 23 (38%) | 19 (31%) | 0.41 |
| Digoxin | 14 (23%) | 11 (18%) | 0.47 |
| Statins | 46 (77%) | 39 (64%) | 0.13 |
| Variable . | Amiodarone (n = 60) . | Amiodarone plus ranolazine (n = 61) . | P value . |
|---|---|---|---|
| Age (years) | 64 ± 9 | 66 ± 11 | 0.56 |
| Men | 29 (48%) | 25 (41%) | 0.41 |
| Number of previous AF episodes | |||
| 0–3 | 37 (62%) | 41 (67%) | 0.54 |
| >3 | 23 (38%) | 20 (33%) | 0.54 |
| Duration of current AF episode (h) | 19 ± 7 | 17 ± 6 | 0.33 |
| Arterial hypertension | 49 (82%) | 44 (72%) | 0.21 |
| Structural heart disease* | 26 (43%) | 30 (49%) | 0.51 |
| Ischemic heart disease | 18 (30%) | 21 (34%) | 0.60 |
| Prior myocardial infarction | 9 (15%) | 14 (23%) | 0.26 |
| Valvular heart disease | 8 (13%) | 6 (10%) | 0.55 |
| Left atrium diameter (cm) | 4.6 ± 0.6 | 4.9 ± 0.8 | 0.44 |
| Left ventricular ejection fraction (%) | 54 ± 10 | 58 ± 7 | 0.32 |
| Left ventricular ejection fraction <50% | 12 (20%) | 15 (25%) | 0.54 |
| Medications | |||
| Beta-blockers | 47 (78%) | 44 (72%) | 0.43 |
| Calcium-channel blockers | 23 (38%) | 19 (31%) | 0.41 |
| Digoxin | 14 (23%) | 11 (18%) | 0.47 |
| Statins | 46 (77%) | 39 (64%) | 0.13 |
Data are expressed as mean ± SD or number (percentage).
*Patients may have had ≥1 condition listed under the ‘structural heart disease’ category.
| Variable . | Amiodarone (n = 60) . | Amiodarone plus ranolazine (n = 61) . | P value . |
|---|---|---|---|
| Age (years) | 64 ± 9 | 66 ± 11 | 0.56 |
| Men | 29 (48%) | 25 (41%) | 0.41 |
| Number of previous AF episodes | |||
| 0–3 | 37 (62%) | 41 (67%) | 0.54 |
| >3 | 23 (38%) | 20 (33%) | 0.54 |
| Duration of current AF episode (h) | 19 ± 7 | 17 ± 6 | 0.33 |
| Arterial hypertension | 49 (82%) | 44 (72%) | 0.21 |
| Structural heart disease* | 26 (43%) | 30 (49%) | 0.51 |
| Ischemic heart disease | 18 (30%) | 21 (34%) | 0.60 |
| Prior myocardial infarction | 9 (15%) | 14 (23%) | 0.26 |
| Valvular heart disease | 8 (13%) | 6 (10%) | 0.55 |
| Left atrium diameter (cm) | 4.6 ± 0.6 | 4.9 ± 0.8 | 0.44 |
| Left ventricular ejection fraction (%) | 54 ± 10 | 58 ± 7 | 0.32 |
| Left ventricular ejection fraction <50% | 12 (20%) | 15 (25%) | 0.54 |
| Medications | |||
| Beta-blockers | 47 (78%) | 44 (72%) | 0.43 |
| Calcium-channel blockers | 23 (38%) | 19 (31%) | 0.41 |
| Digoxin | 14 (23%) | 11 (18%) | 0.47 |
| Statins | 46 (77%) | 39 (64%) | 0.13 |
| Variable . | Amiodarone (n = 60) . | Amiodarone plus ranolazine (n = 61) . | P value . |
|---|---|---|---|
| Age (years) | 64 ± 9 | 66 ± 11 | 0.56 |
| Men | 29 (48%) | 25 (41%) | 0.41 |
| Number of previous AF episodes | |||
| 0–3 | 37 (62%) | 41 (67%) | 0.54 |
| >3 | 23 (38%) | 20 (33%) | 0.54 |
| Duration of current AF episode (h) | 19 ± 7 | 17 ± 6 | 0.33 |
| Arterial hypertension | 49 (82%) | 44 (72%) | 0.21 |
| Structural heart disease* | 26 (43%) | 30 (49%) | 0.51 |
| Ischemic heart disease | 18 (30%) | 21 (34%) | 0.60 |
| Prior myocardial infarction | 9 (15%) | 14 (23%) | 0.26 |
| Valvular heart disease | 8 (13%) | 6 (10%) | 0.55 |
| Left atrium diameter (cm) | 4.6 ± 0.6 | 4.9 ± 0.8 | 0.44 |
| Left ventricular ejection fraction (%) | 54 ± 10 | 58 ± 7 | 0.32 |
| Left ventricular ejection fraction <50% | 12 (20%) | 15 (25%) | 0.54 |
| Medications | |||
| Beta-blockers | 47 (78%) | 44 (72%) | 0.43 |
| Calcium-channel blockers | 23 (38%) | 19 (31%) | 0.41 |
| Digoxin | 14 (23%) | 11 (18%) | 0.47 |
| Statins | 46 (77%) | 39 (64%) | 0.13 |
Data are expressed as mean ± SD or number (percentage).
