-
PDF
- Split View
-
Views
-
Cite
Cite
Gian-Battista Chierchia, Mehdi Namdar, Andrea Sarkozy, Antonio Sorgente, Carlo de Asmundis, Rubén Casado-Arroyo, Lucio Capulzini, Fatih Bayrak, Moisés Rodriguez-Mañero, Danilo Ricciardi, Jayakeerthi Y. Rao, Ingrid Overeinder, Gaetano Paparella, Pedro Brugada, Verification of pulmonary vein isolation during single transseptal cryoballoon ablation: a comparison between the classical circular mapping catheter and the inner lumen mapping catheter, EP Europace, Volume 14, Issue 12, December 2012, Pages 1708–1714, https://doi.org/10.1093/europace/eus189
Close - Share Icon Share
Abstract
Cryoballoon ablation has proven very effective in achieving pulmonary vein isolation (PVI). The novel Achieve inner lumen mapping catheter designed to be used in conjunction with the cryoballoon, serves as both a guidewire and a mapping catheter. To our knowledge, this is the first study comparing the latter to verification of electrical isolation with the ‘traditional’ circular mapping catheter.
We assigned 40 consecutive patients matched for age and left atrial diameter suffering of paroxysmal atrial fibrillation to cryoballoon PVI using either the circular mapping catheter or the Achieve as a mapping catheter. Duration of procedure as well as fluoroscopy times were significantly lower in the Achieve group than in the circular mapping catheter group (111 ± 14 min vs. 126 ± 13 min, P < 0.005 and 22 ± 5 min vs. 29 ± 4 min, P < 0.0001, respectively). There were no significant differences between both groups in terms of mean degree of occlusion, mean minimal temperatures, and PVI. Pulmonary vein isolation could be documented by real-time recordings in 55% of veins in the Achieve group with mean time to isolation of 65 ± 23 s.
Cryoballoon ablation in conjunction with the novel Achieve is feasible, safe, and affords PVI in nearly all veins in similar proportions to the approach with the traditional guidewire. Furthermore, if compared to the procedure with the circular mapping catheter, cryoballoon ablation with the Achieve is significantly faster and associated to shorter fluoroscopy times.
Introduction
Increasing evidence underlines the efficacy of the novel cryoballoon (CB) (Arctic Front, Medtronic, Minnesota, USA) in achieving pulmonary vein isolation (PVI) in the setting of atrial fibrillation (AF) ablation.1–3 Pulmonary vein isolation in the setting of CB ablation is usually documented by positioning a circular mapping catheter (CMC) in the ostium of the veins following cryoenergy application. The novel Achieve (Medtronic, Minnesota, USA) is an inner lumen mapping catheter (ILMC) specifically designed to be used in conjunction with the CB. This catheter serves the double purpose of giving real-time (RT) electrical information during energy application and acting as a supporting guidewire during balloon positioning in the ostium. Although reports on the feasibility of PVI during CB ablation with an ILMC are available in the literature,4,5 no studies comparing the latter with the ‘traditional’ CMC have been carried out.
What's new?
The novel Achieve inner lumen mapping catheter designed to be used in conjunction with the cryoballoon, serves as both a guidewire and a mapping catheter.
The novelty of this paper is that it is the first study comparing the latter to verification of electrical isolation with the ‘traditional’ circular mapping catheter.
Methods
Patients characteristics
All patients provided written informed consent prior to the procedure. Patients were consecutively assigned to CB PVI using either the CMC or the ILMC as a mapping catheter. The two groups were subsequently matched for age and left atrial (LA) diameter and finally 40 patients were included in the analysis (20 patients in each group). Inclusion criteria for PVI with the CB were paroxysmal AF refractory at least to one anti-arrhythmic drug (AAD). To exclude the presence of thrombi in the left atrial appendage (LAA), all patients underwent two-dimensional (2D) trans-esophageal echocardiography (TEE) the day before the procedure, along with a trans-thoracic examination (TTE) enabling assessment of LA dimensions, left ventricular, and valvular function. Also, prior to procedure, detailed information on LA anatomy was obtained by computed tomographic (CT) scan. Anti-arrhythmic drug therapy was not discontinued prior to ablation.
Exclusion criteria were the presence of LA thrombus, severe uncontrolled heart failure, contraindications to general anesthesia and LA dimensions ≥ 50 mm.
