-
PDF
- Split View
-
Views
-
Cite
Cite
Frank Bode, Frank Himmel, Michael Reppel, Kai Mortensen, Heribert Schunkert, Uwe K.H. Wiegand, Should all dysfunctional high-voltage leads be extracted? Results of a single-centre long-term registry, EP Europace, Volume 14, Issue 12, December 2012, Pages 1764–1770, https://doi.org/10.1093/europace/eus202
Close - Share Icon Share
Abstract
A considerable number of lead defects occurs during long-term cardioverter defibrillator therapy. Evidence-based strategies for the handling of chronically implanted, non-functional high-voltage (HV) leads are mandatory.
Patient outcome after abandonment of HV leads was retrospectively compared with patient outcome following other lead revision strategies and following primary implantation. A total of 903 consecutive patients undergoing 997 implantable cardioverter defibrillator (ICD) implantations or lead revisions were followed for a mean period of 48.8 ± 37.8 months. One or more additional HV leads were placed in 60 patients. An additional pace/sense lead was implanted in 13 patients. Extraction and replacement of a dysfunctional HV lead was performed in 21 patients. The overall rate of complications including artefact sensing, ineffective defibrillation, symptomatic subclavian vein thrombosis, and other lead defects did not differ between patients with and without an additional HV lead (10.0 vs. 8.9%, P = 0.32). Survival without lead associated complications did not differ between groups. Results remained unchanged after correction for covariates.
Abandoned HV leads did not increase the risk of ICD system-related complications in the majority of patients. Thus, a general lead extraction policy of dysfunctional HV leads cannot be advised in an average ICD population. Recommendations may not apply for young and physically active patients, in whom HV lead extraction must be considered.
Comparison of three different lead replacement strategies.
Largest database on this topic.
Covering 17 years of ICD therapy.
Lead abandonment appears safe.
Extraction of dysfunctional ICD leads cannot be generally recommended.
Introduction
With expanding indications for implantable cardioverter defibrillator (ICD) therapy, the use of ICD systems has markedly increased over the past decade. As a consequence, the number of patients in long follow-up after ICD implantation is steadily rising. Due to the complexity of its design, the right ventricular high-voltage (HV) lead is the most fragile part of the ICD system, and long-term ICD therapy is accompanied by a considerable incidence of HV lead defects. In recent studies, the estimated survival rate of HV leads ranged from 85 to 95% after 5 years to 60 to 72% after 8 years,1–3 implying that lead failure is common and failure rates may not be linear over time.
A defect of a chronically (>1 year) implanted HV lead can be treated using three major strategies. When the defect can be clearly localized to the pace/sense (P/S) portion of the HV lead proximal to the yoke, it could be adequate to implant a bipolar pacemaker lead in order to replace the defective portion. This strategy is not appropriate for leads under advisory due to potential defects at multiple electrode locations including the HV conductors. In other cases, either extraction or abandonment of the defective HV lead is possible. Lead extraction is a potentially dangerous procedure in chronically implanted leads requiring special experience and tools. Studies suggest that morbidity is higher in ICD lead extraction as compared with pacemaker lead extraction.4,5 Even in experienced hands, lead extraction may lead to serious complications like cardiac tamponade or severe vascular injury.6 The stand-by of a cardiac surgeon is mandatory, yet mortality approaches 1%.7,8 Lead abandonment might also be harmful, since the presence of two or more HV leads may promote a thrombosis of the subclavian vein or complicate future lead removal. Multiple HV leads could theoretically impair proper ICD function by lead-to-lead interaction. Current might be shunted to the abandoned lead.
The aim of the present study was to investigate whether the presence of an abandoned HV lead increases complication risks of ICD therapy. Therefore our institutional database of consecutive pectoral ICD implantations over a 17-year period and the respective follow-up data were analysed.
Patients and methods
Database
A total of 903 patient records of the ICD clinic at the University of Luebeck were evaluated. At the time of inclusion in this database, patients gave informed consent to analyse their data for scientific purposes. All patients receiving transvenous ICD implantations, generator exchanges, and lead revisions between January 1993 and December 2009 were included. Follow-up data were retrieved from the database until December 2010.
