-
PDF
- Split View
-
Views
-
Cite
Cite
Jagmeet P. Singh, Alexander Kulik, Raisa Levin, Patrick T. Ellinor, Jeremy Ruskin, Jerry Avorn, Niteesh K. Choudhry, Renin-angiotensin-system modulators and the incidence of atrial fibrillation following hospitalization for coronary artery disease, EP Europace, Volume 14, Issue 9, September 2012, Pages 1287–1293, https://doi.org/10.1093/europace/eus074
Close - Share Icon Share
Abstract
Previous studies have suggested that upstream medical therapy to modulate the renin-angiotensin axis may facilitate left atrial remodelling and thereby prevent new-onset atrial fibrillation (AF). The purpose of this study was to evaluate the association between angiotensin converting enzyme inhibitor (ACEI) and angiotensin receptor blockers (ARB) on new-onset AF in a large cohort of patients with coronary artery disease (CAD).
This was a population-based study of 28 620 patients, from community-dwelling Medicare beneficiaries who had been hospitalized for acute myocardial infarction or coronary revascularization (1995–2004). All patients, 65 years and older, had a mean follow-up period of upto 3.8 ± 3.0 years. Patients with a history of AF before and during hospitalization were excluded. We compared the incidence of new-onset AF between patients who were (N= 10 918) and were not (N= 17 702) prescribed ACEI and/or ARB within 1 month of hospital discharge following cardiac event. New-onset AF within 5 and 10 years was 39.1 and 61.1%, respectively, in patients who received ACEI/ARB, compared 34.9 and 53.6% in patients who did not receive them [unadjusted hazard ratio (HR): 1.16; 95% confidence interval (CI): 1.11, 1.21]. Multivariable analysis adjusting for patient- and hospital-related characteristics indicated that ACEI/ARB use independently had no impact on the risk of developing new-onset AF compared with non-users (adjusted HR: 0.99; 95% CI: 0.94, 1.04). Adjustment for propensity-score and health-seeking behaviours yielded nearly identical results.
Angiotensin converting enzyme inhibitor/ARB therapy initiated within 1 month after hospital discharge is not associated with a reduction in the risk of new-onset AF after myocardial infarction or coronary revascularization.
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia, affecting over 7 million individuals in North America and the European Union. The incidence of AF continues to increase as the population ages, and it affects 5% of individuals 65 years or older.1 Atrial fibrillation is an independent predictor of morbidity and mortality, and it accounts for more than one-third of all patient discharges with arrhythmia as the principal diagnosis.1–3 Not surprisingly, the costs associated with AF care are substantial.
Current treatment strategies for AF, which include antiarrhythmic drugs and catheter ablation, are limited in their ability to safely and effectively treat this condition. Antiarrhythmic drugs have many adverse effects such as pro-arrhythmia, whereas catheter ablation is invasive and its success depends on the duration and pattern of AF. As a result, increasing attention is being focused on strategies to prevent AF rather than efforts to treat it once it has commenced.
The occurrence of AF is associated with cellular and ionic changes at the level of the atrial myocyte. Several reports have suggested that the renin-angiotensin-system (RAS) may have a significant role in the pathogenesis of AF.4 Pharmacological modulation of the RAS can have a direct impact on ionic currents and atrial electrophysiological properties,5 collagen deposition,6,7 atrial stretch, and autonomic tone, all of which may have a significant impact on the structural and electrical remodelling processes initiating and perpetuating AF.4,8–11 Although previous work has suggested that RAS inhibition may be of value in limiting new onset AF in patients with hypertension12 and congestive heart failure,13,14 its role in coronary artery disease (CAD) has not been adequately evaluated. Therefore, we sought to assess the association between angiotensin converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) therapy and the development of new-onset AF in a large population-based cohort following their hospitalization for the treatment of CAD.
Methods
Study design
Our study included a cohort of Medicare beneficiaries with CAD. The cohort was created by linking Medicare files describing all clinical encounters to complete medication-use data from the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE) and the New Jersey Pharmaceutical Assistance to the Aged and Disabled (PAAD) programmes. These programmes provide prescription drug benefits to individuals aged 65 years or above with a yearly income above the threshold to qualify them for Medicaid.
Data from the PACE, PAAD, and Medicare were assembled into a relational database consisting of claims for all filled prescriptions, physician encounters, hospitalizations, procedures, long-term care admissions and deaths for all patients within the cohort. These data sources have been used extensively in the past to study population-based medication use and health outcomes.15,16 In order to protect the subjects' privacy, all traceable person-specific identifying factors were transformed into anonymous coded study numbers. This study was approved by the institutional review board of the Brigham and Women's Hospital, Boston, MA.
Patient cohort
We included all patients admitted for acute myocardial infarction (MI) (ICD-9 410.01–410.91 or 411), percutaneous coronary intervention (PCI) (ICD-9 36.01–36.09), or coronary artery bypass graft surgery (CABG) (ICD-9 36.1× or 36.2×) between 1 January 1995 and 31 December 2004. The classification of the patients was hierarchical with patients being first ascribed to the CABG group and PCI group, and if neither procedure was performed, the patient was then accordingly classified as post-MI. We excluded patients who died or were re-admitted to hospital within 30 days after hospital discharge and those patients who were not active users of either drug benefit programme. We also excluded patients who had a known diagnosis of AF (ICD-9 427.31) before or during their index hospitalization. In addition, patients prescribed an anti-arrhythmic medication (such as amiodarone or sotalol) in the 1-year period before and within 30 days after CAD hospitalization were excluded. Importantly, prior validation studies have demonstrated that ICD-9 coding for AF has a positive predictive value of 97% and specificity of 99%.17,18 Thirty days after each patient's hospital discharge date was defined as the index date (i.e. the date on which follow-up began). Follow-up was terminated on 31 December 2005.
Clinical covariates
Patient co-morbidities and clinical covariates were identified by examining physician service claims and hospitalization records for pertinent diagnostic codes in the 1-year period before the index date. Using this approach, we identified the following characteristics: age at index date, year of hospitalization, gender, race, duration of hospital stay, congestive heart failure, stroke, peripheral vascular disease, previous CABG, previous PCI, prior MI or acute coronary syndrome, hypertension, diabetes, and chronic kidney disease. We evaluated ACEI or ARB use in the 1-year period before the index CAD hospitalization, as well as within 30 days after the discharge date. The use of the following concurrent medications in the 1-year period before and 30 days after CAD hospitalization was also assessed: β-blockers, calcium channel blockers, clopidogrel, fibrates, diuretics, nitrates, digoxin, statins, and warfarin. Hospitals accredited by the Association of American Medical Colleges were classified as teaching hospitals. All other hospitals were classified as non-teaching hospitals.
