-
PDF
- Split View
-
Views
-
Cite
Cite
Akinori Sato, Yasuhiko Tanabe, Masaomi Chinushi, Yuka Hayashi, Tsuyoshi Yoshida, Eiichi Ito, Daisuke Izumi, Kenichi Iijima, Nobue Yagihara, Hiroshi Watanabe, Hiroshi Furushima, Yoshifusa Aizawa, Analysis of J waves during myocardial ischaemia, EP Europace, Volume 14, Issue 5, May 2012, Pages 715–723, https://doi.org/10.1093/europace/eur323
Close - Share Icon Share
Abstract
The aim of this study was to investigate the relationship between J-wave dynamics and arrhythmias during myocardial ischaemia in patients with vasospastic angina (VSA).
Sixty-seven consecutive patients diagnosed with VSA by a provocation test for coronary spasm were grouped according to whether they had a J wave in the baseline electrocardiograms or not (VSA-JW group, n = 14; VSA-non-JW group: n = 53). We retrospectively studied the associations between J-wave and ST-segment dynamics and induced ventricular fibrillations (VFs) during coronary spasm. In the VSA-JW group, 7 of the 14 patients showed changes in J-wave morphology and/or gains in J-wave voltage, followed by VF in 4 patients. Compared with patients without VF, the four patients with VF showed similar maximal voltage in the baseline J waves but a higher voltage during induced coronary spasms (0.57 ± 0.49 vs. 0.30 ± 0.11 mV; P = 0.011). In three patients with VF, J waves progressively increased and were accompanied by the characteristic coved-type or lambda-shaped ST-segment elevations. In the VSA-non-JW group, only four patients showed new appearances of J waves during coronary spasms and another patient without a distinct J wave developed VF. Ventricular fibrillations were induced more frequently in the VSA-JW group than in the VSA-non-JW group [4/14 (29%) vs. 1/53 (2%); P = 0.012].
J-wave augmentations were caused by myocardial ischaemia during coronary spasms. The presence and augmentation of J waves, especially prominent J waves with the characteristic ST-elevation patterns, were associated with VF.
Introduction
The J wave is denoted by a ‘notch’ or ‘slur’ at the terminal part of the QRS complex, which may be associated with ST-segment elevation.1,2 J waves are common in healthy individuals, and are considered to be benign;3,4 however, prominent J waves may be observed in some clinical situations.5–7 An association between J waves and sudden cardiac death in patients with an apparently healthy heart has been reported in cases of idiopathic ventricular fibrillation (VF)8–11 and Brugada syndrome.12,13 However, it is unknown as to whether J waves are associated with VF in ischaemic heart disease.
Vasospastic angina (VSA) refers to the spasm and subsequent occlusion of coronary arteries, leading to transmural myocardial ischaemia.14 Fatal ventricular arrhythmias, including VF, may develop during spontaneous or induced coronary spasm, causing sudden cardiac death.15–17
In the present study, we investigated the association between J-wave dynamics and arrhythmias during coronary spasm-induced myocardial ischaemia in patients with VSA.
Methods
Study design and patients
This retrospective observational study was conducted between April 2007 and June 2010 with 114 consecutive patients who underwent a coronary spasm provocation test for the diagnosis of VSA at our hospital. All patients suffered from chest pain at rest, but no electrocardiograms (ECGs) were recorded during the incidence of chest pain. To facilitate a diagnosis, the patients underwent coronary angiography with a coronary spasm provocation test. Those patients showing bundle branch blocks, atrial fibrillation, Brugada syndrome, or Wolff–Parkinson–White syndrome were excluded.
The study protocol was approved by our institutional ethics committee. Written informed consent was obtained from all patients.
Coronary spasm provocation test
Patients underwent cardiac catheterization, which included a provocation test for coronary artery spasms. All vasodilator drugs and calcium antagonists were discontinued at least 3 days before cardiac catheterization. After the exclusion of significant stenosis, acetylcholine (ACh; Daiichi Seiyaku, Tokyo, Japan) was injected into the left coronary artery (LCA) in incremental doses of 50 and 100 µg (in 10 mL of 0.9% saline) over 20 s. Acetylcholine was then injected into the right coronary artery (RCA) as described above. If ACh did not induce coronary spasms, 50 µg of intracoronary ergonovine maleate (EM; ASKA Pharmaceutical Co. Ltd, Tokyo, Japan) was injected into the RCA and LCA over a period of 5 min.
Blood pressure monitoring and 12-lead ECGs were recorded continuously during the provocation test. Coronary spasms were verified by coronary angiography 90 s after the injection of ACh or EM. Coronary spasm was defined as total or subtotal occlusion with delayed filling of the distal segment, and was associated with chest pain and/or ischaemic ST-segment elevation on ECG. ST-segment elevation was defined as an elevation ≥0.1 mV at 40 ms after the J point in two or more leads. The site of coronary artery spasm was determined to be either the left anterior descending coronary artery (LAD), left circumflex coronary artery (LCx), or RCA. The endpoint was defined as either the induction of coronary spasms or completion of the study protocol. Isosorbide dinitrate was injected to relieve spasms and/or to exclude organic coronary artery lesions. Left ventriculography was performed at the end of the study.
Electrocardiogram analysis
Standard 12-lead ECGs recorded at rest and during the provocation test for coronary spasms were scanned and magnified to 500% to measure the ECG parameters with Adobe Photoshop (version 7.0, 2002; Adobe Systems Inc., San Jose, CA, USA). The ECGs were read by two independent cardiologists blinded to the clinical characteristics and results of catheterization.
J waves were considered present when the positive deflection at the J point was ≥0.1 mV above the isoelectric line in two or more contiguous leads. The J waves were then classified as either the ‘notch-type’ or ‘slur-type’. A notch-type J wave is a positive deflection inscribed on the S wave; in comparison, a slur-type J wave is a smooth transition from the QRS segment to the ST segment (Figure 1). In this study, the peak amplitude of the J wave was measured in each ECG lead presenting a J wave, and the J wave showing the highest voltage among the 12 leads was defined as the maximal J wave. Morphological changes and/or voltage gains (≥0.1 mV) were considered to be an augmentation of the J wave.