Institutional strategy of lead revision
The revision strategy for chronically (>1 year) implanted HV leads remained unchanged throughout the data assessment period. In case of lead or pocket infection, the whole ICD system including generator and leads was explanted. Lead removal was attempted primarily by transvenous closed-chest extraction. When large thrombi adherent to the leads were present or transvenous extraction had failed, leads were removed by surgical open-chest intervention. In case of conductor or insulation defect, the HV lead was abandoned and a new HV lead was implanted. There were two exceptions from this strategy: (i) When the lead defect could be localized to the proximal P/S portion of the HV lead proximal to the yoke and the lead was not under advisory due to potential defects at multiple electrode locations, it was recommended to implant an additional P/S lead only and to continue using the HV part of the ICD lead. (ii) In younger patients (typically <50 years of age) complete replacement of the HV lead was proposed. In each case, the final revision strategy was left to the discretion of the implanting physician after informed consent of the patient.
Endpoints and follow-up
Patients were followed by our outpatient clinic in 3–6-month intervals. In patients lost to follow-up, mortality data were retrieved from the population record section at the time of database closure. Adverse events were defined as: (i) oversensing of myopotentials and artefacts identified either by stored electrogram episodes that were inappropriately classified as sustained or non-sustained ventricular tachycardia/ventricular fibrillation or by a significant number of short ventricular intervals, (ii) lead defects, (iii) inefficient defibrillation therapy, and (iv) symptomatic thrombosis of the subclavian vein verified by phlebography. Patient survival without any of these adverse events defined the combined endpoint. Patients were subdivided into four groups according to the type of intervention: first implants (A), complete extraction of an infectious or dysfunctional HV lead and placement of a new lead (B), implantation of an additional P/S lead (C), and placement of an additional HV lead (D). Apart from intergroup comparisons, patients with a single right ventricular lead were compared with patients with two or more right ventricular leads. Patient follow-up was censored after the first adverse event.
Statistical analysis
Normally distributed values were compared by Student's t-test. Multiple comparisons were performed by one-way analysis of variance and post hoc analysis using the Bonferoni procedure. Non-parametric values were analysed by Wilcoxon test and multiple comparisons by Kolgerow–Smirnov test with post hoc analysis using the Neminye procedure. Frequencies were compared by χ2 test or Fisher's exact test. Survival curves were analysed by Cox regression curves and corrected for confounders like patient age, sex, left ventricular ejection fraction, presence of coronary artery disease, diabetes mellitus, renal insufficiency, history of former generator exchange or operative lead revision, and type of ICD generator (single chamber, dual chamber, or CRT-D). These confounders were included in a multivariate analysis to identify predictors of survival without an adverse event. A P value >0.05 was considered significant.
Results
Baseline characteristics
A total of 997 HV lead implantations were performed in 903 patients. The study population included 903 first ICD implantations and 94 reoperations for HV lead defects. Of these 94 patients, 60 received an additional HV lead and 13 an additional P/S lead using the HV part of the previously implanted HV lead. In another 21 patients, the HV lead was completely removed and a new one was implanted. Table 1 shows the baseline characteristics of the patient population. At the time of ICD implantation, mean age was 64 ± 11 years, 81% of patients were male. Mean left ventricular ejection fraction was 33 ± 11%. A total of 63% of patients had coronary artery disease. Diabetes mellitus was present in 18% and severe renal insufficiency in 12% of patients, indicated by a serum creatinin level ≥2.5 mg/dL. Single-chamber ICDs were implanted in 50%, dual-chamber devices in 28%, and CRT–ICDs in 22% of patients (Table 1). Generator exchanges were performed in 196 patients (22%). The use of HV leads with elevated failure rate, manufacturer advisory, or safety alert9 was not different between groups.