Statistical analysis
We compared the baseline characteristics of patients who did and did not fill an ACEI/ARB prescription within the 30 days after hospital discharge using Student's t-tests, Fisher's exact tests, or χ2 trend tests, as appropriate. Statistical significance was defined as P< 0.05. Our primary outcome was the occurrence of new-onset AF among patients with no prior documented history of AF before or during the index hospitalization. This outcome was defined based on any documented diagnosis of AF during follow-up that occurred as an outpatient or as an inpatient during a subsequent hospital admission.
Rates of new-onset AF among ACEI/ARB users and non-users were compared using Kaplan–Meier method and the log-rank test. Patients were censored at the end of follow-up or if they developed the outcome of interest. Multivariable Cox proportional hazards models were used to adjust for potentially important differences between ACEI/ARB users and non-users including age, gender, race, year of index hospitalization, treatment in a teaching hospital, length of hospital stay, history of peripheral vascular disease, hypertension, congestive heart failure, chronic kidney disease, previous stroke, previous myocardial infarction, previous coronary revascularization (PCI or CABG), diabetes mellitus, medication use in the 1-year period before hospital admission (ACEI/ARB, statin, beta-blocker), and medication use within 30 days after hospital discharge (statin, β-blocker, calcium-channel blocker, digoxin). SAS version 8.2 (SAS Institute, Cary, NC) was used to perform all the analyses.
Sensitivity analysis
We repeated our analyses using propensity-score matching. We developed a propensity score for post-hospitalization ACEI/ARB use with logistic regression incorporating the following covariates: teaching hospital, age, gender, white race, year of index hospitalization, history of congestive heart failure, diabetes mellitus, peripheral vascular disease, stroke, and ACEI/ARB use in the 1-year period before the index hospitalization. Angiotensin converting enzyme inhibitor/ARB users were matched 1:1 to non-users based on the propensity score. The univariate and multivariable analyses were then repeated within this propensity-matched cohort.
To ascertain whether the impact of ACEI/ARB on rates of AF differed by inclusion criteria, we repeated our analyses in each CAD subgroup (MI, PCI, or CABG). We also repeated the analysis to specifically evaluate the impact of ACEI therapy alone, ARB therapy alone, or combined therapy, on rates of AF. To assess the association between ACEI/ARB use and ambulatory AF, we excluded inpatient AF events from our outcome measures. As patients who use ACEI/ARB and other preventive medications may be more likely to adopt other health-seeking behaviours that affect clinical outcomes, we repeated our analyses by including covariates in our multivariable Cox models that adjust for the ‘healthy-user effect’.16 These healthy-user markers were assessed during the 1-year period before the index date and included influenza vaccination, pneumococcal vaccination, mammography, bone mineral densitometry, faecal occult blood testing, and prostate-specific antigen testing. We also repeated our analyses restricting our cohort to individuals newly initiating an ACEI or ARB after hospital discharge, but excluding patients who filled a prescription for either of these medications in the 12 months before their index hospitalization. Finally, to test whether the effect of ACEI/ARB use differed for patients with or without CHF, we tested the statistical significance of the interaction term between drug use and this clinical comorbidity.
Results
Patient cohort
The patient cohort consisted of 28 620 patients with no previous history of AF who were admitted for MI (N= 12 061), PCI (N= 11 596), or CABG (N= 4963). The mean age of the cohort was 78.1 ± 7.0 years; 72.9% of patients were female. A large proportion of patients were cared for at teaching hospitals (62.3%). Within 1 month of hospital discharge, 38.1% of patients were prescribed ACEI or ARB therapy (33.7% ACEI only, 4.4% ARB only, 0.1% ACEI and ARB concurrently). The mean follow-up for the entire cohort was 3.8 ± 3.0 years (maximum 10.9 years).
Clinical characteristics
Table 1 summarizes the clinical characteristics of patients who did and did not fill prescriptions for ACEI/ARB therapy within 1 month of hospital discharge. Angiotensin converting enzyme inhibitor/ARB users were more likely than non-users to have received ACEI/ARB therapy before hospitalization, and to have filled prescriptions for other cardiac medications, including statins, β-blockers, and clopidogrel, both before and after hospitalization (all P< 0.05). Although ACEI/ARB users were more likely to have diabetes mellitus or hypertension, non-users were more likely to have chronic kidney disease or peripheral vascular disease and had significantly longer hospital stays (all P< 0.05). Propensity-matching substantially improved balance between patient- and hospital-related characteristics.
