-
PDF
- Split View
-
Views
-
Cite
Cite
Naiara Calvo, Hugo Arguedas, Juan Pablo Canepa, Ignacio García-Bolao, Endocardial left ventricular lead placement from the left subclavian vein approach, EP Europace, Volume 16, Issue 12, December 2014, Pages 1857–1859, https://doi.org/10.1093/europace/euu123
Close - Share Icon Share
Abstract
In up to 10–15% of cases, the traditional epicardial approach for left ventricular (LV) lead placement is not feasible and surgical implantation is considered the alternative. We present the implantation of a transseptal LV lead through a left subclavian access.
Through the left subclavian vein access and using a system which includes a guiding catheter, a puncture screw catheter and a puncture stylet, access to the LV was achieved and the LV stimulation lead was successfully implanted.
We describe the implantation of a transseptal LV stimulation lead through a left subclavian access.
Placement of a lead into the coronary venous system (CS) may fail due to anatomical constraints within the CS, lead instability, or phrenic nerve stimulation. In these situations, surgical epicardial lead implantation is considered the alternative. However, this is a more invasive procedure.
Advantages of the endocardial approach include the improved ability to select a left ventricular lead position and a superior haemodynamic benefit compared with conventional cardiac resynchronization therapy.
Previous described methods for implantation of a transseptal left ventricular lead include superior approach via jugular vein or superior vena cava and/or inferior access via the femoral vein.
We describe the implantation of a transseptal left ventricular stimulation lead, through a subclavian access.
This technique represents the usual access for cardiac leads implantation and avoids the need of an inferior access as well as subcutaneous tunnelling of the lead over the clavicle if a neck vein access is used.
Introduction
Cardiac resynchronization therapy (CRT) is a well-established treatment for patients with moderate-to-severe heart failure (HF) and a wide QRS complex. In conventional CRT, the left ventricle (LV) is paced from the epicardial aspect through a lead placed in a tributary branch of the coronary sinus (CS). However, in some patients, this conventional approach is not feasible due to anatomic abnormalities in the CS and its branches or due to high pacing thresholds or phrenic nerve stimulation. In these cases, surgical implantation of a lead onto the LV epicardium via thoracotomy or thoracoscopy is considered the alternative.1,2
However, this is a more invasive procedure, which limits its applicability in some patients with very low LV ejection fraction and high anaesthesia risk.
Endocardial LV lead placement via a transseptal approach has been recently proposed as an alternative approach for LV pacing in CRT. However, only a few cases have been performed worldwide, with different approaches.
Here, we describe the implantation of a transseptal LV stimulation lead through a superior access.
Case report
We present a case of a 77-year-old male patient with permanent atrial fibrillation and severe LV dysfunction due to ischaemic cardiomyopathy who had undergone surgical myocardial revascularization in 1999. Three years ago, an internal cardiac defibrillator (ICD) was implanted. During that procedure, the right ventricular lead was implanted in the apex and the LV lead implantation was tried, which was unsuccessful due to anatomic anomalies in the CS.
He attended our outpatient clinic complaining of shortness of breath and deterioration of dyspnoea to New York Heart Association Class III/IV. The patient was on acenocumarol, angiotensin-converting enzyme inhibitors, beta-blockers, statins, and diuretics.
The patient was admitted to our unit and a stress echocardiography was performed which confirmed a severe LV dysfunction, with no signs of ischaemia.
After a multidisciplinary evaluation, we decided to implant the LV lead via transseptal approach.
An access was obtained to the right femoral vein via a 9F and to the right femoral artery via a 5F introducers. An intracardiac echocardiography (ICE) catheter was inserted into the right atrium (RA) and a reference pigtail catheter was placed into the aortic root.
Left subclavian vein access was obtained using a 12F introducer. A 40 cm steerable (Agilis™, St Jude Medical) introducer with dilator was inserted over a guidewire into the RA. The wire and dilator were subsequently exchanged for the left atrial (LA) Crosse™ Transseptal Set, which includes a guiding catheter, a transseptal puncture screw catheter and a puncture stylet (Figure 1).
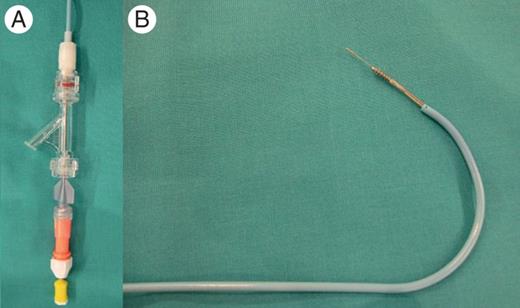
Left atrial Crosse™ transseptal set which includes a guiding catheter, the TPSC and the puncture stylet: (A) distal end of the system. (B) Proximal end of the system.
The ICE probe was positioned in the RA to obtain optimal septal viewing. The LA Crosse™/Agilis™ catheter system was directed towards the septum, guided by the ICE probe. The LA Crosse™ system was extended by 15 mm beyond the distal tip of the Agilis™ and directed towards the septum. To achieve tenting, the LA Crosse™ system was extended an additional 5 mm beyond the distal tip of the Agilis™ (Figure 2A). Tenting was confirmed by contrast injection and visualization on ICE. The TSPSC was then gradually screwed across the septum while applying forward force until LA access was achieved.