*Patients may have had ≥1 condition listed under the ‘structural heart disease’ category.
Efficacy of the amiodarone plus ranolazine combination
The proportion of patients in the amiodarone plus ranolazine group who converted to normal SR within 24 h was 87% (53 of 61 patients) as compared with 70% in the amiodarone group (42 of 60 patients; P = 0.024) (Figure 1A). The conversion rate at 12 h was also significantly higher in the amiodarone plus ranolazine compared with the amiodarone group (52 vs. 32%, respectively; P = 0.021; Figure 1B). Among patients who met the primary endpoint, SR was maintained at 24 h without relapse of AF in all (53 of 53; 100%) patients in the amiodarone plus ranolazine group vs. 93% of patients in the amiodarone group (39 of 42 patients; P = 0.05). Time to conversion was shorter in the amiodarone plus ranolazine group as compared with the amiodarone-only group (10.2 ± 3.3 vs. 13.3 ± 4.1 h; P = 0.001) (Figure 2).
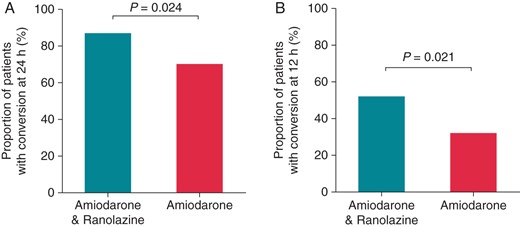
Rate of conversion of AF to SR at 24 h (A) and at 12 h (B) in patients treated with amiodarone plus ranolazine or with amiodarone alone.
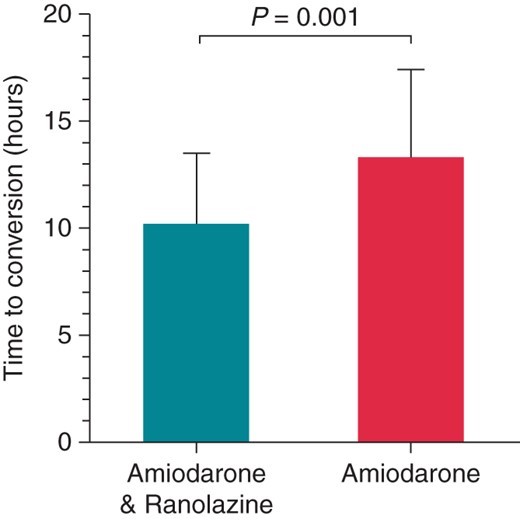
Time to conversion of AF to SR in patients treated with amiodarone plus ranolazine or with amiodarone alone.
An ancillary analysis assessed the 24 h conversion rate in patients of each treatment group stratified according to LA diameter by TTE above or below the median of all patients' LA diameter values (46 mm). The 24 h efficacy of the two treatment modes did not differ in patients with LA diameter <46 mm, (P = 0.77; Figure 3A). In sharp contrast, among patients with LA diameter ≥46 mm, the 24 h conversion rate was higher in patients who received the combination treatment compared with amiodarone monotherapy (81 vs. 54%, respectively; P = 0.02) (Figure 3A). Figure 3B shows the cumulative progression of AF conversion in patients stratified according to study intervention and LA diameter above or below the median value of 46 mm. Very similar results were obtained when patients were dichotomized in relation to LA area by TTE above or below the median (28 mm2) of all LA area values (see Supplementary material online, Figure S1).