| Characteristic . | Entire study cohort . | Propensity-matched cohort . | ||
|---|---|---|---|---|
| . | Non-ACEI/ARB users (N = 17 702) . | ACEI/ARB users (N = 10 918) . | Non-ACEI/ARB users (N = 7587) . | ACEI/ARB users (N = 7587) . |
| Cohort entry criteria, % | ||||
| Myocardial infarction | 39.4 | 46.5 | 43.2 | 45.4 |
| PCI | 41.5 | 38.9 | 37.0 | 39.6 |
| CABG | 19.0 | 14.6 | 19.8 | 15.0 |
| Demographics | ||||
| Age, mean ± SD | 78.1 ± 7.0 | 78.3 ± 7.0* | 78.3 ± 7.1 | 78.2 ± 7.0 |
| Female, % | 72.2 | 74.2* | 75.0 | 75.5 |
| White race, % | 91.4 | 90.7 | 91.1 | 92.0 |
| Co-morbid conditions, % | ||||
| Prior myocardial infarction | 24.1 | 21.6* | 26.6 | 21.9* |
| Congestive heart failure | 44.8 | 58.0* | 59.0 | 57.5 |
| Stroke | 7.6 | 7.1 | 9.0 | 8.0* |
| Peripheral vascular disease | 5.5 | 4.6* | 6.5 | 6.0 |
| Hypertension | 82.0 | 86.7* | 88.7 | 86.4* |
| Diabetes | 41.5 | 48.2* | 49.4 | 47.9 |
| Chronic kidney disease | 27.8 | 25.0* | 35.6 | 25.4* |
| Previous CABG | 0.6 | 0.7 | 0.8 | 0.6 |
| Previous PCI | 3.3 | 3.0 | 3.9 | 2.8* |
| Pre-hospital medication use, % | ||||
| Prior statin | 34.0 | 39.1* | 38.1 | 38.2 |
| ACEI/ARB | 30.9 | 80.6* | 72.1 | 72.1 |
| Clopidogrel | 24.7 | 29.1* | 25.4 | 27.4* |
| Beta-blocker | 56.5 | 61.6* | 58.2 | 59.6 |
| Calcium-channel blocker | 53.4 | 50.2* | 52.7 | 50.4* |
| Digoxin | 9.4 | 15.0* | 12.8 | 14.3* |
| Diuretics | 9.6 | 12.5* | 12.5 | 12.4 |
| Fibrate | 3.7 | 3.4 | 3.9 | 3.1* |
| Nitrates | 62.9 | 66.9* | 63.5 | 64.4 |
| Warfarin | 6.8 | 8.6* | 6.8 | 8.1* |
| Post-hospital medication use, % | ||||
| Clopidogrel | 19.4 | 27.7* | 19.5 | 29.3* |
| Beta-blocker | 39.2 | 51.6* | 37.0 | 52.5* |
| Calcium-channel blocker | 24.2 | 20.6* | 20.8 | 20.7 |
| Digoxin | 4.9 | 11.6* | 5.4 | 11.9* |
| Diuretics | 2.4 | 5.1* | 2.8 | 5.0* |
| Fibrate | 1.2 | 1.5 | 1.1 | 1.4 |
| Nitrates | 37.6 | 49.6* | 35.0 | 49.7* |
| Statin | 20.9 | 32.1* | 20.6 | 32.9* |
| Warfarin | 4.3 | 6.8* | 4.3 | 7.0* |
| Hospital characteristics | ||||
| Teaching hospital, % | 63.0 | 61.2* | 62.8 | 63.4 |
| Length of stay, mean ± SD | 7.1 ± 6.2 | 6.8 ± 4.8* | 7.6 ± 6.4 | 6.9 ± 4.8* |
| Mean follow-up, years | 4.0 ± 3.1 | 3.5 ± 2.7* | 3.4 ± 2.8 | 3.5 ± 2.8* |
| Characteristic . | Entire study cohort . | Propensity-matched cohort . | ||
|---|---|---|---|---|
| . | Non-ACEI/ARB users (N = 17 702) . | ACEI/ARB users (N = 10 918) . | Non-ACEI/ARB users (N = 7587) . | ACEI/ARB users (N = 7587) . |
| Cohort entry criteria, % | ||||
| Myocardial infarction | 39.4 | 46.5 | 43.2 | 45.4 |
| PCI | 41.5 | 38.9 | 37.0 | 39.6 |
| CABG | 19.0 | 14.6 | 19.8 | 15.0 |
| Demographics | ||||
| Age, mean ± SD | 78.1 ± 7.0 | 78.3 ± 7.0* | 78.3 ± 7.1 | 78.2 ± 7.0 |
| Female, % | 72.2 | 74.2* | 75.0 | 75.5 |
| White race, % | 91.4 | 90.7 | 91.1 | 92.0 |
| Co-morbid conditions, % | ||||
| Prior myocardial infarction | 24.1 | 21.6* | 26.6 | 21.9* |
| Congestive heart failure | 44.8 | 58.0* | 59.0 | 57.5 |
| Stroke | 7.6 | 7.1 | 9.0 | 8.0* |
| Peripheral vascular disease | 5.5 | 4.6* | 6.5 | 6.0 |
| Hypertension | 82.0 | 86.7* | 88.7 | 86.4* |
| Diabetes | 41.5 | 48.2* | 49.4 | 47.9 |
| Chronic kidney disease | 27.8 | 25.0* | 35.6 | 25.4* |
| Previous CABG | 0.6 | 0.7 | 0.8 | 0.6 |
| Previous PCI | 3.3 | 3.0 | 3.9 | 2.8* |
| Pre-hospital medication use, % | ||||
| Prior statin | 34.0 | 39.1* | 38.1 | 38.2 |
| ACEI/ARB | 30.9 | 80.6* | 72.1 | 72.1 |
| Clopidogrel | 24.7 | 29.1* | 25.4 | 27.4* |
| Beta-blocker | 56.5 | 61.6* | 58.2 | 59.6 |
| Calcium-channel blocker | 53.4 | 50.2* | 52.7 | 50.4* |
| Digoxin | 9.4 | 15.0* | 12.8 | 14.3* |
| Diuretics | 9.6 | 12.5* | 12.5 | 12.4 |
| Fibrate | 3.7 | 3.4 | 3.9 | 3.1* |
| Nitrates | 62.9 | 66.9* | 63.5 | 64.4 |
| Warfarin | 6.8 | 8.6* | 6.8 | 8.1* |
| Post-hospital medication use, % | ||||
| Clopidogrel | 19.4 | 27.7* | 19.5 | 29.3* |
| Beta-blocker | 39.2 | 51.6* | 37.0 | 52.5* |
| Calcium-channel blocker | 24.2 | 20.6* | 20.8 | 20.7 |
| Digoxin | 4.9 | 11.6* | 5.4 | 11.9* |
| Diuretics | 2.4 | 5.1* | 2.8 | 5.0* |
| Fibrate | 1.2 | 1.5 | 1.1 | 1.4 |
| Nitrates | 37.6 | 49.6* | 35.0 | 49.7* |
| Statin | 20.9 | 32.1* | 20.6 | 32.9* |
| Warfarin | 4.3 | 6.8* | 4.3 | 7.0* |
| Hospital characteristics | ||||
| Teaching hospital, % | 63.0 | 61.2* | 62.8 | 63.4 |
| Length of stay, mean ± SD | 7.1 ± 6.2 | 6.8 ± 4.8* | 7.6 ± 6.4 | 6.9 ± 4.8* |
| Mean follow-up, years | 4.0 ± 3.1 | 3.5 ± 2.7* | 3.4 ± 2.8 | 3.5 ± 2.8* |
SD, standard deviation.