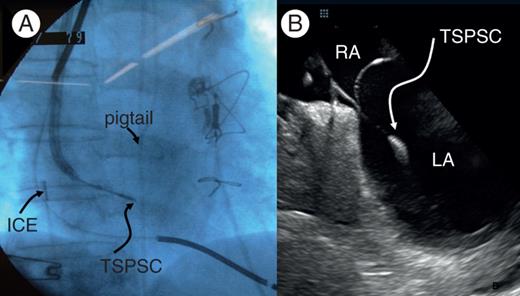
Images during the procedure. (A) Right anterior oblique view showing the LA Crosse™/Agilis™ catheter system directed towards the septum with the LA cross™ system extended by 20 mm beyond the distal tip of the Agilis™ sheath. (B) Intracardiac echocardiography (ICE) images showing the LA Cross™ system across the septum with the distal tip in the LA. ICE, intracardiac echocardiography; RA, right atrium; LA, left atrium.
Once LA access was achieved (Figure 2B), the patient was administered with an intravenous injection of 5000 IU of heparin, followed by additional boluses to maintain an activated clotting time of 250–300 s. Access into the LA was secured with the LA Crosse™ guidewire. Next, the LA Crosse™ TSPSC was unscrewed from the septum and removed together with the guiding catheter from the vasculature, while retaining the LA Crosse™ guidewire within the LA.
The LA Crosse™ guidewire was next advanced into the LV by using the deflection feature of the Agilis™ Steerable Introducer. Once the LA Crosse™ guidewire was positioned within the LV, the Agilis™ Steerable Introducer was exchanged for a straight 45 cm slittable CS delivery sheath.
Finally, a standard bipolar screw-in lead (St Jude Medical 2088TC) was subsequently implanted through the delivery sheath in the posterior lateral wall (Figure 3). Electrical parameters of the LV lead were as follows: Mean LV amplitude 12.3 mV, LV pacing threshold 0.75 V at impulse width of 0.5 ms, and mean LV lead impedance 580 Ω.
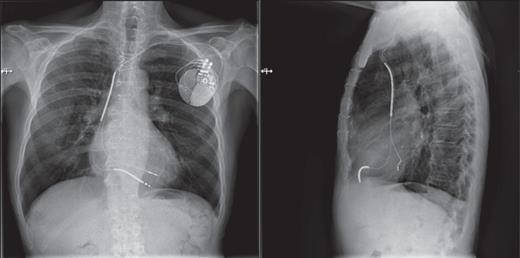
Anteroposterior and lateral RX images showing the final position of the left endocardial ventricular lead and the right ventricular lead.
The echocardiographic study after implant did not show a transseptal shunt, pericardial fluid, or any other complication. There was no increase in mitral regurgitation.
After 6 months of follow-up, sensing and pacing parameters have remained stable, there have not been clinical signs of thrombo-embolic events and patient has improved his functional class.
Discussion
We describe a successful transseptal implantation of a LV lead in a patient with a previous failed transvenous CS implant and a high surgical risk.
Cardiac resynchronization therapy is a well-established therapy for selected patients with HF through the transvenous lead implantation in one of the posterolateral branches of the coronary venous system. However, in up to 10–15% of cases, placement of a lead into the coronary venous system may fail due to anatomical constraints within the CS, lead instability, high stimulation threshold, phrenic nerve stimulation, or a combination of the above.3
Surgical epicardial lead implantation is considered the standard alternative in these situations. However, this is a more invasive procedure and often not suitable for many patients.
Endocardial LV lead placement via a transseptal approach is a novel alternative approach for LV pacing in CRT. A few centres have described different approaches for endocardial LV pacing. However, up to now there is not a standardized approach because the implant techniques described to date are technically difficult.4–6
Previous described methods for permanent implantation of a transseptal LV stimulation lead include superior approach via jugular vein or superior vena cava, inferior access via the femoral vein or the most common mixed approach.7–12
Therapeutic implication
In our case, the transseptal access was acquired via left subclavian vein, which represents the usual vascular access for cardiac leads implantation, and, therefore could permit to perform successively in the same procedure the standard right ventricular and RA lead implantation and the transseptal endocardial LV lead placement. In addition, this technique avoids the need of an inferior access as well as subcutaneous tunnelling of the lead over the clavicle if a neck vein access is used. To avoid the potential difficulties in manoeuvres when using a 12F introducer for LV endocardial lead, we recommend to use the cephalic vein approach for implanting ventricular pacemaker and ICD leads and to perform two independent subclavian punctures for atrial pacing and LV lead.
Advantages of the endocardial approach include the improved ability to select a LV lead position, a superior haemodynamic benefit compared with conventional CRT delivered from the CS,13,14 a more physiologic electrical activation of the LV, with endocardial to epicardial activation with a lower risk of proarrhythmia, and a more rapid LV activation as endocardial conduction is faster than epicardial conduction.
However, there are some concerns regarding the safety of the endocardial lead placement, which include the risk of development of mitral regurgitation, interatrial shunts, and the potential for thrombo-embolic complications associated with endocardial lead placement. Furthermore, the long-term risks of LV endocardial pacing remain undetermined.
With the current evidence and perspectives, transseptal left-sided implantation of endocardial leads should be reserved for patients with end-stage HF who failed endocardial lead placement and have no contraindication for lifelong anticoagulation with warfarin, as an alternative to epicardial surgical approach.
Conclusions
We describe the implantation of a transseptal LV stimulation lead through a left subclavian access. Controlled clinical trials involving larger numbers of patients are now required to establish the safety and effectiveness of this method. Further development of dedicated tools for use during implantation will allow the implant procedure to be further widespread.
Conflict of interest: none declared.