Cryoballoon ablation procedure with the circular mapping catheter
All procedures were performed under general anesthesia and as has been described in detail previously.6 Through a single transseptal puncture (TSP) a 20 pole CMC (Lasso, Biosense-Webster, Inc., Diamond Bar, CA, USA) was positioned sequentially in each PV ostium to gather baseline electrical information. Following the mapping, a 23 mm or 28 mm CB (Arctic Front, Medtronic, Minnesota) was inserted through a steerable 15 F over-the-wire sheath (FlexCath, Medtronic) in the LA. The choice of the balloon diameter was determined by the LA and PV anatomy observed on the pre-procedural CT scan. However, whenever possible, the larger diameter CB was preferred. Once inflated and wedged in the PV ostium, dye was injected and vessel occlusion was evaluated according to a semiquantitative grading ranging from grade 0 (very poor occlusion) to grade 4 (perfect occlusion). For each vein, cryoablation consisted of a minimum of two applications lasting 4 min each. In order to avoid phrenic nerve palsy, a quadripolar catheter was inserted in the superior vena cava and diaphragmatic stimulation was achieved by pacing the ipsilateral phrenic nerve with a 1200 ms cycle at an output of 20 mA. During the whole procedure activated clotting time was maintained between 250 and 350 s.
Assessment of electrical isolation with the circular mapping catheter
Pulmonary vein activity was recorded with the CMC before, immediately after, and 20 min following ablation. In order to memorize the location of recorded potentials, positions of the mapping catheter were recorded fluoroscopically in left lateral and anteroposterior views in each PV ostium before ablation. Successful PVI was achieved when all PV potentials (PVP) were abolished or dissociated from atrial activity. If needed, pacing from the distal and/or proximal coronary sinus was performed to distinguish eventual farfield atrial signals from PVP recorded on the mapping catheter, respectively, for left- and right-sided PVs. Moreover, after having retrieved the 15 french sheath to the right atrium while keeping the Lasso in the left superior pulmonary vein (LSPV), a bipolar catheter was introduced through the transseptal access in the LAA and pacing was performed in order to distinguish LAA activity from PVP.
The Achieve inner lumen circular mapping catheter
The Achieve catheter is an ILMC designed to use in conjunction with CB to obtain RT PV electrograms during cryoenergy application. It is inserted in the inner lumen of the CB and placed distally to the catheter in the vein with the double purpose of being a mapping catheter and a supporting guidewire. Its shaft is composed of three different calibers: a proximal 0.034″ 132 cm long portion, a 1 cm 0.043″ segment, and a distal 0.037″ 13 cm segment. The distal part of the catheter consists in a circular loop composed of eight evenly spaced electrodes. Two loop diameters are today available on the market: 15 and 20 mm. The interelectrode distance varies between 4 and 6 mm (respectively for the 15 and 20 mm diameter).
Cryoballoon ablation procedure with the inner lumen mapping catheter
Transseptal (TS) puncture, introduction of the Flexcath sheath, and PV angiogram were already described previously. Before introducing the balloon catheter in the sheath a 20 mm diameter ILMC was inserted in the lumen of the CB. The choice of using only the 20 mm and not the 15 mm was based on the assumption that the larger diameter might yield a higher chance of contact with the PV ostium consequently enhancing the possibility of observing RT isolation. The whole system was then advanced into the LA with the ILMC used as a guidewire. Before ablation, the ILMC was positioned in the venous ostium to record baseline electrical activity. Then, the ILMC was advanced more distally similarly to a guidewire. After, the CB was wedged in the ostium and occlusion was tested with dye injection. The ablation procedure was carried out as mentioned above.
Assessment of electrical isolation with the inner lumen mapping catheter
PV activity was recorded with the ILMC at a proximal site in the ostium prior to ablation in each vein. If PVPs were visible during energy application, time to isolation was recorded when PVPs completely disappeared or were dissociated from LA activity (Figure 1). If PVPs were not visible during ablation due to a distal positioning of the ILMC, the latter was immediately retracted after completion of energy application to a more proximal position, in which PV activity had been recorded prior to ablation. Pacing maneuvers for the differentiation of eventual non-PV sources were applied as mentioned above.
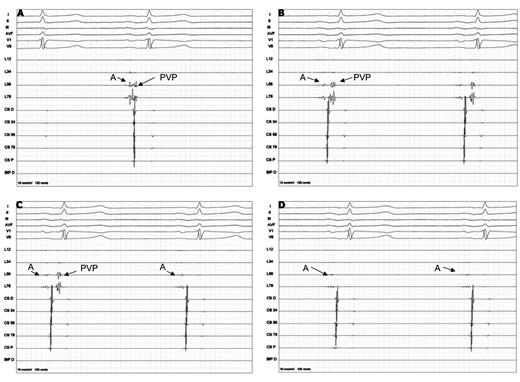
(A) Before ablation farfield left atrial signals (A) and the pulmonary vein potential are connected. (B) Progressive prolongation between the left atrial signal and the pulmonary vein potential during ablation. (C) Real-time 2:1 left atrial-pulmonary vein conduction during ablation (D) Electrical isolation during the freeze documented by real-time recordings.