*P< 0.05.
| Characteristic . | Entire study cohort . | Propensity-matched cohort . | ||
|---|---|---|---|---|
| . | Non-ACEI/ARB users (N = 17 702) . | ACEI/ARB users (N = 10 918) . | Non-ACEI/ARB users (N = 7587) . | ACEI/ARB users (N = 7587) . |
| Cohort entry criteria, % | ||||
| Myocardial infarction | 39.4 | 46.5 | 43.2 | 45.4 |
| PCI | 41.5 | 38.9 | 37.0 | 39.6 |
| CABG | 19.0 | 14.6 | 19.8 | 15.0 |
| Demographics | ||||
| Age, mean ± SD | 78.1 ± 7.0 | 78.3 ± 7.0* | 78.3 ± 7.1 | 78.2 ± 7.0 |
| Female, % | 72.2 | 74.2* | 75.0 | 75.5 |
| White race, % | 91.4 | 90.7 | 91.1 | 92.0 |
| Co-morbid conditions, % | ||||
| Prior myocardial infarction | 24.1 | 21.6* | 26.6 | 21.9* |
| Congestive heart failure | 44.8 | 58.0* | 59.0 | 57.5 |
| Stroke | 7.6 | 7.1 | 9.0 | 8.0* |
| Peripheral vascular disease | 5.5 | 4.6* | 6.5 | 6.0 |
| Hypertension | 82.0 | 86.7* | 88.7 | 86.4* |
| Diabetes | 41.5 | 48.2* | 49.4 | 47.9 |
| Chronic kidney disease | 27.8 | 25.0* | 35.6 | 25.4* |
| Previous CABG | 0.6 | 0.7 | 0.8 | 0.6 |
| Previous PCI | 3.3 | 3.0 | 3.9 | 2.8* |
| Pre-hospital medication use, % | ||||
| Prior statin | 34.0 | 39.1* | 38.1 | 38.2 |
| ACEI/ARB | 30.9 | 80.6* | 72.1 | 72.1 |
| Clopidogrel | 24.7 | 29.1* | 25.4 | 27.4* |
| Beta-blocker | 56.5 | 61.6* | 58.2 | 59.6 |
| Calcium-channel blocker | 53.4 | 50.2* | 52.7 | 50.4* |
| Digoxin | 9.4 | 15.0* | 12.8 | 14.3* |
| Diuretics | 9.6 | 12.5* | 12.5 | 12.4 |
| Fibrate | 3.7 | 3.4 | 3.9 | 3.1* |
| Nitrates | 62.9 | 66.9* | 63.5 | 64.4 |
| Warfarin | 6.8 | 8.6* | 6.8 | 8.1* |
| Post-hospital medication use, % | ||||
| Clopidogrel | 19.4 | 27.7* | 19.5 | 29.3* |
| Beta-blocker | 39.2 | 51.6* | 37.0 | 52.5* |
| Calcium-channel blocker | 24.2 | 20.6* | 20.8 | 20.7 |
| Digoxin | 4.9 | 11.6* | 5.4 | 11.9* |
| Diuretics | 2.4 | 5.1* | 2.8 | 5.0* |
| Fibrate | 1.2 | 1.5 | 1.1 | 1.4 |
| Nitrates | 37.6 | 49.6* | 35.0 | 49.7* |
| Statin | 20.9 | 32.1* | 20.6 | 32.9* |
| Warfarin | 4.3 | 6.8* | 4.3 | 7.0* |
| Hospital characteristics | ||||
| Teaching hospital, % | 63.0 | 61.2* | 62.8 | 63.4 |
| Length of stay, mean ± SD | 7.1 ± 6.2 | 6.8 ± 4.8* | 7.6 ± 6.4 | 6.9 ± 4.8* |
| Mean follow-up, years | 4.0 ± 3.1 | 3.5 ± 2.7* | 3.4 ± 2.8 | 3.5 ± 2.8* |
| Characteristic . | Entire study cohort . | Propensity-matched cohort . | ||
|---|---|---|---|---|
| . | Non-ACEI/ARB users (N = 17 702) . | ACEI/ARB users (N = 10 918) . | Non-ACEI/ARB users (N = 7587) . | ACEI/ARB users (N = 7587) . |
| Cohort entry criteria, % | ||||
| Myocardial infarction | 39.4 | 46.5 | 43.2 | 45.4 |
| PCI | 41.5 | 38.9 | 37.0 | 39.6 |
| CABG | 19.0 | 14.6 | 19.8 | 15.0 |
| Demographics | ||||
| Age, mean ± SD | 78.1 ± 7.0 | 78.3 ± 7.0* | 78.3 ± 7.1 | 78.2 ± 7.0 |
| Female, % | 72.2 | 74.2* | 75.0 | 75.5 |
| White race, % | 91.4 | 90.7 | 91.1 | 92.0 |
| Co-morbid conditions, % | ||||
| Prior myocardial infarction | 24.1 | 21.6* | 26.6 | 21.9* |
| Congestive heart failure | 44.8 | 58.0* | 59.0 | 57.5 |
| Stroke | 7.6 | 7.1 | 9.0 | 8.0* |
| Peripheral vascular disease | 5.5 | 4.6* | 6.5 | 6.0 |
| Hypertension | 82.0 | 86.7* | 88.7 | 86.4* |
| Diabetes | 41.5 | 48.2* | 49.4 | 47.9 |
| Chronic kidney disease | 27.8 | 25.0* | 35.6 | 25.4* |
| Previous CABG | 0.6 | 0.7 | 0.8 | 0.6 |
| Previous PCI | 3.3 | 3.0 | 3.9 | 2.8* |
| Pre-hospital medication use, % | ||||
| Prior statin | 34.0 | 39.1* | 38.1 | 38.2 |
| ACEI/ARB | 30.9 | 80.6* | 72.1 | 72.1 |
| Clopidogrel | 24.7 | 29.1* | 25.4 | 27.4* |
| Beta-blocker | 56.5 | 61.6* | 58.2 | 59.6 |
| Calcium-channel blocker | 53.4 | 50.2* | 52.7 | 50.4* |
| Digoxin | 9.4 | 15.0* | 12.8 | 14.3* |
| Diuretics | 9.6 | 12.5* | 12.5 | 12.4 |
| Fibrate | 3.7 | 3.4 | 3.9 | 3.1* |
| Nitrates | 62.9 | 66.9* | 63.5 | 64.4 |
| Warfarin | 6.8 | 8.6* | 6.8 | 8.1* |
| Post-hospital medication use, % | ||||
| Clopidogrel | 19.4 | 27.7* | 19.5 | 29.3* |
| Beta-blocker | 39.2 | 51.6* | 37.0 | 52.5* |
| Calcium-channel blocker | 24.2 | 20.6* | 20.8 | 20.7 |
| Digoxin | 4.9 | 11.6* | 5.4 | 11.9* |
| Diuretics | 2.4 | 5.1* | 2.8 | 5.0* |
| Fibrate | 1.2 | 1.5 | 1.1 | 1.4 |
| Nitrates | 37.6 | 49.6* | 35.0 | 49.7* |
| Statin | 20.9 | 32.1* | 20.6 | 32.9* |
| Warfarin | 4.3 | 6.8* | 4.3 | 7.0* |
| Hospital characteristics | ||||
| Teaching hospital, % | 63.0 | 61.2* | 62.8 | 63.4 |
| Length of stay, mean ± SD | 7.1 ± 6.2 | 6.8 ± 4.8* | 7.6 ± 6.4 | 6.9 ± 4.8* |
| Mean follow-up, years | 4.0 ± 3.1 | 3.5 ± 2.7* | 3.4 ± 2.8 | 3.5 ± 2.8* |
SD, standard deviation.