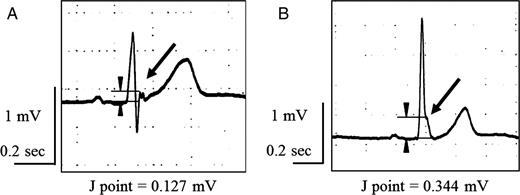
Electrocardiograms showing J-wave morphologies. (A) A notch-type J wave (arrow) is a positive deflection inscribed on the S wave. The amplitude of the J wave was measured as 0.127 mV. (B) A slur-type J wave (arrow) is a smooth transition from the QRS segment to the ST segment. The amplitude of the J wave was measured as 0.344 mV. The J wave was defined as positive when the J point was ≥0.1 mV above the isoelectric line ≥2 contiguous leads (arrowheads and lines).
The sites of the J waves were indicated in the inferior leads (II, III, and aVF), right precordial leads (V1 and V2), left precordial leads (V3–V6), and left lateral leads (I and aVL). In this study, we analysed the presence/absence or dynamics of the J wave in all 12 leads, including the right precordial leads. Baseline ECGs indicating Brugada syndrome, which is characterized by a J-point elevation (≥0.2 mV) with a coved-type ST-segment elevation in the right precordial leads,13 were not observed in any patients.
Data and statistical analyses
To determine whether J waves are affected by myocardial ischaemia during coronary spasms, we examined eligible patients diagnosed with VSA using the positive provocation test for coronary spasms. The J-wave prevalence at baseline was resolved, and the patients were divided into two groups: those with J waves and those without (VSA-JW and VSA-non-JW groups, respectively). Changes in the J–ST morphology and gains in the maximal J-wave voltage were then evaluated in ECGs recorded at baseline and during induced coronary spasms. We analysed the relationship between the J-wave location and coronary artery perfusion area in which the spasm was induced. When VF was induced during the provocation test for coronary spasms, serial changes in J–ST morphology were investigated.
Continuous variables are presented as the mean ± standard deviation and were compared between groups using unpaired Student's t-tests. Categorical variables, expressed as absolute numbers and percentages, were compared using the χ2 test or Fisher's exact test. All statistical analyses were performed using SPSS II software (version 11.0; SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered to be statistically significant.
Results
Induction of coronary artery spasms
Of the 114 patients, 67 (59%) were diagnosed with VSA based on a positive provocation test result for coronary spasms. Intracoronary ACh injected into the RCA caused coronary spasms in the RCA (n = 41), while injection into the LCA caused coronary spasms in the LAD (n = 23), LCx (n = 7), or both (n = 20). Spasms in two or more vessels were induced by ACh in 30 patients. Intracoronary EM infusion was performed in 18 patients, and coronary spasms were induced in four RCAs, one LAD, and one LCx. Ergonovine maleate infusion did not provoke multi-vessel spasms. During coronary spasm, all patients complained of chest pain, and distinct ECG changes were observed. Coronary angiography demonstrated coronary spasms, which were relieved by an intracoronary infusion of isosorbide dinitrate.
Ventricular fibrillation developed following coronary spasms in five patients; three cases were caused by spasm of the RCA, and the others were caused by simultaneous spasms of the LAD and LCx. A direct-current shock was necessary to restore the sinus rhythm from VF in two of the five cases. None of the patients experienced complications related to these diagnostic catheterizations, including the provocation test for coronary spasms.
J waves at baseline
Of the 67 patients who experienced coronary spasms, 14 (21%) had J waves in their baseline ECG (VSA-JW group: 5 with notch-type and 9 with slur-type at the end of the QRS complex), while 53 patients did not have J waves (VSA-non-JW group). None of the patients had a family history of sudden death, but three patients in the VSA-non-JW group had syncopal episodes. The clinical data and baseline ECG findings were similar between patients in both groups (Table 1). In the VSA-JW group, J waves were located in the inferior leads in seven patients and in the left lateral leads in two patients. The remaining five patients had J waves both in the inferior leads and another site (Table 2). Among the baseline ECGs, none of the patients showed a J wave and ST elevation in the right precordial lead (Brugada-like ECG abnormality).
Clinical characteristics of vasospastic-angina patients with and without J wave at baseline
| . | VSA-JW group . | VSA-non-JW group . | . |
|---|---|---|---|
| Variable . | (n = 14) . | (n = 53) . | P value . |
| Age (years) | 61.5 ± 8.2 | 63.2 ± 8.9 | 0.510 |
| Male (%) | 12 (86%) | 40 (75%) | 0.719 |
| Syncope (%) | 0 (0%) | 3 (6%) | 1.000 |
| HR (bpm) | 61.1 ± 12.5 | 65.4 ± 13.2 | 0.281 |
| PQ (ms) | 170 ± 37 | 174 ± 31 | 0.627 |
| QRS (ms) | 100 ± 8 | 101 ± 47 | 0.956 |
| QRS-axis (°) | 47 ± 32 | 40 ± 35 | 0.480 |
| QT (ms) | 418 ± 32 | 400 ± 49 | 0.183 |
| QTc (ms) | 418 ± 27 | 414 ± 49 | 0.765 |
| Organic stenosis ≥75% (%) | 3 (21%) | 12 (22%) | 1.000 |
| LVEF (%) | 63.4 ± 4.4 | 64.3 ± 6.7 | 0.627 |
| . | VSA-JW group . | VSA-non-JW group . | . |
|---|---|---|---|
| Variable . | (n = 14) . | (n = 53) . | P value . |
| Age (years) | 61.5 ± 8.2 | 63.2 ± 8.9 | 0.510 |
| Male (%) | 12 (86%) | 40 (75%) | 0.719 |
| Syncope (%) | 0 (0%) | 3 (6%) | 1.000 |
| HR (bpm) | 61.1 ± 12.5 | 65.4 ± 13.2 | 0.281 |
| PQ (ms) | 170 ± 37 | 174 ± 31 | 0.627 |
| QRS (ms) | 100 ± 8 | 101 ± 47 | 0.956 |
| QRS-axis (°) | 47 ± 32 | 40 ± 35 | 0.480 |
| QT (ms) | 418 ± 32 | 400 ± 49 | 0.183 |
| QTc (ms) | 418 ± 27 | 414 ± 49 | 0.765 |
| Organic stenosis ≥75% (%) | 3 (21%) | 12 (22%) | 1.000 |
| LVEF (%) | 63.4 ± 4.4 | 64.3 ± 6.7 | 0.627 |
The values are the mean ± SD or numbers (%). VSA, vasospastic angina; JW, J wave; HR, heart rate; QTc, corrected QT interval; LVEF, left ventricular ejection fraction.