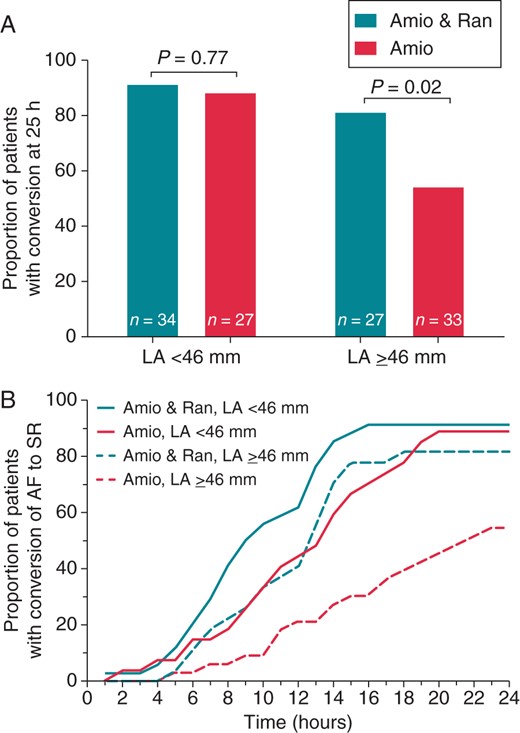
(A) Conversion rate of AF to SR at 24 h in patients stratified according to LA diameter by TTE < 46 mm (left) vs. ≥46 mm (right) and according to treatment with amiodarone plus ranolazine (Amio and Ran) vs. amiodarone alone (Amio). (B) Cumulative progression of conversion of AF in relation to LA diameter and treatment group. Note that the addition of ranolazine to amiodarone led to faster conversion, an effect that was particularly pronounced in patients with larger LA diameter. LA, left atrium.
The 24 h conversion rate tended to be higher in patients with AF duration <24 h compared with patients with AF duration ≥24 h (86 vs. 72%, respectively; P = 0.06). Among patients who were treated with the amiodarone plus ranolazine combination, the 24 h conversion rate did not differ in relation to AF duration <24 vs. ≥24 h (P = 0.20; see Supplementary material online,Supplementary Data).
Safety of the amiodarone plus ranolazine combination
Two patients experienced presumed allergic reactions immediately following initiation of amiodarone infusion: one patient in the combination group manifested flushing and mild dyspnoea, and one in the amiodarone group complained of severe acute lower back pain; amiodarone infusion was discontinued permanently, and both patients had an uneventful recovery. Transient hypotension (systolic blood pressure <85 mmHg) was observed in 25 and 19%, respectively, of patients in the amiodarone plus ranolazine and the amiodarone-only group; the decrease of blood pressure was fully reversible with intravenous normal saline administration. Three patients in the combination group reported dizziness and/or nausea, which were presumably side-effects of ranolazine; these effects gradually resolved. One patient in the amiodarone group developed atrial flutter; the patient remained haemodynamically stable and converted to SR spontaneously. There were no cases of ventricular fibrillation, polymorphic or sustained VT, or TdP in either group throughout the study period.
In both the groups, the QTc interval increased over the 24 h study period (P< 0.001 for both the groups). The QTc did not differ significantly between the two groups at baseline (P = 0.89) and at 24 h (P = 0.33), but the increase of QTc from baseline to 24 h was higher in the amiodarone plus ranolazine group compared with the amiodarone group (24.4 ± 7.1 vs. 19.1 ± 7.3 ms, respectively; P = 0.001) (Figure 4). No patient had excessive QTc prolongation >550 ms.
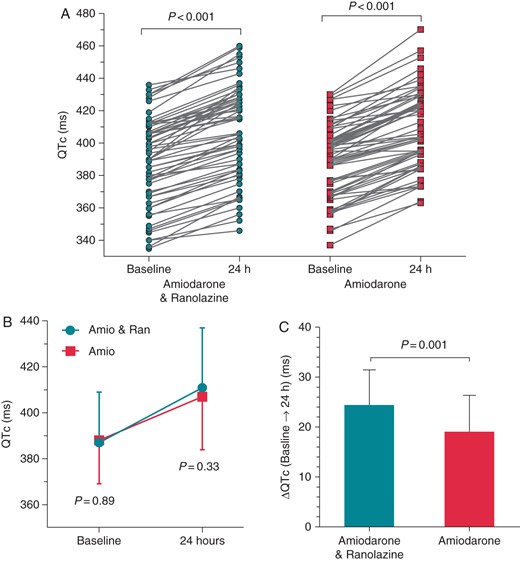
Individual (A) and mean values (B) of the QTc interval at baseline and at 24 h in patients in the amiodarone plus ranolazine group and in the amiodarone-only group. The change of the QTc (ΔQTc) from baseline to 24 h was higher in the amiodarone plus ranolazine group (C). LA, left atrium.