*P< 0.05.
Impact of angiotensin converting enzyme inhibitor /angiotensin receptor blocker on new-onset atrial fibrillation
Freedom from new-onset AF at 1, 5, and 10 years was 87.5, 60.9, and 38.9%, respectively, in patients who received ACEI/ARB therapy, compared with 88.3, 65.1, and 46.4%, respectively, in patients who did not receive ACEI/ARB therapy. In univariate analysis, ACEI/ARB therapy was associated with a significantly higher incidence of new-onset AF [hazard ratio (HR) 1.16; 95% confidence interval (CI) 1.11, 1.21]. However, after adjusting for patient- and hospital-related characteristics, ACEI/ARB use was not independently associated with an increased risk of AF (HR 0.99; 95% CI 0.94, 1.04; Figure 1; Table 2). Similar unadjusted and adjusted results were seen in each of the CAD subgroups (Table 3). Used alone or in combination, ACEI and ARB therapy was not independently associated with the risk of new-onset AF (ACEI alone: HR 0.98; 95% CI 0.93, 1.04; ARB alone: HR 1.05; 95% CI 0.93, 1.18; ACEI and ARB combined: HR 1.06; 95% CI 0.53, 2.13).
Predictors of new-onset atrial fibrillation after hospital admission for coronary artery disease
| Characteristic . | Hazard ratio for new-onset AF
(95% confidence interval) . | |
|---|---|---|
| . | Entire cohort (N = 28 620) . | Propensity-matched cohort (N = 15 174) . |
| Univariate analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 1.16 (1.11–1.21)* | 1.01 (0.95–1.07) |
| Multivariable analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 0.99 (0.94–1.04) | 1.01 (0.95–1.07) |
| Patient characteristics | ||
| Patient age (per additional year) | 1.03 (1.02–1.03)* | 1.03 (1.02–1.03)* |
| Male gender | 1.19 (1.13–1.25)* | 1.23 (1.15–1.31)* |
| White race | 1.16 (1.08–1.26)* | 1.21 (1.08–1.35)* |
| Year of admission (per additional year) | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) |
| Diabetes mellitus | 1.16 (1.11–1.21)* | 1.16 (1.09–1.23)* |
| Peripheral vascular disease | 1.14 (1.03–1.26)* | 1.12 (1.00–1.27)* |
| Congestive heart failure | 1.57 (1.49–1.64)* | 1.58 (1.48–1.69)* |
| Stroke | 1.17 (1.08–1.27)* | 1.16 (1.04–1.30)* |
| Previous myocardial infarction | 0.93 (0.88–0.98) | 0.94 (0.87–1.01) |
| Previous CABG or PCI | 1.04 (0.92–1.16) | 1.04 (0.90–1.21) |
| Chronic kidney disease | 1.22 (1.16–1.28)* | 1.24 (1.16–1.32)* |
| Hypertension | 1.03 (0.97–1.10) | 1.04 (0.95–1.14) |
| Hospital length of stay (per additional day) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) |
| Hospital characteristic | ||
| Teaching hospital | 0.96 (0.92–1.00) | 0.98 (0.92–1.04) |
| Pre-hospital medications | ||
| ACEI/ARB | 1.14 (1.08–1.20)* | 1.15 (1.08, 1.24)* |
| Beta-blocker | 0.98 (0.94–1.03) | 0.96 (0.90–1.02) |
| Statin | 0.98 (0.93–1.04) | 0.97 (0.90–1.04) |
| Post-hospital medications | ||
| Statin | 0.92 (0.86–0.98) | 0.94 (0.86–1.02) |
| Beta-blocker | 0.94 (0.89–0.98) | 0.98 (0.92–1.05) |
| Calcium channel blockers | 1.13 (1.08–1.19)* | 1.10 (1.02–1.18)* |
| Digoxin | 1.42 (1.32–1.53)* | 1.35 (1.22–1.48)* |
| Characteristic . | Hazard ratio for new-onset AF
(95% confidence interval) . | |
|---|---|---|
| . | Entire cohort (N = 28 620) . | Propensity-matched cohort (N = 15 174) . |
| Univariate analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 1.16 (1.11–1.21)* | 1.01 (0.95–1.07) |
| Multivariable analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 0.99 (0.94–1.04) | 1.01 (0.95–1.07) |
| Patient characteristics | ||
| Patient age (per additional year) | 1.03 (1.02–1.03)* | 1.03 (1.02–1.03)* |
| Male gender | 1.19 (1.13–1.25)* | 1.23 (1.15–1.31)* |
| White race | 1.16 (1.08–1.26)* | 1.21 (1.08–1.35)* |
| Year of admission (per additional year) | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) |
| Diabetes mellitus | 1.16 (1.11–1.21)* | 1.16 (1.09–1.23)* |
| Peripheral vascular disease | 1.14 (1.03–1.26)* | 1.12 (1.00–1.27)* |
| Congestive heart failure | 1.57 (1.49–1.64)* | 1.58 (1.48–1.69)* |
| Stroke | 1.17 (1.08–1.27)* | 1.16 (1.04–1.30)* |
| Previous myocardial infarction | 0.93 (0.88–0.98) | 0.94 (0.87–1.01) |
| Previous CABG or PCI | 1.04 (0.92–1.16) | 1.04 (0.90–1.21) |
| Chronic kidney disease | 1.22 (1.16–1.28)* | 1.24 (1.16–1.32)* |
| Hypertension | 1.03 (0.97–1.10) | 1.04 (0.95–1.14) |
| Hospital length of stay (per additional day) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) |
| Hospital characteristic | ||
| Teaching hospital | 0.96 (0.92–1.00) | 0.98 (0.92–1.04) |
| Pre-hospital medications | ||
| ACEI/ARB | 1.14 (1.08–1.20)* | 1.15 (1.08, 1.24)* |
| Beta-blocker | 0.98 (0.94–1.03) | 0.96 (0.90–1.02) |
| Statin | 0.98 (0.93–1.04) | 0.97 (0.90–1.04) |
| Post-hospital medications | ||
| Statin | 0.92 (0.86–0.98) | 0.94 (0.86–1.02) |
| Beta-blocker | 0.94 (0.89–0.98) | 0.98 (0.92–1.05) |
| Calcium channel blockers | 1.13 (1.08–1.19)* | 1.10 (1.02–1.18)* |
| Digoxin | 1.42 (1.32–1.53)* | 1.35 (1.22–1.48)* |
*P< 0.05.