Clinical characteristics of vasospastic-angina patients with and without J wave at baseline
| . | VSA-JW group . | VSA-non-JW group . | . |
|---|---|---|---|
| Variable . | (n = 14) . | (n = 53) . | P value . |
| Age (years) | 61.5 ± 8.2 | 63.2 ± 8.9 | 0.510 |
| Male (%) | 12 (86%) | 40 (75%) | 0.719 |
| Syncope (%) | 0 (0%) | 3 (6%) | 1.000 |
| HR (bpm) | 61.1 ± 12.5 | 65.4 ± 13.2 | 0.281 |
| PQ (ms) | 170 ± 37 | 174 ± 31 | 0.627 |
| QRS (ms) | 100 ± 8 | 101 ± 47 | 0.956 |
| QRS-axis (°) | 47 ± 32 | 40 ± 35 | 0.480 |
| QT (ms) | 418 ± 32 | 400 ± 49 | 0.183 |
| QTc (ms) | 418 ± 27 | 414 ± 49 | 0.765 |
| Organic stenosis ≥75% (%) | 3 (21%) | 12 (22%) | 1.000 |
| LVEF (%) | 63.4 ± 4.4 | 64.3 ± 6.7 | 0.627 |
| . | VSA-JW group . | VSA-non-JW group . | . |
|---|---|---|---|
| Variable . | (n = 14) . | (n = 53) . | P value . |
| Age (years) | 61.5 ± 8.2 | 63.2 ± 8.9 | 0.510 |
| Male (%) | 12 (86%) | 40 (75%) | 0.719 |
| Syncope (%) | 0 (0%) | 3 (6%) | 1.000 |
| HR (bpm) | 61.1 ± 12.5 | 65.4 ± 13.2 | 0.281 |
| PQ (ms) | 170 ± 37 | 174 ± 31 | 0.627 |
| QRS (ms) | 100 ± 8 | 101 ± 47 | 0.956 |
| QRS-axis (°) | 47 ± 32 | 40 ± 35 | 0.480 |
| QT (ms) | 418 ± 32 | 400 ± 49 | 0.183 |
| QTc (ms) | 418 ± 27 | 414 ± 49 | 0.765 |
| Organic stenosis ≥75% (%) | 3 (21%) | 12 (22%) | 1.000 |
| LVEF (%) | 63.4 ± 4.4 | 64.3 ± 6.7 | 0.627 |
The values are the mean ± SD or numbers (%). VSA, vasospastic angina; JW, J wave; HR, heart rate; QTc, corrected QT interval; LVEF, left ventricular ejection fraction.
Clinical and electrocardiogram data in 19 patients with a J wave at baseline or during provoked coronary spasms, and/or with ventricular fibrillation
| Patient . | Age (years) /sex . | At baseline . | During induced coronary spasm . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Leads of J wave . | Type of J wave . | Voltage (mV)/lead of Max-J wave . | Lead of augmented J wave . | Type of augmented J wave . | Voltage (mV)/lead of Max-J wave . | Drug/coronary resulting in augmented J wave . | Drug/coronary resulting in coronary spasm . | ST elevation . | VF . | Type of trigger premature beat . | R-on-T pattern . | Long-short sequence . |
| VSA-JW group with an augmented J wave | ||||||||||||||
| 1 | 66/M | Inf | Slur | 0.228/II | RP | Notch (coved) | 0.365/V2 | ACh/RCA | ACh/LAD, LCx, RCA | (+) | (+) | LBBB/NA | (+) | (+) |
| 2 | 43/M | Inf, LP | Slur | 0.218/aVF | RP | Notch (coved) | 0.487/V1 | ACh/RCA | ACh/ RCA | (+) | (+) | LBBB/LA | (+) | No |
| 3 | 59/M | Inf, LP | Slur | 0.105/aVF | Inf | Notch | 0.141/III | EM/RCA | EM/ RCA | (+) | (+) | LBBB/NA | (+) | No |
| 4 | 67/M | Inf, LP | Slur | 0.404/V4 | LP, LL | Slur (lambda) | 1.268/V5 | ACh/LCA | ACh/LAD, LCx | (+) | (+)a | LBBB/LA | (+) | (+) |
| 5 | 64/F | Inf | Slur | 0.158/aVF | Inf | Notch | 0.124/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 6 | 53/M | Inf | Notch | 0.141/III | Inf | Notch | 0.327/III | EM/RCA | EM/ RCA | (+) | No | |||
| 7 | 59/M | Inf, LL | Notch | 0.218/III | LP, LL | Notch | 0.467/I | ACh/LCA | ACh/LAD | No | No | |||
| VSA-JW group without an augmented J wave | ||||||||||||||
| 8 | 68/M | Inf | Slur | 0.179/III | (−) | (−) | 0.141/III | (−) | ACh/LAD, RCA | (+) | No | |||
| 9 | 57/M | Inf | Slur | 0.303/aVF | (−) | (−) | 0.320/aVF | (−) | ACh/ LCx | (+) | No | |||
| 10 | 71/M | Inf | Slur | 0.474/III | (−) | (−) | 0.442/III | (−) | ACh/LAD, LCx, RCA | (+) | No | |||
| 11 | 75/M | LL | Slur | 0.421/aVL | (−) | (−) | 0.390/aVL | (−) | ACh/ RCA | (+) | No | |||
| 12 | 64/F | Inf | Notch | 0.290/aVF | (−) | (−) | 0.252/aVF | (−) | ACh/LDA, LCx | (+) | No | |||
| 13 | 54/M | LL | Notch | 0.284/V5 | (−) | (−) | 0.293/V5 | (−) | ACh/ RCA | (+) | No | |||
| 14 | 61/M | Inf, RP | Notch | 0.294/V3 | (−) | (−) | 0.280/V3 | (−) | ACh/LCx | (+) | No | |||
| VSA-non-JW group with a new appearance of J wave | ||||||||||||||