Discussion
This study shows that the combination of ranolazine plus amiodarone is safe and more effective than amiodarone alone for conversion of recent-onset AF. Patients who received the combination treatment had significantly higher conversion rates, and faster restoration of SR compared with patients treated with amiodarone monotherapy. Subgroup analysis demonstrated that the efficacy benefit of the combination treatment was more accentuated in patients with LA enlargement as determined by TTE, whereas amiodarone alone was as highly efficient as the combination treatment in patients with smaller LA size. Thus, the present results affirm and advance preliminary findings of an earlier pilot investigation,18 and they provide validation of a marked synergistic clinical effect of amiodarone plus ranolazine for conversion of AF.
Increasing evidence suggests that ranolazine exhibits remarkable antiarrhythmic properties.5–9,24,25 Ranolazine has previously demonstrated a beneficial effect for prevention and treatment of AF across several clinical settings, including AF prevention following an acute coronary syndrome;10,11 prevention of post-operative AF after coronary revascularization;12 maintenance of SR in recurrent AF;26 and facilitation of electrical AF cardioversion.13 In an earlier pilot study, we found that the addition of ranolazine tended to increase amiodarone's efficacy for conversion of AF.18 Those preliminary results were hypothesis generating, but the difference in the primary efficacy endpoint did not reach statistical significance, likely due to the small sample size (n = 51) that was determined by convenient sampling.18 The present study was designed to overcome those major limitations, and it now advances our earlier proof-of-concept findings in two important ways. First, the larger sample, determined by power analysis, allows for clearer and more robust demonstration of the superiority of the ranolazine plus amiodarone combination over amiodarone alone. Secondly, we show here that the relative efficacy benefit associated with the addition of ranolazine to amiodarone may occur particularly in patients with LA enlargement, defined as LA diameter ≥46 mm by TTE. If confirmed in larger studies, these novel findings may have implications for more effective, risk-tailored rhythm control of AF in subjects with large LA size—a challenging group of patients who are less likely to convert to SR with amiodarone alone, as demonstrated in earlier studies4,19 and affirmed herein.
Among all drugs currently available for conversion of AF, amiodarone is recommended primarily for patients with abnormal left ventricular function or ischaemia.1,2,27 While the lack of significant pro-arrhythmia is an important advantage, amiodarone has two major, clinically crucial limitations: (i) the inferior efficacy for SR restoration compared with other antiarrhythmic drugs; and (ii) the poor performance for early cardioversion due to it delayed action.4,22 The present study shows for the first time that the addition of a single dose of oral ranolazine to conventional amiodarone therapy led to a significantly higher proportion of patients who converted to normal SR. Therefore, in patients who would otherwise be treated with amiodarone for pharmacological AF conversion according to current guidelines,1,2 the addition of ranolazine may represent a more effective therapeutic alternative. Direct-current cardioversion is clearly more effective in this setting, but it is limited by the need for a fasting state and for sedation or anaesthesia.28
We also found that the addition of ranolazine led to a modest, but statistically significant and clinically meaningful reduction of time to conversion. Faster AF conversion offers multiple, important advantages including faster alleviation of symptoms, reduction of the need for atrioventricular node blocking drugs for rate control, and the amelioration of electrophysiological atrial remodelling.1–3 Other drugs (e.g. ibutilide, flecainide, or vernakalant),1–3 alone or in combination,29 may be much more effective for rapid AF conversion; those drugs, however, are indicated only when there is little or no underlying structural heart disease, and they convey a non-negligible risk of serious pro-arrhythmia.2,3 We therefore suggest that the amiodarone plus ranolazine combination may accelerate SR restoration in patients, whose clinical profile would render amiodarone the drug of choice over other, more effective, antiarrhythmic drugs or over electrical cardioversion.