Predictors of new-onset atrial fibrillation after hospital admission for coronary artery disease
| Characteristic . | Hazard ratio for new-onset AF
(95% confidence interval) . | |
|---|---|---|
| . | Entire cohort (N = 28 620) . | Propensity-matched cohort (N = 15 174) . |
| Univariate analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 1.16 (1.11–1.21)* | 1.01 (0.95–1.07) |
| Multivariable analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 0.99 (0.94–1.04) | 1.01 (0.95–1.07) |
| Patient characteristics | ||
| Patient age (per additional year) | 1.03 (1.02–1.03)* | 1.03 (1.02–1.03)* |
| Male gender | 1.19 (1.13–1.25)* | 1.23 (1.15–1.31)* |
| White race | 1.16 (1.08–1.26)* | 1.21 (1.08–1.35)* |
| Year of admission (per additional year) | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) |
| Diabetes mellitus | 1.16 (1.11–1.21)* | 1.16 (1.09–1.23)* |
| Peripheral vascular disease | 1.14 (1.03–1.26)* | 1.12 (1.00–1.27)* |
| Congestive heart failure | 1.57 (1.49–1.64)* | 1.58 (1.48–1.69)* |
| Stroke | 1.17 (1.08–1.27)* | 1.16 (1.04–1.30)* |
| Previous myocardial infarction | 0.93 (0.88–0.98) | 0.94 (0.87–1.01) |
| Previous CABG or PCI | 1.04 (0.92–1.16) | 1.04 (0.90–1.21) |
| Chronic kidney disease | 1.22 (1.16–1.28)* | 1.24 (1.16–1.32)* |
| Hypertension | 1.03 (0.97–1.10) | 1.04 (0.95–1.14) |
| Hospital length of stay (per additional day) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) |
| Hospital characteristic | ||
| Teaching hospital | 0.96 (0.92–1.00) | 0.98 (0.92–1.04) |
| Pre-hospital medications | ||
| ACEI/ARB | 1.14 (1.08–1.20)* | 1.15 (1.08, 1.24)* |
| Beta-blocker | 0.98 (0.94–1.03) | 0.96 (0.90–1.02) |
| Statin | 0.98 (0.93–1.04) | 0.97 (0.90–1.04) |
| Post-hospital medications | ||
| Statin | 0.92 (0.86–0.98) | 0.94 (0.86–1.02) |
| Beta-blocker | 0.94 (0.89–0.98) | 0.98 (0.92–1.05) |
| Calcium channel blockers | 1.13 (1.08–1.19)* | 1.10 (1.02–1.18)* |
| Digoxin | 1.42 (1.32–1.53)* | 1.35 (1.22–1.48)* |
| Characteristic . | Hazard ratio for new-onset AF
(95% confidence interval) . | |
|---|---|---|
| . | Entire cohort (N = 28 620) . | Propensity-matched cohort (N = 15 174) . |
| Univariate analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 1.16 (1.11–1.21)* | 1.01 (0.95–1.07) |
| Multivariable analysis | ||
| Post-discharge ACEI/ARB use within 1 month | 0.99 (0.94–1.04) | 1.01 (0.95–1.07) |
| Patient characteristics | ||
| Patient age (per additional year) | 1.03 (1.02–1.03)* | 1.03 (1.02–1.03)* |
| Male gender | 1.19 (1.13–1.25)* | 1.23 (1.15–1.31)* |
| White race | 1.16 (1.08–1.26)* | 1.21 (1.08–1.35)* |
| Year of admission (per additional year) | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) |
| Diabetes mellitus | 1.16 (1.11–1.21)* | 1.16 (1.09–1.23)* |
| Peripheral vascular disease | 1.14 (1.03–1.26)* | 1.12 (1.00–1.27)* |
| Congestive heart failure | 1.57 (1.49–1.64)* | 1.58 (1.48–1.69)* |
| Stroke | 1.17 (1.08–1.27)* | 1.16 (1.04–1.30)* |
| Previous myocardial infarction | 0.93 (0.88–0.98) | 0.94 (0.87–1.01) |
| Previous CABG or PCI | 1.04 (0.92–1.16) | 1.04 (0.90–1.21) |
| Chronic kidney disease | 1.22 (1.16–1.28)* | 1.24 (1.16–1.32)* |
| Hypertension | 1.03 (0.97–1.10) | 1.04 (0.95–1.14) |
| Hospital length of stay (per additional day) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) |
| Hospital characteristic | ||
| Teaching hospital | 0.96 (0.92–1.00) | 0.98 (0.92–1.04) |
| Pre-hospital medications | ||
| ACEI/ARB | 1.14 (1.08–1.20)* | 1.15 (1.08, 1.24)* |
| Beta-blocker | 0.98 (0.94–1.03) | 0.96 (0.90–1.02) |
| Statin | 0.98 (0.93–1.04) | 0.97 (0.90–1.04) |
| Post-hospital medications | ||
| Statin | 0.92 (0.86–0.98) | 0.94 (0.86–1.02) |
| Beta-blocker | 0.94 (0.89–0.98) | 0.98 (0.92–1.05) |
| Calcium channel blockers | 1.13 (1.08–1.19)* | 1.10 (1.02–1.18)* |
| Digoxin | 1.42 (1.32–1.53)* | 1.35 (1.22–1.48)* |
*P< 0.05.