| 15 | 63/M | (−) | (−) | 0.061/IIIb | Inf | Slur | 0.143/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 16 | 63/M | (−) | (−) | 0.052/IIIb | Inf | Notch | 0.123/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 17 | 47/F | (−) | (−) | 0.024/IIIb | Inf | Notch | 0.167/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 18 | 60/M | (−) | (−) | 0.010/aVFb | Inf | Notch | 0.140/aVF | ACh/RCA | ACh/ RCA | (+) | No | |||
| 19c | 64/M | (−) | (−) | 0.013/aVLb | aVL | Notch | 0.083/aVLb | ACh/LCA | ACh/LAD, LCx | No | (+)a | NBBB/NA | (+) | No |
| Patient . | Age (years) /sex . | At baseline . | During induced coronary spasm . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Leads of J wave . | Type of J wave . | Voltage (mV)/lead of Max-J wave . | Lead of augmented J wave . | Type of augmented J wave . | Voltage (mV)/lead of Max-J wave . | Drug/coronary resulting in augmented J wave . | Drug/coronary resulting in coronary spasm . | ST elevation . | VF . | Type of trigger premature beat . | R-on-T pattern . | Long-short sequence . |
| VSA-JW group with an augmented J wave | ||||||||||||||
| 1 | 66/M | Inf | Slur | 0.228/II | RP | Notch (coved) | 0.365/V2 | ACh/RCA | ACh/LAD, LCx, RCA | (+) | (+) | LBBB/NA | (+) | (+) |
| 2 | 43/M | Inf, LP | Slur | 0.218/aVF | RP | Notch (coved) | 0.487/V1 | ACh/RCA | ACh/ RCA | (+) | (+) | LBBB/LA | (+) | No |
| 3 | 59/M | Inf, LP | Slur | 0.105/aVF | Inf | Notch | 0.141/III | EM/RCA | EM/ RCA | (+) | (+) | LBBB/NA | (+) | No |
| 4 | 67/M | Inf, LP | Slur | 0.404/V4 | LP, LL | Slur (lambda) | 1.268/V5 | ACh/LCA | ACh/LAD, LCx | (+) | (+)a | LBBB/LA | (+) | (+) |
| 5 | 64/F | Inf | Slur | 0.158/aVF | Inf | Notch | 0.124/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 6 | 53/M | Inf | Notch | 0.141/III | Inf | Notch | 0.327/III | EM/RCA | EM/ RCA | (+) | No | |||
| 7 | 59/M | Inf, LL | Notch | 0.218/III | LP, LL | Notch | 0.467/I | ACh/LCA | ACh/LAD | No | No | |||
| VSA-JW group without an augmented J wave | ||||||||||||||
| 8 | 68/M | Inf | Slur | 0.179/III | (−) | (−) | 0.141/III | (−) | ACh/LAD, RCA | (+) | No | |||
| 9 | 57/M | Inf | Slur | 0.303/aVF | (−) | (−) | 0.320/aVF | (−) | ACh/ LCx | (+) | No | |||
| 10 | 71/M | Inf | Slur | 0.474/III | (−) | (−) | 0.442/III | (−) | ACh/LAD, LCx, RCA | (+) | No | |||
| 11 | 75/M | LL | Slur | 0.421/aVL | (−) | (−) | 0.390/aVL | (−) | ACh/ RCA | (+) | No | |||
| 12 | 64/F | Inf | Notch | 0.290/aVF | (−) | (−) | 0.252/aVF | (−) | ACh/LDA, LCx | (+) | No | |||
| 13 | 54/M | LL | Notch | 0.284/V5 | (−) | (−) | 0.293/V5 | (−) | ACh/ RCA | (+) | No | |||
| 14 | 61/M | Inf, RP | Notch | 0.294/V3 | (−) | (−) | 0.280/V3 | (−) | ACh/LCx | (+) | No | |||
| VSA-non-JW group with a new appearance of J wave | ||||||||||||||
| 15 | 63/M | (−) | (−) | 0.061/IIIb | Inf | Slur | 0.143/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 16 | 63/M | (−) | (−) | 0.052/IIIb | Inf | Notch | 0.123/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 17 | 47/F | (−) | (−) | 0.024/IIIb | Inf | Notch | 0.167/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 18 | 60/M | (−) | (−) | 0.010/aVFb | Inf | Notch | 0.140/aVF | ACh/RCA | ACh/ RCA | (+) | No | |||
| 19c | 64/M | (−) | (−) | 0.013/aVLb | aVL | Notch | 0.083/aVLb | ACh/LCA | ACh/LAD, LCx | No | (+)a | NBBB/NA | (+) | No |
VSA, vasospastic angina; JW, J wave; M, male; F, female; Inf, inferior leads; RP, right precordial leads; LP, left precordial leads; LL, left lateral lead; Ach, acetylcholine; EM, ergonovine maleate; LCA, left coronary artery; RCA, right coronary artery; LAD, left anterior descending coronary artery; LCx, circumflex coronary artery; VF, ventricular fibrillation; LBBB, left bundle branch block type; NBBB, non-bundle branch block type; NA, normal axis; LA, left axis deviation; Max-J wave, maximal J wave.
aA direct-current shock was needed to restore the sinus rhythm after VF.
bThe voltage of the Max-J wave was less than the positive criteria (≥0.1 mV).
cPatient 19 in the VSA-non JW group developed VF during coronary spasm with an embryonic J wave only in aVL.