The present findings are consistent with important evidence from basic research. Ranolazine suppresses AF in cultured cells and in isolated animal and human tissue via atrial-selective inhibition of several INa-related parameters.5–9,24,25 Notably, ranolazine potently augments the efficacy of amiodarone for INa blockade and suppression of experimental AF,15,16 likely due to synergistic INa-blocking action of the two drugs: ranolazine preferentially blocks open-state sodium channels,30 whereas amiodarone predominantly blocks inactivated-state sodium channels.31 This marked synergistic electrophysiological effect may account in part for our present clinical observations.
One can speculate on possible mechanisms that might explain our results pertaining to LA size. Left atrial dilatation favours AF through increased conduction delay and shortening of the atrial-effective refractory period.32 The susceptibility to AF might be mediated in part by enhanced INa activation in myocytes of enlarged atria.33–35 Ranolazine—a potent, atrial-selective INa blocker7,8—strongly enhances the effect of INa blockade in experimental models of AF induced by atrial dilatation, thereby leading to substantial prolongation of atrial post-repolarization refractoriness (aPRR), slowing of conduction time, and pronounced AF suppression.36 Of note, pharmacological INa blockade has been reported to cause more marked prolongation of aPRR and local conduction delay in dilated compared with non-dilated atria.37 Importantly, and relevant to our present findings, the addition of ranolazine to amiodarone strongly potentiates the effect of INa blockade, thereby leading to enhanced aPRR and more effective AF suppression compared with amiodarone alone in experimental AF triggered by atrial stretching.17 While clinical extrapolation of experimental results requires caution, those prior mechanistic insights might explain in part our observation that the addition of ranolazine improves the efficacy of amiodarone for AF conversion particularly in patients with LA enlargement. In contrast, amiodarone alone may be highly efficacious for AF suppression in a favourable electrophysiological milieu of smaller atrial with presumably less advanced remodelling; this might explain why the efficacy of amiodarone monotherapy for AF termination was very high in patients without LA enlargement in our study, and also in previous relevant reports.19
Possible side-effects in this study were not evaluated by an independent safety endpoint committee; therefore, our present results concerning the safety and tolerability of the amiodarone plus ranolazine combination are presented as indicative rather than definitive findings. These findings, however, are consistent with ranolazine's excellent safety profile as documented previously in larger studies with long-term administration of ranolazine.10,11,38,39 As expected based on the two drugs' electrophysiological properties, we found modest QTc prolongation in both treatment groups that was more marked in the combination group, but this effect did not lead to pro-arrhythmic events. Previous pre-clinical and clinical studies consistently demonstrated that ranolazine suppresses arrhythmic activity despite the slight QT prolongation,10,40 and that ranolazine and amiodarone, alone or in combination, have rapid unbinding kinetics from the INa and produce a shift in the balance of currents that does not precipitate ventricular pro-arrhythmia.41,42
Several limitations should be considered with the interpretation of the present findings. As per protocol, this was a single-blinded, single-centre study. Larger, double-blinded, placebo-controlled studies may be needed to definitively confirm the present findings. Evaluation of safety endpoints by an independent safety endpoint committee would have provided the gold standard for assessing the safety of the amiodarone plus ranolazine combination; potential bias was substantially limited, however, through assessment of side-effects by investigators blinded to study allocation. As in all relevant studies, the time of AF onset was based on self-reported symptoms and thus, it could not be determined with absolute certainty; possible errors were minimized, however, by including only patients with convincingly symptomatic episodes of AF. The present study did not include a ranolazine-only arm; further studies may be needed to assess the potential efficacy of ranolazine alone for AF conversion, as suggested by experimental5–9 and preliminary clinical investigations.43
In conclusion, the combination of ranolazine with amiodarone demonstrated efficacy superior to amiodarone alone in terms of both higher 24 h conversion and earlier termination of recent-onset AF. The addition of ranolazine may provide a more efficacious and faster acting treatment alternative when amiodarone is selected over more effective drugs for pharmacological conversion of AF. Larger, double-blinded, placebo-controlled trials may be needed to definitively confirm these observations.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
The authors gratefully acknowledge the support of the GD Behrakis Research Fellowship to K.C.K.
Conflicts of interest: none declared.
References
Author notes
The first two authors contributed equally to this work.