Post-procedural management
All patients were dismissed the day following ablation. A 2D TTE was performed in all individuals in order to exclude postprocedural pericardial effusion. Low molecular weight heparine (LMWH) was started the same day following ablation. Oral anticoagulation (OAC) was started the day following procedure. Patients were dismissed on both OAC and LMWH. When a target international normalized ratio of 2–3 was reached LMWH was stopped and only OAC was continued. Oral anticoagulation was discontinued 3 months after the procedure. Anti-arrhythmic drug therapy was administered for 2 months following the procedure and discontinued if the patient was free of AF relapse.
Follow-up
All patients underwent Holter recordings after 1 month and subsequently every 3 months for the first year, as well as clinical evaluation as routinely performed in our center. All documented episodes of AF ≥ 30 s were considered a recurrence. A post-ablation ‘blanking period’ of 2 months was considered.
Statistical analysis
Statistical differences were calculated using χ2 test or Fisher's exact test when appropriate for discrete variables and t-test for continuous variables (expressed as mean values and standard deviation). Continuous variables were compared by analysis of variance for repeated measurements. Receiver operating curve analysis was performed to assess ablation time yielding the highest sensitivity and specificity for predicting a sustainable PVI. Analyses were performed using SPSS 17.0 software. A P value of <0.05 was considered statistically significant.
Results
Baseline population characteristics
There were no statistical differences in the baseline characteristics (Table 1). Mean age of the total population was 57 ± 8 years (33 male), there were no differences with regard to mean age between the two patient groups. At the preprocedural 2D TEE no patient exhibited pericardial effusion, mean LA diameter did not differ significantly between the two patient groups. A total of 160 veins were depicted on the preprocedural CT scan. No middle or accessory vein was identified. A 23 mm CB was used in nine patients (five in the ILMC group and four in the CMC group).
| Parameter . | ILMC . | CMC . | P value . |
|---|---|---|---|
| Age (years) | 56 ± 10 | 56 ± 9 | NS |
| Gender, male | 17 | 16 | NS |
| Left atrial diameter (mm) | 40 ± 3.3 | 40 ± 4 | NS |
| LV-EF (%) | 58 ± 3 | 57 ± 3 | NS |
| HTN | 3 | 4 | NS |
| Mitral valvulopathy | 1 | 0 | NS |
| Procedure time (min) | 111 ± 14 | 126 ± 13 | <0.005 |
| Fluoroscopy time (min) | 22 ± 5 | 29 ± 4 | <0.0001 |
| Parameter . | ILMC . | CMC . | P value . |
|---|---|---|---|
| Age (years) | 56 ± 10 | 56 ± 9 | NS |
| Gender, male | 17 | 16 | NS |
| Left atrial diameter (mm) | 40 ± 3.3 | 40 ± 4 | NS |
| LV-EF (%) | 58 ± 3 | 57 ± 3 | NS |
| HTN | 3 | 4 | NS |
| Mitral valvulopathy | 1 | 0 | NS |
| Procedure time (min) | 111 ± 14 | 126 ± 13 | <0.005 |
| Fluoroscopy time (min) | 22 ± 5 | 29 ± 4 | <0.0001 |
Values are expressed as mean ± standard deviation.
LV-EF, left ventricular ejection fraction; HTN, arterial hypertension.
| Parameter . | ILMC . | CMC . | P value . |
|---|---|---|---|
| Age (years) | 56 ± 10 | 56 ± 9 | NS |
| Gender, male | 17 | 16 | NS |
| Left atrial diameter (mm) | 40 ± 3.3 | 40 ± 4 | NS |
| LV-EF (%) | 58 ± 3 | 57 ± 3 | NS |
| HTN | 3 | 4 | NS |
| Mitral valvulopathy | 1 | 0 | NS |
| Procedure time (min) | 111 ± 14 | 126 ± 13 | <0.005 |
| Fluoroscopy time (min) | 22 ± 5 | 29 ± 4 | <0.0001 |
| Parameter . | ILMC . | CMC . | P value . |
|---|---|---|---|
| Age (years) | 56 ± 10 | 56 ± 9 | NS |
| Gender, male | 17 | 16 | NS |
| Left atrial diameter (mm) | 40 ± 3.3 | 40 ± 4 | NS |
| LV-EF (%) | 58 ± 3 | 57 ± 3 | NS |
| HTN | 3 | 4 | NS |
| Mitral valvulopathy | 1 | 0 | NS |
| Procedure time (min) | 111 ± 14 | 126 ± 13 | <0.005 |
| Fluoroscopy time (min) | 22 ± 5 | 29 ± 4 | <0.0001 |
Values are expressed as mean ± standard deviation.