Unadjusted and adjusted hazard ratios for angiotensin converting enzyme inhibitor/angiotensin receptor blocker use and new-onset atrial fibrillation in the entire cohort and each of the coronary artery disease sub-groups
| Analysis . | Unadjusted hazard ratio (95% confidence interval) . | Adjusted hazard ratio (95% confidence interval)a . |
|---|---|---|
| Entire cohort (N= 28 620) | 1.16 (1.11–1.21)* | 0.99 (0.94–1.04) |
| CABG cohort (N= 4963) | 1.16 (1.04–1.30)* | 1.00 (0.88–1.14) |
| PCI cohort (N= 11 596) | 1.13 (1.05–1.21)* | 0.95 (0.87–1.04) |
| MI cohort (N= 12 061) | 1.08 (1.01–1.15)* | 0.95 (0.88, 1.03) |
| Analysis . | Unadjusted hazard ratio (95% confidence interval) . | Adjusted hazard ratio (95% confidence interval)a . |
|---|---|---|
| Entire cohort (N= 28 620) | 1.16 (1.11–1.21)* | 0.99 (0.94–1.04) |
| CABG cohort (N= 4963) | 1.16 (1.04–1.30)* | 1.00 (0.88–1.14) |
| PCI cohort (N= 11 596) | 1.13 (1.05–1.21)* | 0.95 (0.87–1.04) |
| MI cohort (N= 12 061) | 1.08 (1.01–1.15)* | 0.95 (0.88, 1.03) |
aAdjusted for age, gender, race, previous myocardial infarction, previous CABG or PCI, history of congestive heart failure, diabetes mellitus, peripheral vascular disease, stroke, hypertension, chronic kidney disease, pre-hospital medication use (statin, β-blocker, angiotensin converting enzyme inhibitor, or angiotensin II receptor blocker), post-hospital medication use within 30 days of discharge (β-blocker, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker, calcium channel blocker, digoxin), year of index hospitalization, teaching hospital, and length of hospital stay.
*P< 0.05.
Unadjusted and adjusted hazard ratios for angiotensin converting enzyme inhibitor/angiotensin receptor blocker use and new-onset atrial fibrillation in the entire cohort and each of the coronary artery disease sub-groups
| Analysis . | Unadjusted hazard ratio (95% confidence interval) . | Adjusted hazard ratio (95% confidence interval)a . |
|---|---|---|
| Entire cohort (N= 28 620) | 1.16 (1.11–1.21)* | 0.99 (0.94–1.04) |
| CABG cohort (N= 4963) | 1.16 (1.04–1.30)* | 1.00 (0.88–1.14) |
| PCI cohort (N= 11 596) | 1.13 (1.05–1.21)* | 0.95 (0.87–1.04) |
| MI cohort (N= 12 061) | 1.08 (1.01–1.15)* | 0.95 (0.88, 1.03) |
| Analysis . | Unadjusted hazard ratio (95% confidence interval) . | Adjusted hazard ratio (95% confidence interval)a . |
|---|---|---|
| Entire cohort (N= 28 620) | 1.16 (1.11–1.21)* | 0.99 (0.94–1.04) |
| CABG cohort (N= 4963) | 1.16 (1.04–1.30)* | 1.00 (0.88–1.14) |
| PCI cohort (N= 11 596) | 1.13 (1.05–1.21)* | 0.95 (0.87–1.04) |
| MI cohort (N= 12 061) | 1.08 (1.01–1.15)* | 0.95 (0.88, 1.03) |
aAdjusted for age, gender, race, previous myocardial infarction, previous CABG or PCI, history of congestive heart failure, diabetes mellitus, peripheral vascular disease, stroke, hypertension, chronic kidney disease, pre-hospital medication use (statin, β-blocker, angiotensin converting enzyme inhibitor, or angiotensin II receptor blocker), post-hospital medication use within 30 days of discharge (β-blocker, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker, calcium channel blocker, digoxin), year of index hospitalization, teaching hospital, and length of hospital stay.
*P< 0.05.
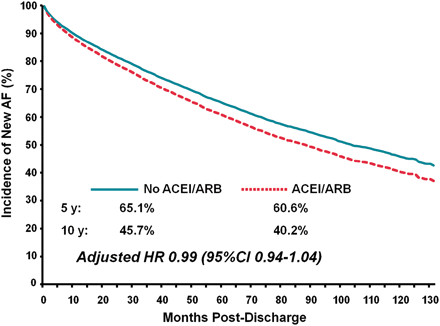
New-onset atrial fibrillation after hospital admission for coronary artery disease, stratified by angiotensin converting enzyme inhibitor/angiotensin receptor blocker use within 1 month of hospital discharge.
After adjusting for predictors of health-seeking behaviour in the multivariable analysis, ACEI/ARB use was not significantly associated with new-onset AF (HR 0.99; 95% CI 0.94, 1.04). Similarly, in the propensity-matched cohorts, ACEI/ARB use was not independently associated with new-onset AF in univariate analysis (HR 1.01; 95% CI 0.95, 1.07) or in multivariate analysis (HR 1.01; 95% CI 0.95, 1.07). When we limited the analysis to the outcome of ambulatory new-onset AF, ACEI/ARB therapy was associated a higher risk of AF on univariate analysis (HR 1.17; 95% CI 1.11, 1.23), but not in the multivariable analysis (HR 0.99; 95% CI 0.93, 1.05). Analysis restricted to patients newly initiating an ACEI/ARB after hospital discharge yielded similar results (see Appendix). Finally, the impact of ACEI/ARB on rates of AF did not differ for patients with and without heart failure (P value for interaction = 0.39).
Discussion
Although the use of ACEI and ARB therapy has been shown to reduce cardiovascular events and enhance survival in patients with CAD, their association with new-onset AF is less well understood. To our knowledge, this study is among the first to evaluate the impact of ACEI/ARB therapy on the occurrence of AF in patients with CAD. We found no independent relationship between ACEI/ARB therapy and the development of new-onset AF following hospitalization for CAD, either in the entire patient cohort or within each of the CAD subgroups.