| . | First implant (n = 903) . | Additional HV lead (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Age (years) | 64.4 ± 11.4 | 65.8 ± 10.6 | 61.9 ± 9.6 | 57.2 ± 14.2 | 0.02 |
| Male gender (%) | 80.8 | 75.0 | 69.2 | 90.5% | 0.31 |
| LVEF (%) | 32.5 ± 10.9 | 36.6 ± 11.3 | 30.3 ± 5.4 | 36.3 ± 17.4 | 0.02 |
| Coronary artery disease (%) | 62.5 | 60.0 | 38.5 | 57.1 | 0.33 |
| Diabetes mellitus (%) | 18.1 | 10.0 | 23.1 | 38.1 | 0.04 |
| Serum creatinine ≥2.5 mg/dL (%) | 11.5 | 3.3 | 0 | 9.5 | 0.14 |
| 1/2/3 chamber devices (%) | 50/28/22 | 58/25/17 | 23/46/31 | 67/24/10 | 0.20 |
| Lead designs with known material deficiency (%) | 9.6 | 8.3 | 0 | 9.5 | 0.67 |
| Follow-up period (months) | 49.1 ± 37.8 | 46.8 ± 37.3 | 39.7 ± 39.9 | 48.9 ± 37.8 | 0.79 |
| . | First implant (n = 903) . | Additional HV lead (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Age (years) | 64.4 ± 11.4 | 65.8 ± 10.6 | 61.9 ± 9.6 | 57.2 ± 14.2 | 0.02 |
| Male gender (%) | 80.8 | 75.0 | 69.2 | 90.5% | 0.31 |
| LVEF (%) | 32.5 ± 10.9 | 36.6 ± 11.3 | 30.3 ± 5.4 | 36.3 ± 17.4 | 0.02 |
| Coronary artery disease (%) | 62.5 | 60.0 | 38.5 | 57.1 | 0.33 |
| Diabetes mellitus (%) | 18.1 | 10.0 | 23.1 | 38.1 | 0.04 |
| Serum creatinine ≥2.5 mg/dL (%) | 11.5 | 3.3 | 0 | 9.5 | 0.14 |
| 1/2/3 chamber devices (%) | 50/28/22 | 58/25/17 | 23/46/31 | 67/24/10 | 0.20 |
| Lead designs with known material deficiency (%) | 9.6 | 8.3 | 0 | 9.5 | 0.67 |
| Follow-up period (months) | 49.1 ± 37.8 | 46.8 ± 37.3 | 39.7 ± 39.9 | 48.9 ± 37.8 | 0.79 |
HV, high voltage; P/S, pace/sense; LVEF, left ventricular ejection fraction.
| . | First implant (n = 903) . | Additional HV lead (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Age (years) | 64.4 ± 11.4 | 65.8 ± 10.6 | 61.9 ± 9.6 | 57.2 ± 14.2 | 0.02 |
| Male gender (%) | 80.8 | 75.0 | 69.2 | 90.5% | 0.31 |
| LVEF (%) | 32.5 ± 10.9 | 36.6 ± 11.3 | 30.3 ± 5.4 | 36.3 ± 17.4 | 0.02 |
| Coronary artery disease (%) | 62.5 | 60.0 | 38.5 | 57.1 | 0.33 |
| Diabetes mellitus (%) | 18.1 | 10.0 | 23.1 | 38.1 | 0.04 |
| Serum creatinine ≥2.5 mg/dL (%) | 11.5 | 3.3 | 0 | 9.5 | 0.14 |
| 1/2/3 chamber devices (%) | 50/28/22 | 58/25/17 | 23/46/31 | 67/24/10 | 0.20 |
| Lead designs with known material deficiency (%) | 9.6 | 8.3 | 0 | 9.5 | 0.67 |
| Follow-up period (months) | 49.1 ± 37.8 | 46.8 ± 37.3 | 39.7 ± 39.9 | 48.9 ± 37.8 | 0.79 |
| . | First implant (n = 903) . | Additional HV lead (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Age (years) | 64.4 ± 11.4 | 65.8 ± 10.6 | 61.9 ± 9.6 | 57.2 ± 14.2 | 0.02 |
| Male gender (%) | 80.8 | 75.0 | 69.2 | 90.5% | 0.31 |
| LVEF (%) | 32.5 ± 10.9 | 36.6 ± 11.3 | 30.3 ± 5.4 | 36.3 ± 17.4 | 0.02 |
| Coronary artery disease (%) | 62.5 | 60.0 | 38.5 | 57.1 | 0.33 |
| Diabetes mellitus (%) | 18.1 | 10.0 | 23.1 | 38.1 | 0.04 |
| Serum creatinine ≥2.5 mg/dL (%) | 11.5 | 3.3 | 0 | 9.5 | 0.14 |
| 1/2/3 chamber devices (%) | 50/28/22 | 58/25/17 | 23/46/31 | 67/24/10 | 0.20 |
| Lead designs with known material deficiency (%) | 9.6 | 8.3 | 0 | 9.5 | 0.67 |
| Follow-up period (months) | 49.1 ± 37.8 | 46.8 ± 37.3 | 39.7 ± 39.9 | 48.9 ± 37.8 | 0.79 |
HV, high voltage; P/S, pace/sense; LVEF, left ventricular ejection fraction.