Clinical and electrocardiogram data in 19 patients with a J wave at baseline or during provoked coronary spasms, and/or with ventricular fibrillation
| Patient . | Age (years) /sex . | At baseline . | During induced coronary spasm . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Leads of J wave . | Type of J wave . | Voltage (mV)/lead of Max-J wave . | Lead of augmented J wave . | Type of augmented J wave . | Voltage (mV)/lead of Max-J wave . | Drug/coronary resulting in augmented J wave . | Drug/coronary resulting in coronary spasm . | ST elevation . | VF . | Type of trigger premature beat . | R-on-T pattern . | Long-short sequence . |
| VSA-JW group with an augmented J wave | ||||||||||||||
| 1 | 66/M | Inf | Slur | 0.228/II | RP | Notch (coved) | 0.365/V2 | ACh/RCA | ACh/LAD, LCx, RCA | (+) | (+) | LBBB/NA | (+) | (+) |
| 2 | 43/M | Inf, LP | Slur | 0.218/aVF | RP | Notch (coved) | 0.487/V1 | ACh/RCA | ACh/ RCA | (+) | (+) | LBBB/LA | (+) | No |
| 3 | 59/M | Inf, LP | Slur | 0.105/aVF | Inf | Notch | 0.141/III | EM/RCA | EM/ RCA | (+) | (+) | LBBB/NA | (+) | No |
| 4 | 67/M | Inf, LP | Slur | 0.404/V4 | LP, LL | Slur (lambda) | 1.268/V5 | ACh/LCA | ACh/LAD, LCx | (+) | (+)a | LBBB/LA | (+) | (+) |
| 5 | 64/F | Inf | Slur | 0.158/aVF | Inf | Notch | 0.124/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 6 | 53/M | Inf | Notch | 0.141/III | Inf | Notch | 0.327/III | EM/RCA | EM/ RCA | (+) | No | |||
| 7 | 59/M | Inf, LL | Notch | 0.218/III | LP, LL | Notch | 0.467/I | ACh/LCA | ACh/LAD | No | No | |||
| VSA-JW group without an augmented J wave | ||||||||||||||
| 8 | 68/M | Inf | Slur | 0.179/III | (−) | (−) | 0.141/III | (−) | ACh/LAD, RCA | (+) | No | |||
| 9 | 57/M | Inf | Slur | 0.303/aVF | (−) | (−) | 0.320/aVF | (−) | ACh/ LCx | (+) | No | |||
| 10 | 71/M | Inf | Slur | 0.474/III | (−) | (−) | 0.442/III | (−) | ACh/LAD, LCx, RCA | (+) | No | |||
| 11 | 75/M | LL | Slur | 0.421/aVL | (−) | (−) | 0.390/aVL | (−) | ACh/ RCA | (+) | No | |||
| 12 | 64/F | Inf | Notch | 0.290/aVF | (−) | (−) | 0.252/aVF | (−) | ACh/LDA, LCx | (+) | No | |||
| 13 | 54/M | LL | Notch | 0.284/V5 | (−) | (−) | 0.293/V5 | (−) | ACh/ RCA | (+) | No | |||
| 14 | 61/M | Inf, RP | Notch | 0.294/V3 | (−) | (−) | 0.280/V3 | (−) | ACh/LCx | (+) | No | |||
| VSA-non-JW group with a new appearance of J wave | ||||||||||||||
| 15 | 63/M | (−) | (−) | 0.061/IIIb | Inf | Slur | 0.143/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 16 | 63/M | (−) | (−) | 0.052/IIIb | Inf | Notch | 0.123/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 17 | 47/F | (−) | (−) | 0.024/IIIb | Inf | Notch | 0.167/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 18 | 60/M | (−) | (−) | 0.010/aVFb | Inf | Notch | 0.140/aVF | ACh/RCA | ACh/ RCA | (+) | No | |||
| 19c | 64/M | (−) | (−) | 0.013/aVLb | aVL | Notch | 0.083/aVLb | ACh/LCA | ACh/LAD, LCx | No | (+)a | NBBB/NA | (+) | No |
| Patient . | Age (years) /sex . | At baseline . | During induced coronary spasm . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Leads of J wave . | Type of J wave . | Voltage (mV)/lead of Max-J wave . | Lead of augmented J wave . | Type of augmented J wave . | Voltage (mV)/lead of Max-J wave . | Drug/coronary resulting in augmented J wave . | Drug/coronary resulting in coronary spasm . | ST elevation . | VF . | Type of trigger premature beat . | R-on-T pattern . | Long-short sequence . |
| VSA-JW group with an augmented J wave | ||||||||||||||
| 1 | 66/M | Inf | Slur | 0.228/II | RP | Notch (coved) | 0.365/V2 | ACh/RCA | ACh/LAD, LCx, RCA | (+) | (+) | LBBB/NA | (+) | (+) |
| 2 | 43/M | Inf, LP | Slur | 0.218/aVF | RP | Notch (coved) | 0.487/V1 | ACh/RCA | ACh/ RCA | (+) | (+) | LBBB/LA | (+) | No |
| 3 | 59/M | Inf, LP | Slur | 0.105/aVF | Inf | Notch | 0.141/III | EM/RCA | EM/ RCA | (+) | (+) | LBBB/NA | (+) | No |
| 4 | 67/M | Inf, LP | Slur | 0.404/V4 | LP, LL | Slur (lambda) | 1.268/V5 | ACh/LCA | ACh/LAD, LCx | (+) | (+)a | LBBB/LA | (+) | (+) |
| 5 | 64/F | Inf | Slur | 0.158/aVF | Inf | Notch | 0.124/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 6 | 53/M | Inf | Notch | 0.141/III | Inf | Notch | 0.327/III | EM/RCA | EM/ RCA | (+) | No | |||
| 7 | 59/M | Inf, LL | Notch | 0.218/III | LP, LL | Notch | 0.467/I | ACh/LCA | ACh/LAD | No | No | |||
| VSA-JW group without an augmented J wave | ||||||||||||||