Renin-angiotensin-system and atrial fibrillation
Atrial fibrillation is a progressive disease, associated with structural and electrical remodelling of the left atrium. Many of these changes are a consequence of alterations in preload or afterload states, often accompanying hypertension and heart failure. Clinical and experimental studies suggest that complex interactions between different pathways involving inflammation,19 oxidative stress, the RAS,4,10 and transforming growth factor-β1 (TGF-B1) over-expression may be involved.20 The activation of the RAS is associated with the generation of angiotensin II, which has been shown to stimulate collagen synthesis and reduce collagenase activity in cardiac fibroblasts.8 Post-MI stimulation of angiotensin II production promotes cardiac fibrosis and remodelling of the left ventricle. Similarly, rapid atrial rates cause the increase of plasma angiotensin converting enzyme and angiotensin II levels, which may in turn result in the activation of fibroblasts, fibrosis, and consequent heterogeneity in atrial conduction tissue.10,21
As ACEI and ARB therapy prevents fibroblast growth,4,8 theoretically they may have a role in preventing new onset AF and reducing recurrent AF. Also, angiotensin II is involved in atrial electrical remodelling, through enhancement of the slow component of the delayed rectifier K+ current (IKs) resulting in shortening of the atrial refractoriness.22 Renin-angiotensin-system inhibition in turn, can exert several beneficial effects through its haemodynamic changes and modulation of the autonomic tone, as well as directly through the reduction of atrial fibrosis, modulation of refractoriness, and ion channel function.4,9
Cardiac substrate, renin-angiotensin-system and atrial fibrillation
The potential benefit of modulating the RAS has been shown in patients with hypertension and high cardiovascular risk.23,24 A 33% reduction in new-onset AF was found in the Losartan Intervention for Endpoint Reduction in Hypertension study, AF over a 4.8 year follow-up period. The larger reduction of AF incidence with ARB (vs. atenolol) was associated with a greater reduction of left atrial size and increased regression of the left ventricular hypertrophy.24 There is evidence from hypertension trials that patients with structural and/or functional abnormalities benefit most from RAS inhibition.12,23 Most studies with RAS modulation in patients with left ventricular dysfunction demonstrate a reduction in AF burden.25,26 Reduced cardiac function is associated with atrial distension, which in turn is linked to the opening of stretch-activated channels, which may be responsible for increasing atrial vulnerability via shortening of the atrial refractory period.27 Renin-angiotensin-system inhibition reduces afterload, left ventricular systolic stress, and left atrial pressure.13 Recent trials have shown that RAS inhibition in CHF results in an overall 44% relative risk reduction for the development of AF.26 Of note, a direct relation has been shown between the extent of cardiac dysfunction and degree of benefit from RAS modulation.
However, a recently published meta-analysis found substantial heterogeneity in the studies evaluating the relationship between RAS inhibition and AF, even when the analysis was restricted to trials of patients with hypertension or heart failure.28 Furthermore, because the number of existing studies is somewhat limited, there is a possibility that the observed benefits of RAS inhibition are over-estimated because of publication bias. Consistent with this, in post-MI patients, the results of RAS inhibition on AF have been variable, and are affected by the presence of co-existing left ventricular dysfunction. The Trandolapril Cardiac Evaluation study found a benefit of trandolapril on AF burden in post-MI patients who had associated heart failure.29 In contrast, the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca-Heart Failure-3 (GISSI-3) study did not demonstrate any association between ACEI therapy and the incidence of AF in post-MI patients, the vast majority of whom did not have heart failure.30 Recent work from the GISSI-AF investigators showed that ARBs do not prevent the recurrence of AF in 1442 patients with a history of AF and an established substrate.31 Although this study examined the impact of ARBs on AF recurrence, it did raise the hypothesis that earlier intervention with an ARB could prevent AF. The fact that atrial fibrosis may occur even before AF becomes clinically manifest emphasizes the potential of upstream therapy as an even more relevant strategy, as it deals with the processes involved in the development of the substrate that supports AF.
The absence of benefit from modulating the RAS in our study cannot be ascribed to the absence of significant structural changes in our cohort, since approximately half of the patients had CHF and the impact of ACEI/ARB did not differ in the subgroups with and without CHF. Age may have played a role, as the mean age in our study was 78 years and it is possible that ACEI and ARB therapy may have little effect on the distribution and extent of atrial fibrosis in the elderly. Also, in contrast to previous reports, the majority of the patients in our study were women. Gender differences in the extent of the atrial pathology and the degree of response to ACEI/ARB therapy remain to be examined. Although the haemodynamic, electrophysiological and structural effects of ACEI and ARB therapy are considered quite comparable, it is possible that differences in specific properties of these agents may account for some of the variability in the extent of atrial remodelling and AF recurrence. Nevertheless, RAS modulation did not impact the occurrence of AF in our study, suggesting that the use of ACEI or ARB therapy in CAD should be primarily for reasons other than AF prevention.
Limitations and strengths
Despite the statistical adjustments applied to control for potential selection bias, including control of the ‘healthy-user effect’,32 it is possible that unmeasured or unknown confounders may have influenced the results in this observational study. Similarly, potential differences between RAS inhibitors and interactions or synergisms with other cardioactive medications may have influenced our results. Our administrative data does not contain detailed clinical information, such as blood pressure changes during treatment.
Our study included elderly patients enrolled in Medicare and the PACE and PAAD prescription drug benefit plans, thereby impacting the generalizability of our results to other cohorts with different patient characteristics. It is quite possible that the remodelling response to ACEI and ARB therapy is different among different age groups. Prior work has suggested that the response to angiotensin inhibition in the elderly may be different from that in younger patients, and that it may not be associated with any significant change in mechanistic measures such as aortic distensibility or improvement in long-term clinical responses in older patients.32
There could have been some level of variance in the detection of AF in some patients vs. that in others, even though the underlying incidence was the same in both groups. However, detection or surveillance bias would have been more likely to identify AF in patients receiving more clinical scrutiny and/or aggressive care. Thus, surveillance bias would have caused the appearance of a higher, not lower, incidence of AF in patients taking ACEI/ARB therapy. Although the drugs and dosages used by the patients in our cohort are reflective of real-world clinical practice, this study cannot rule out a modest protective effect of ACEI or ARB therapy as demonstrated previously by randomized clinical trials.
Conclusion
In summary, ACEI/ARB therapy initiated within 1 month after discharge following CAD hospitalization was not associated with a reduction in the incidence of new-onset AF. These findings are in contrast to recent studies that have suggested a potential anti-arrhythmic effect of ACEI/ARB, and need to be confirmed in prospective randomized studies.
Acknowledgements
All authors read and approved the final manuscript. J.P.S. conceived the study, participated in the protocol design and data analysis, and drafted the manuscript. A.K. helped in the analysis and interpretation of the data, and participated in the writing of the manuscript. R.L. performed the data analysis. P.T.E., J.A., and J.N.R. contributed to the analysis and interpretation of the data and the preparation of the manuscript. N.K.C. is the principal investigator and conceived the study, designed the protocol and drafted the manuscript. N.K.C. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: J.P.S., A.K., R.L., P.T.E., J.A., and N.K.C. have no relevant disclosures. J.R. has consulted for Pfizer, Novartis, AstraZeneca, Sanofi Aventis, Squibb, Forrest Labs, and Novo Nordisk.