Some baseline characteristics were inadequately distributed between the four intervention groups (Table 1). Patients undergoing lead extraction were younger than the other patient groups, had a higher left ventricular ejection fraction and a higher incidence of diabetes mellitus. Other parameters showed no significant differences between groups.
Patient outcome with different lead replacement strategies
The incidence of adverse events with different HV lead replacement strategies as compared with first implants is shown in Tables 2 and 3. The risk of symptomatic thrombosis of the subclavian vein tended to be higher after implantation of an additional HV lead than in the other groups. During a mean follow-up period of 48.8 ± 37.8 months, mechanical defects of the HV lead requiring replacement occurred in 7.3% of patients without significant difference between the groups. Lead defects manifested predominantly by artefact sensing (67%). Sensing of artefacts tended to be more frequent in patients who already had received a lead replacement, irrespective of whether a P/S lead or an additional HV lead was placed. Patients after lead extraction and subsequent reimplantation of a new HV lead were prone to artefact sensing in particular. Electromyopotential oversensing was due to insulation defect of the HV lead in 94% of cases and required operative lead replacement. Ineffective shock delivery due to an increase of defibrillation threshold was not documented after any lead replacement. In 98% of cases, electromyopotential oversensing was associated with other indicators of HV lead defects. A HV lead defect was associated with unsuccessful shock delivery in one patient.
| . | First implant (n = 903) . | Additional HV lead(s) (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts | 42 (4.7%) | 4 (6.7%) | 1 (7.7%) | 4 (19.0%) | 0.11 |
| HV lead defect | 63 (7.0%) | 4 (6.7%) | 1 (7.7%) | 3 (14.3%) | 0.64 |
| Unsuccessful HV delivery | 3 (0.3%) | 0 | 0 | 0 | 0.96 |
| Symptomatic thrombosis of subclavian vein | 8 (0.9%) | 2 (3.3%) | 0 | 0 | 0.29 |
| Combined endpoint | 78 (8.6%) | 6 (10.0%) | 1 (7.7%) | 4 (19.0%) | 0.42 |
| Death | 142 (15.7%) | 6 (10.0%) | 2 (15.4%) | 2 (9.5%) | 0.54 |
| . | First implant (n = 903) . | Additional HV lead(s) (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts | 42 (4.7%) | 4 (6.7%) | 1 (7.7%) | 4 (19.0%) | 0.11 |
| HV lead defect | 63 (7.0%) | 4 (6.7%) | 1 (7.7%) | 3 (14.3%) | 0.64 |
| Unsuccessful HV delivery | 3 (0.3%) | 0 | 0 | 0 | 0.96 |
| Symptomatic thrombosis of subclavian vein | 8 (0.9%) | 2 (3.3%) | 0 | 0 | 0.29 |
| Combined endpoint | 78 (8.6%) | 6 (10.0%) | 1 (7.7%) | 4 (19.0%) | 0.42 |
| Death | 142 (15.7%) | 6 (10.0%) | 2 (15.4%) | 2 (9.5%) | 0.54 |
HV, high voltage; P/S, pace/sense.