| 8 | 68/M | Inf | Slur | 0.179/III | (−) | (−) | 0.141/III | (−) | ACh/LAD, RCA | (+) | No | |||
| 9 | 57/M | Inf | Slur | 0.303/aVF | (−) | (−) | 0.320/aVF | (−) | ACh/ LCx | (+) | No | |||
| 10 | 71/M | Inf | Slur | 0.474/III | (−) | (−) | 0.442/III | (−) | ACh/LAD, LCx, RCA | (+) | No | |||
| 11 | 75/M | LL | Slur | 0.421/aVL | (−) | (−) | 0.390/aVL | (−) | ACh/ RCA | (+) | No | |||
| 12 | 64/F | Inf | Notch | 0.290/aVF | (−) | (−) | 0.252/aVF | (−) | ACh/LDA, LCx | (+) | No | |||
| 13 | 54/M | LL | Notch | 0.284/V5 | (−) | (−) | 0.293/V5 | (−) | ACh/ RCA | (+) | No | |||
| 14 | 61/M | Inf, RP | Notch | 0.294/V3 | (−) | (−) | 0.280/V3 | (−) | ACh/LCx | (+) | No | |||
| VSA-non-JW group with a new appearance of J wave | ||||||||||||||
| 15 | 63/M | (−) | (−) | 0.061/IIIb | Inf | Slur | 0.143/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 16 | 63/M | (−) | (−) | 0.052/IIIb | Inf | Notch | 0.123/III | ACh/RCA | ACh/LAD, LCx, RCA | (+) | No | |||
| 17 | 47/F | (−) | (−) | 0.024/IIIb | Inf | Notch | 0.167/III | ACh/RCA | ACh/LAD, RCA | (+) | No | |||
| 18 | 60/M | (−) | (−) | 0.010/aVFb | Inf | Notch | 0.140/aVF | ACh/RCA | ACh/ RCA | (+) | No | |||
| 19c | 64/M | (−) | (−) | 0.013/aVLb | aVL | Notch | 0.083/aVLb | ACh/LCA | ACh/LAD, LCx | No | (+)a | NBBB/NA | (+) | No |
VSA, vasospastic angina; JW, J wave; M, male; F, female; Inf, inferior leads; RP, right precordial leads; LP, left precordial leads; LL, left lateral lead; Ach, acetylcholine; EM, ergonovine maleate; LCA, left coronary artery; RCA, right coronary artery; LAD, left anterior descending coronary artery; LCx, circumflex coronary artery; VF, ventricular fibrillation; LBBB, left bundle branch block type; NBBB, non-bundle branch block type; NA, normal axis; LA, left axis deviation; Max-J wave, maximal J wave.
aA direct-current shock was needed to restore the sinus rhythm after VF.
bThe voltage of the Max-J wave was less than the positive criteria (≥0.1 mV).
cPatient 19 in the VSA-non JW group developed VF during coronary spasm with an embryonic J wave only in aVL.
J-wave dynamics during coronary spasm
J-wave augmentation during coronary spasm was noted in 7 of the 14 patients in the VSA-JW group (Patients 1–7, Table 2). An analysis of the J-wave location indicated that J-wave augmentation in the inferior or right precordial leads was associated with coronary spasms in the RCA, while those in the left precordial and left lateral leads were associated with coronary spasms in the LAD and LCx. The coronary spasms caused J-wave augmentations to be accompanied by ST-segment elevations in all but one case (Patient 7).
Slur-type J waves at baseline
During coronary spasm, slur-type J waves changed to notch-type J waves in four of nine patients who had slur-type J waves at baseline (Patients 1–3 and 5). Among these four patients, two patients (1 and 2) showed prominent gains in J-wave voltage accompanied by a characteristic ST-segment elevation in the right precordial leads (coved-type J–ST elevation) during coronary spasm in the RCA (Figure 2). On the other hand, J waves with ST elevation in the inferior leads at baseline disappeared. These notched-type J waves in the right precordial leads were morphologically similar to the coved-type J–ST elevation found in patients with Brugada syndrome. In one of the five remaining patients (Patient 4), new slur-type J waves developed with prominent gains in J-wave voltage in the left precordial and left lateral leads during spasms in the LAD and LCx (Figure 3). This J-wave dynamic was accompanied by a direct steep downward-sloping ST-segment elevation, with a configuration resembling the Greek character ‘lambda’. Conversely, the J waves at baseline (Leads III and aVF) disappeared.

Electrocardiogram of Patient 2. (A) Baseline electrocardiogram shows slur-type J waves with ST-segment elevations in the inferior and V6 leads (arrows). (B) After induction of spasms in the right coronary artery by acetylcholine, these J waves became inconspicuous, while a new J wave appeared in Lead V1. This J wave increased rapidly in amplitude and changed to a coved-type J–ST elevation (arrow heads). A premature beat with a close coupling interval (*) then developed and led to ventricular fibrillation 2.5 min after intracoronary administration of acetylcholine.
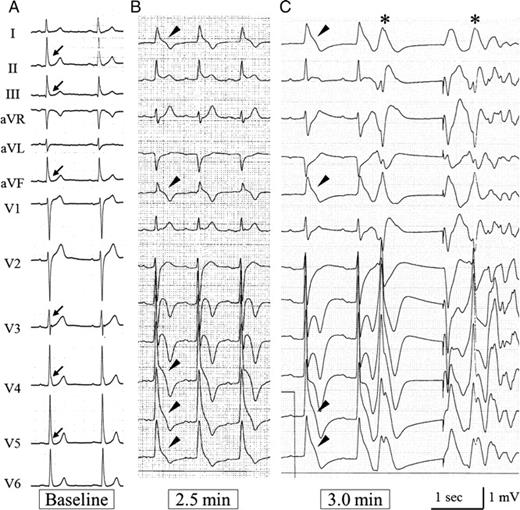
Electrocardiogram of Patient 4. (A) Baseline electrocardiogram shows slur-type J waves in the inferior and left precordial leads (arrows). (B) At 2.5 min after injection of acetylcholine into the left coronary artery, spasms were provoked in the left anterior descending and circumflex arteries and J waves developed in I, aVL, and V4–V6 leads (arrow heads). (C) These J waves were progressively augmented with a steep downward-sloping ST-segment elevation, and changed into a lambda-shaped pattern. After a long–short sequence, closely coupled premature beats (*) triggered ventricular fibrillation at 3.0 min.
Notch-type J waves at baseline
A total of two of five patients with notch-type J waves at baseline (Patients. 6 and 7) showed no change in morphology; however, they showed an increase in the maximal J-wave voltage (≥0.1 mV).
Absence of a J wave at baseline
In 4 (8%) of the 53 patients in the VSA-non-JW group (Patients 15–18), J waves developed during coronary spasms in the RCA (notch type in 3 patients and slur type in the fourth patient) that were accompanied by ST elevation in the inferior leads.
J–ST pattern and ventricular fibrillation
In the VSA-JW group, four of seven patients with J-wave augmentation developed VF (Patients 1–4, Table 2). In the VSA-JW group, the occurrence of VF was more common in patients with J-wave augmentation than in those without, but it was not significant [4/7 (57%) vs. 0/7 (0%); P = 0.070]. Immediately after J-wave augmentation with ST-segment elevation, VF was triggered by closely coupled premature beats in all cases, which presented as left bundle branch block patterns. Of the four patients with VF, three showed progressive J-wave augmentation with a steep downward-sloping ST-segment elevation (coved-type or lambda-shaped pattern), as described above. The remaining case of VF was a patient who showed a change in J-wave morphology from slur type to notch type in the inferior leads during spasms of the RCA. As compared with the 10 patients who had J waves at baseline but did not develop VF, the 4 patients with VF had similar maximal J-wave voltages in their baseline ECGs (0.24 ± 0.12 vs. 0.27 ± 0.11 mV; P = 0.984), but reached higher voltages during the induced coronary spasms (0.57 ± 0.49 vs. 0.30 ± 0.11 mV; P = 0.011).
In the VSA-non-JW group, only one patient developed VF during spasms in the LAD and LCx (Patient 19). Ventricular fibrillation was preceded by an embryonic J wave, which was 0.08 mV in the Lead aVL alone (Figure 4). Ventricular fibrillation developed more frequently in the VSA-JW group than in the VSA-non-JW group [4/14 (29%) vs. 1/53 (2%); P = 0.012]. In those patients who developed VF during induced coronary spasms, intracoronary infusions of isosorbide dinitrate were administered promptly, and coronary angiography confirmed the release of the spasms. Following the resolution of VF (either spontaneously or by directed current), no recurrence was detected; the J-wave augmentations and ST elevations then gradually diminished and returned to baseline conditions.
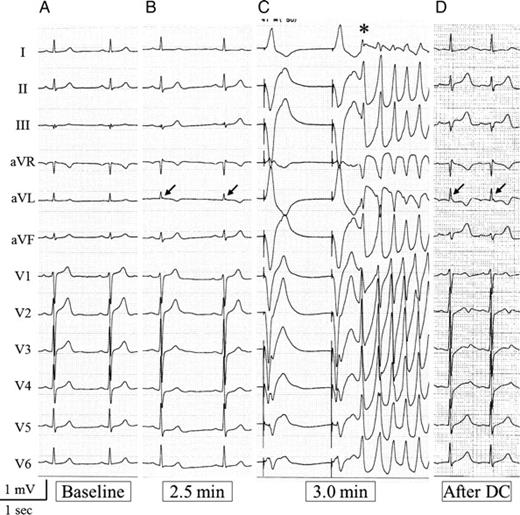
Electrocardiogram of Patient 19. (A) Baseline electrocardiogram shows no J wave. (B) At 2.5 min after injection of acetylcholine into the left coronary artery, spasms were provoked in the left anterior descending and circumflex arteries and an embryonic J wave (= 0.08 mV) developed only in the Lead aVL alone (arrow). (C) At 3.0 min, pacing beats were followed by a premature beat (*), which triggered ventricular fibrillation. (D) The small J wave (arrow) remained in the Lead aVL even after direct-current shock for restoration to sinus rhythm.
Patient follow-up
All patients with VSA were treated with a calcium channel blocker and/or nitrovasodilator; however, none of them received antiarrhythmic agents or an implantable cardioverter defibrillator. The clinical course was uneventful during the follow-up period of 26 ± 10 months.
Discussion
J-wave prevalence
In this study, 67 of 114 patients were diagnosed with VSA based on a positive provocation test result for coronary spasms, and J waves were found at baseline in 14 (21%) of the 67 patients. Since new J waves developed during the induced coronary spasms in 4 (8%) of 53 patients, 27% of the patients with VSA [(14 + 4)/67 patients] were found to have J waves at baseline and/or in the provocation study.