| . | First implant (n = 903) . | Additional HV lead(s) (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts | 42 (4.7%) | 4 (6.7%) | 1 (7.7%) | 4 (19.0%) | 0.11 |
| HV lead defect | 63 (7.0%) | 4 (6.7%) | 1 (7.7%) | 3 (14.3%) | 0.64 |
| Unsuccessful HV delivery | 3 (0.3%) | 0 | 0 | 0 | 0.96 |
| Symptomatic thrombosis of subclavian vein | 8 (0.9%) | 2 (3.3%) | 0 | 0 | 0.29 |
| Combined endpoint | 78 (8.6%) | 6 (10.0%) | 1 (7.7%) | 4 (19.0%) | 0.42 |
| Death | 142 (15.7%) | 6 (10.0%) | 2 (15.4%) | 2 (9.5%) | 0.54 |
| . | First implant (n = 903) . | Additional HV lead(s) (n = 60) . | Additional P/S lead (n = 13) . | Lead extraction (n = 21) . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts | 42 (4.7%) | 4 (6.7%) | 1 (7.7%) | 4 (19.0%) | 0.11 |
| HV lead defect | 63 (7.0%) | 4 (6.7%) | 1 (7.7%) | 3 (14.3%) | 0.64 |
| Unsuccessful HV delivery | 3 (0.3%) | 0 | 0 | 0 | 0.96 |
| Symptomatic thrombosis of subclavian vein | 8 (0.9%) | 2 (3.3%) | 0 | 0 | 0.29 |
| Combined endpoint | 78 (8.6%) | 6 (10.0%) | 1 (7.7%) | 4 (19.0%) | 0.42 |
| Death | 142 (15.7%) | 6 (10.0%) | 2 (15.4%) | 2 (9.5%) | 0.54 |
HV, high voltage; P/S, pace/sense.
| . | One HV lead (n = 937) . | Additional HV lead(s) (n = 60) . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts (%) | 47 (5.0%) | 4 (6.7%) | 1.35 | 0.47–3.89 | 0.57 |
| HV lead defect (%) | 68 (7.3%) | 4 (6.7%) | 0.91 | 0.32–2.59 | 0.94 |
| Unsuccessful HV delivery (%) | 3 (0.3%) | 0 | – | – | 0.83 |
| Symptomatic thrombosis of subclavian vein (%) | 8 (0.9%) | 2 (3.3%) | 4.00 | 0.83–19.29 | 0.12 |
| Combined endpoint (%) | 83 (8.9%) | 6 (10.0%) | 0.71 | 0.36–1.39 | 0.32 |
| Death (%) | 146 (15.6%) | 6 (10.0%) | 0.62 | 0.25–1.43 | 0.29 |
| . | One HV lead (n = 937) . | Additional HV lead(s) (n = 60) . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts (%) | 47 (5.0%) | 4 (6.7%) | 1.35 | 0.47–3.89 | 0.57 |
| HV lead defect (%) | 68 (7.3%) | 4 (6.7%) | 0.91 | 0.32–2.59 | 0.94 |
| Unsuccessful HV delivery (%) | 3 (0.3%) | 0 | – | – | 0.83 |
| Symptomatic thrombosis of subclavian vein (%) | 8 (0.9%) | 2 (3.3%) | 4.00 | 0.83–19.29 | 0.12 |
| Combined endpoint (%) | 83 (8.9%) | 6 (10.0%) | 0.71 | 0.36–1.39 | 0.32 |
| Death (%) | 146 (15.6%) | 6 (10.0%) | 0.62 | 0.25–1.43 | 0.29 |
HV, high voltage; HR, hazard ratio; CI, confidence interval.
| . | One HV lead (n = 937) . | Additional HV lead(s) (n = 60) . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts (%) | 47 (5.0%) | 4 (6.7%) | 1.35 | 0.47–3.89 | 0.57 |
| HV lead defect (%) | 68 (7.3%) | 4 (6.7%) | 0.91 | 0.32–2.59 | 0.94 |
| Unsuccessful HV delivery (%) | 3 (0.3%) | 0 | – | – | 0.83 |
| Symptomatic thrombosis of subclavian vein (%) | 8 (0.9%) | 2 (3.3%) | 4.00 | 0.83–19.29 | 0.12 |
| Combined endpoint (%) | 83 (8.9%) | 6 (10.0%) | 0.71 | 0.36–1.39 | 0.32 |
| Death (%) | 146 (15.6%) | 6 (10.0%) | 0.62 | 0.25–1.43 | 0.29 |
| . | One HV lead (n = 937) . | Additional HV lead(s) (n = 60) . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Sensing of artefacts (%) | 47 (5.0%) | 4 (6.7%) | 1.35 | 0.47–3.89 | 0.57 |
| HV lead defect (%) | 68 (7.3%) | 4 (6.7%) | 0.91 | 0.32–2.59 | 0.94 |
| Unsuccessful HV delivery (%) | 3 (0.3%) | 0 | – | – | 0.83 |
| Symptomatic thrombosis of subclavian vein (%) | 8 (0.9%) | 2 (3.3%) | 4.00 | 0.83–19.29 | 0.12 |
| Combined endpoint (%) | 83 (8.9%) | 6 (10.0%) | 0.71 | 0.36–1.39 | 0.32 |
| Death (%) | 146 (15.6%) | 6 (10.0%) | 0.62 | 0.25–1.43 | 0.29 |
HV, high voltage; HR, hazard ratio; CI, confidence interval.
Univariate and multivariate analysis of patient survival without an adverse event are displayed in Figures 1 and 2. Lead revision strategy had no influence on event-free survival. However, several clinical parameters contributed to the occurrence of the combined endpoint: lower left ventricular ejection fraction, diabetes mellitus, coronary artery disease, and severe renal insufficiency significantly reduced event-free survival (Table 4).
| . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| Patient age (per year of age) | 1.01 | 0.99–1.03 | 0.06 |
| Male gender | 1.08 | 0.77–1.55 | 0.68 |
| LV ejection fraction (per per cent) | 0.97 | 0.95–0.98 | <0.001 |
| Coronary artery disease | 1.40 | 1.02–1.90 | 0.04 |
| Diabetes mellitus | 1.47 | 1.08–2.01 | 0.04 |
| Serum creatinin ≥2.5 mg/dL | 2.93 | 2.08–3.98 | <0.001 |
| Additional right ventricular HV lead | 1.06 | 0.52–2.11 | 0.79 |
| Dual- or triple-chamber ICD | 1.31 | 0.68–2.32 | 0.53 |
| Prior generator exchange or lead revision | 1.29 | 0.89–2.07 | 0.22 |
| . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| Patient age (per year of age) | 1.01 | 0.99–1.03 | 0.06 |
| Male gender | 1.08 | 0.77–1.55 | 0.68 |
| LV ejection fraction (per per cent) | 0.97 | 0.95–0.98 | <0.001 |
| Coronary artery disease | 1.40 | 1.02–1.90 | 0.04 |
| Diabetes mellitus | 1.47 | 1.08–2.01 | 0.04 |
| Serum creatinin ≥2.5 mg/dL | 2.93 | 2.08–3.98 | <0.001 |
| Additional right ventricular HV lead | 1.06 | 0.52–2.11 | 0.79 |
| Dual- or triple-chamber ICD | 1.31 | 0.68–2.32 | 0.53 |
| Prior generator exchange or lead revision | 1.29 | 0.89–2.07 | 0.22 |
HV, high voltage; CI, confidence interval; LV, left ventricular.
| . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| Patient age (per year of age) | 1.01 | 0.99–1.03 | 0.06 |
| Male gender | 1.08 | 0.77–1.55 | 0.68 |
| LV ejection fraction (per per cent) | 0.97 | 0.95–0.98 | <0.001 |
| Coronary artery disease | 1.40 | 1.02–1.90 | 0.04 |
| Diabetes mellitus | 1.47 | 1.08–2.01 | 0.04 |
| Serum creatinin ≥2.5 mg/dL | 2.93 | 2.08–3.98 | <0.001 |
| Additional right ventricular HV lead | 1.06 | 0.52–2.11 | 0.79 |
| Dual- or triple-chamber ICD | 1.31 | 0.68–2.32 | 0.53 |
| Prior generator exchange or lead revision | 1.29 | 0.89–2.07 | 0.22 |
| . | Hazard ratio . | 95% CI . | P value . |
|---|---|---|---|
| Patient age (per year of age) | 1.01 | 0.99–1.03 | 0.06 |
| Male gender | 1.08 | 0.77–1.55 | 0.68 |
| LV ejection fraction (per per cent) | 0.97 | 0.95–0.98 | <0.001 |
| Coronary artery disease | 1.40 | 1.02–1.90 | 0.04 |
| Diabetes mellitus | 1.47 | 1.08–2.01 | 0.04 |
| Serum creatinin ≥2.5 mg/dL | 2.93 | 2.08–3.98 | <0.001 |
| Additional right ventricular HV lead | 1.06 | 0.52–2.11 | 0.79 |
| Dual- or triple-chamber ICD | 1.31 | 0.68–2.32 | 0.53 |
| Prior generator exchange or lead revision | 1.29 | 0.89–2.07 | 0.22 |
HV, high voltage; CI, confidence interval; LV, left ventricular.