In a study conducted at our hospital, J waves were found in 11.5% of the Japanese control subjects and were observed significantly more often in males than females (14.7 vs. 8.1%).18 Although the subjects in the present study were predominantly male and might not have had entirely healthy hearts, the incidence of J waves in this study seems to be higher than that found in the general Japanese population.18,19 Hence, the prevalence of J waves in patients with ischaemic heart disease, particularly VSA, has not been reported. To our knowledge, only some reports that Brugada syndrome showing J waves in the right precordial leads is easy to combine with VSA described an association between J waves and VSA.20,21
While the reason for the unexpectedly high prevalence of J waves among patients with VSA remains to be determined, we showed that in most cases VF was induced not only in patients with J waves at baseline, but also in those with J-wave augmentation during coronary spasms. Experimental studies using perfused ventricular wedge preparations revealed that the transient outward-current (Ito)-mediated prominent action potential notch in the epicardium, but not in the endocardium, causes a transmural voltage gradient resulting in J waves on the surface ECG.22,23 Therefore, the presence of a J wave at baseline may be related to the heterogeneous distribution of (transmural) ventricular repolarization in patients with latent myocardial ischaemia due to coronary spasm.
J-wave and ST-segment dynamics during coronary spasm
A prominent J-wave and ST-segment elevation have been reproduced in animal models of myocardial ischaemia using perfused ventricular wedge preparations.22,24,25 Adenosine triphosphate (ATP) depletion during myocardial ischaemia causes ATP-sensitive potassium channels to open and shorten the duration of action potentials.26–28 A cascade of pathophysiological events via ischaemia decreases the inward sodium and calcium currents. These changes lead to augmentation of the transient outward current (Ito), creating transmural or spatial heterogeneity of voltage gradients and resulting in J-waves and ST-segment elevations on the ECG. A voltage gradient during early acute myocardial ischaemia can produce Phase 2 reentry and subsequent VF.25 Thus, prominent J waves with ST-segment elevation may be a warning sign for the development of VF in myocardial ischaemia as well as in Brugada syndrome.
During coronary spasm, we observed serial changes in the J waves and ST segments. Upon spasm induction, the J waves were augmented first in leads associated with ischaemic areas, followed by ST-segment elevation (including a coved-type or lambda-shaped pattern; Figures 2 and 3). These serial changes are in agreement with previous experimental findings.25,29,30 We observed that (i) VF was induced more frequently in VSA patients showing a J wave at baseline and (ii) those patients who developed VF during coronary spasm showed greater J-wave augmentation than patients without VF, suggesting an association between ischaemia-induced VF and significant heterogeneity in action potentials. In addition, the finding that most cases of VF were triggered by closely coupled premature beats originating in the ischaemic area is consistent with the above mechanism of Phase 2 reentry.25 Recently, Jastrzebski and Kukla31 and Kukla et al.32 denoted this characteristic J–ST elevation pattern as ischaemic J waves and considered it to be a warning sign for VF.
There was some discrepancy between those leads showing a J wave at baseline and those leads with an augmented J wave before the initiation of VF. For example, in Patient 2 (Figure 2), spasm of the RCA would cause the appearance of characteristic J-point and ST-segment elevations in the right precordial leads (coved-type ST elevation), followed by VF. On the other hand, the J waves in the inferior leads at baseline decreased and disappeared. Additionally, in Patient 4 (Figure 3), coronary spasms, both in the LAD and LCx, caused prominent J–ST elevation in the left precordial and left lateral leads (lambda-shaped ST elevation) due to myocardial ischaemia in parts of the anterior and lateral walls of the heart, while the slur-type J waves in Leads III and aVF present at baseline disappeared. It is reasonable to suggest that myocardial ischaemia modified the myocardial action potential configuration resulting in a larger voltage gradient through the ventricular wall and between the ischaemic and non-ischaemic myocardial lesions both in the systolic and diastolic periods of the cardiac cycle. Therefore, in those leads corresponding to the ischaemic myocardium, it is possible that the J-point and ST-segment elevation was much more obvious than the simple J-wave augmentation in these cases. On the other hand, the J waves observed at baseline (in the inferior leads in these cases) might have been concealed by the large changes in depolarization and repolarization in the surrounding myocardium during ischaemia. Since acute myocardial ischaemia due to coronary spasm can dynamically alter both the depolarization and repolarization patterns in the heart, a J wave at baseline may either be concealed/minimized or manifested/augmented depending on the severity of myocardial ischaemia and/or segment of the coronary spasm.
Interestingly, VF developed in four of the seven patients whose J waves were augmented by myocardial ischaemia due to coronary spasms, but VF was not induced in the seven patients without J-wave augmentation. Therefore, J-wave dynamics during myocardial ischaemia are clearly more important in triggering VF than is the presence of a J wave at baseline.
In the same way that a family history of sudden death or genetic mutations bears significance in J-wave-related idiopathic VF,12,33 it has been suggested that one's family history or the presence of a particular genetic alteration is a risk factor for primary VF during the acute phase of myocardial infarction.34–36 Whether one's genetic background plays a role in ischaemia-induced J–ST changes or the development of VF in patients with VSA is an important question to be explored in the future.
Study limitations
This study was retrospective and included a small number of patients. However, the J-wave augmentations with a characteristic ST-segment elevation induced by myocardial ischaemia were consistent with previous reports. Because the extent and duration of myocardial ischaemia induced by coronary spasm could not be controlled in this clinical study, the effect of myocardial ischaemia on the augmentation of J waves and the development of VF might not be the same in every patient. However, three of five patients developed VF during spasm in a single vessel (the RCA); not all cases of VF were induced by multi-vessel spasms. These results imply that even extensive ischaemia did not cause VF. However, the relationship between the extent of induced ischaemia, J-wave augmentation, and the development of VF needs to be clarified.
Conclusions
This study demonstrated that myocardial ischaemia during provoked coronary spasms altered J-wave dynamics, including the augmentation and development of J waves. The presence and augmentation of J waves, especially prominent J waves with characteristic ST-segment elevations, were often associated with ischaemia-induced VF.
Conflict of interest: none declared.