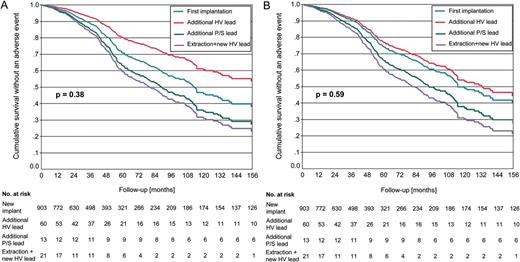
Cumulative survival without lead associated complications according to lead replacement strategy before (A) and after correction for covariates (B). No significant differences were found between patients after first implantation and patients after additional high-voltage lead placement, patients after additional pace/sense lead placement, and patients after extraction and placement of a new high-voltage lead.
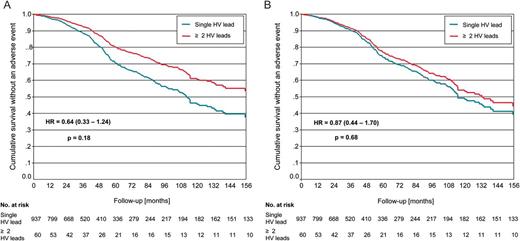
Cumulative survival without lead associated complications according to the presence of one or more high-voltage leads before (A) and after correction for covariates (B). No significant difference was found between the presence of single or multiple high-voltage leads.
Discussion
Our study in over 900 patients shows that abandoned ICD leads do not expose a significant risk and might therefore be left in place in a majority of patients. Current guidelines recommend extraction of a failing lead when implantation of another lead would result in more than four leads on one side or more than five leads through the superior vena cava.10 Extraction is also advised in rare cases where the lead imposes a severe risk, i.e. malignant arrhythmia induction. In the absence of clear guideline recommendations for the majority of lead failures where fewer leads are involved, the management of defective HV leads varies largely between different centres.11 Whether chronically implanted, inactive ICD leads should be generally extracted is an ongoing debate.
Durability of ICD leads has shown to be one of the major shortcomings of ICD therapy. Recent registries showed a 1.5–2.0% annual risk of HV lead failure which may increase during longer follow-up periods.1–3 Recent low diameter lead designs like the Sprint Fidelis™ (Medtronic, MN, USA) showed even higher rates of lead failure.9,12 Thus, every centre implanting cardioverter defibrillators faces this problem and has to develop a revision strategy. There are four major options to handle a damage of a HV lead: Direct comparisons of lead handling strategies are rare. In the present study, long-term outcome after placement of an additional HV lead was not impaired as compared with patients with a single right ventricular ICD lead. This applied to patient and lead survival as well as ineffective or inadequate defibrillator therapies. Patients after HV lead extraction even tended to show a higher incidence of artefact sensing and defects of the replacing HV lead (Figure 2). This was most likely due to the selection of younger patients for lead extraction, in whom a higher physical activity may result in more mechanical stress to the HV lead.
Repair of an insulation defect by coating it with a silicon tube: This technique may be suitable for leads with visible damage of the outer insulation layer. This approach will not be feasible in the vast majority of HV lead defects. Systematic data on long-term outcome of this technique have never been published.
Abandonment of the damaged HV lead and implantation of a new lead: Advantages and disadvantages of this approach are discussed controversially by experts. Most data on this topic originate from pacemaker therapy. Furman et al.13 reported an uncomplicated long-term course of retained pacemaker leads. In contrast, Bohm et al.14 reported about a 20% of complications in patients with abandoned leads, mainly due to lead migration and skin erosion, complications that were not observed to this extent in our study group or by other investigators.13,15,16 Long-term persistence of abandoned leads, a large number of abandoned leads (e.g. three or more), and young patient age have been identified as risk factors for venous thrombosis.15 In our patient group, the incidence of thrombosis was not significantly increased after lead abandonment. Likewise, Glikson et al.17 did not observe any symptomatic thrombosis of the subclavian vein. An additional risk might be posed by contact of two HV leads which could result in artefact sensing and inadequate ICD therapies.18 It is therefore recommended to avoid placement of the new HV lead collaterally to the old one. This is of particular importance in integrated bipolar leads with sensing between the tip electrode and the distal shock coil. Respecting this placement rule, we did not observe any artefact sensing in our patients with two or more HV leads. Abandoned HV leads were also accused to increase defibrillation threshold. Study data regarding this problem are rare. Glikson et al.17 found no change of defibrillation threshold in 43 patients before and after revision with lead abandonment. In our study, ineffective defibrillation did not occur in any patient with two or more HV leads. In support of this, no case has been described in literature where ineffective defibrillation therapy was attributable to an abandoned HV lead. Thus, abandonment of HV leads appears to be safe in the vast majority of ICD recipients.
Implantation of an additional P/S lead: This approach is possible in true bipolar leads when a lead defect can be clearly attributed to the P/S connector or to the P/S lead portion proximal to the yoke, where P/S and HV portions converge. It might also be considered in non-advisory leads when exit block or loss of sensing occurs without evidence of a mechanical lead defect. Data on this strategy are rare. Wollman et al.19reported a high incidence of mid-term complications after placement of an additional P/S lead in patients with pacing or sensing defects of integrated bipolar ICD leads. There, almost 70% of complications observed were due to a progressive defect of the HV lead documented either by oversensing or impedance rise of the HV conductor. Therefore it is not recommendable to place only a P/S lead when the location of the defect is unclear or when the HV coil is involved in sensing due to integrated bipolar electrode design. In our population, we did not observe an excess rate of reoperations in patients after clear defect localization proximal to the yoke and additional P/S lead placement.
Extraction of the HV lead: Complete lead removal appears to be the most desirable approach to handle a defective HV lead. It avoids any problem of lead interaction, reduces the potential risk of venous thrombosis, and facilitates future removal of the new HV lead. Using a sheath for removal, it may be possible to place a new lead even when the subclavian vein is occluded. However, extraction of a chronically implanted HV lead may lead to serious and potentially life-threatening complications in up to 10% of patients.5,20,21 In very experienced cardiosurgical centres, complication rates of HV lead extraction appear to be lower.4 Yet, it is unlikely that all dysfunctional leads will be extracted by experienced operators only, given the increasing number of implanting centres and a considerable electrode failure rate averaging 3% per year.
Only two registries investigated the long-term outcome of patients with HV lead failure. In concordance with our findings, Glikson et al. reported on 78 patients after abandonment of a HV ICD lead and observed no sensing malfunctions during a mean follow-up of 3 years. Defibrillation threshold did not increase.17 Wollmann et al.22 reported comparable survival and complication rates in 86 patients with HV lead defects after the lead had been either replaced or an additional HV lead had been placed. These registry data cover mean follow-up periods not exceeding 4 years. Randomized trials comparing the outcome of patients with abandoned or replaced HV leads are lacking. Thus, present evidence supports no general extraction policy of dysfunctional HV leads.
Limitations
Our data derive from a single-centre registry. The institutional policy as described in the method section had a clear influence on our results. Except for pocket or lead infection, lead extraction was avoided in patients of higher age. In result, patients undergoing lead extraction were significantly younger than those with abandoned HV leads. This was in accordance with other investigators who favoured extraction of HV leads in young and active patients,22 but might have caused a potential bias to our results. Thus, endpoints were compared not only between patients with different lead revision strategies but also with a large patient group after primary ICD implantation, and multivariate analysis was performed to correct for potential confounders.
During the 17-year follow-up covered by our registry, a considerable number of HV lead defects occurred in lead designs with specific types of material deficiency. This might have influenced the individual lead revision strategy. However, this limitation cannot be overcome by any study design and the percentage of leads with high failure rates did not differ between study groups.
Conclusions and clinical implications
Adandonment of a non-functional, non-infected HV lead, and additional implantation of a new lead did not increase the risk of subsequent major complications in our ICD patients. For a majority of ICD patients, this strategy might be recommended, particularly when patients are old or suffer from advanced cardiac disease. In young and physically active patients, however, extraction of non-functional leads remains advisable for theoretical concerns until more data on this subgroup exists.
Conflict of interest: none declared.