LV-EF, left ventricular ejection fraction; HTN, arterial hypertension.
Procedural parameters
Procedure, fluoroscopy, and ablation time
Duration of procedure (considered from first groin puncture to complete sheath extraction) was significantly lower in the ILMC group (111 ± 14 min vs. 126 ± 13 min, P < 0.005). Similarly, the fluoroscopic time was significantly shorter in the Achieve group (22 ± 5 min vs. 29 ± 4 min, P < 0.0001). There was no statistical difference in the two groups in terms of ablation time when comparing homologous veins.
Degree of occlusion
In a total 131 PVs (82%), a grade 4 occlusion could be obtained with a direct approach. A grade 3 occlusion could be documented in 26 veins (16%) and in the other three a grade 2 (2%). In all grade 3 and 2 occlusions, alternative techniques such as the ‘hockey stick’ and ‘pull-down’ techniques were performed (Table 2). In only one patient the ILMC had to be exchanged with a traditional guidewire because of very poor stability of the CB in the right inferior pulmonary vein (RIPV) ostium.
| . | °C . | Occlusion grade 4 (n) . | Occlusion grade 3 (n) . | Isolation (n) . | Isol live (s) . | Time to isol (s) . | Total time (mm) . | Big diameter (mm) . | Small diameter (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| LSPV total | −51 ± 7 | 36 | 4 | 38 | – | – | 486 ± 38 | 20.5 ± 1.8 | 14.6 ± 1.9 |
| LSPV ILMC | −51 ± 6 | 19 | 1 | 19 | 14 | 61 ± 26 | 480 ± 0 | 20.4 ± 2.2 | 14.7 ± 2.2 |
| LSPV CMC | −51 ± 9 | 17 | 3 | 19 | – | – | 492 ± 54 | 20.6 ± 1.5 | 14.5 ± 1.7* |
| LIPV total | −46 ± 7 | 31 | 9 | 37 | – | – | 546 ± 109 | 18.2 ± 1.3 | 12.3 ± 1.6 |
| LIPV ILMC | −46 ± 7 | 16 | 4 | 19 | 11 | 67 ± 23 | 516 ± 88 | 18.3 ± 1.4 | 12.6 ± 1.6 |
| LIPV CMC | −47 ± 7 | 15 | 5 | 18 | – | – | 576 ± 121 | 18.1 ± 1.2 | 12.1 ± 1.5* |
| RSPV total | −51 ± 6 | 38 | 2 | 40 | – | – | 486 ± 66 | 21 ± 1.4 | 17.8 ± 1.4 |
| RSPV ILMC | −51 ± 6 | 19 | 1 | 20 | 13 | 60 ± 24 | 480 ± 0 | 20.4 ± 1.5 | 17.8 ± 1.6 |
| RSPV CMC | −50 ± 6 | 19 | 1 | 20 | – | – | 492 ± 95 | 21.4 ± 1.1 | 17.8 ± 1.1* |
| RIPV total | −45 ± 6 | 22 | 18 | 35 | – | – | 564 ± 128 | 18.0 ± 1.7 | 16.0 ± 1.7 |
| RIPV ILMC | −46 ± 6** | 12 | 8 | 17 | 6 | 76 ± 31*** | 588 ± 145** | 17.8 ± 1.6** | 15.8 ± 1.5** |
| RIPV CMC | −44 ± 6** | 10 | 10 | 18 | – | – | 540 ± 107** | 18.3 ± 1.7** | 16.1 ± 1.8*,** |
| . | °C . | Occlusion grade 4 (n) . | Occlusion grade 3 (n) . | Isolation (n) . | Isol live (s) . | Time to isol (s) . | Total time (mm) . | Big diameter (mm) . | Small diameter (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| LSPV total | −51 ± 7 | 36 | 4 | 38 | – | – | 486 ± 38 | 20.5 ± 1.8 | 14.6 ± 1.9 |
| LSPV ILMC | −51 ± 6 | 19 | 1 | 19 | 14 | 61 ± 26 | 480 ± 0 | 20.4 ± 2.2 | 14.7 ± 2.2 |
| LSPV CMC | −51 ± 9 | 17 | 3 | 19 | – | – | 492 ± 54 | 20.6 ± 1.5 | 14.5 ± 1.7* |
| LIPV total | −46 ± 7 | 31 | 9 | 37 | – | – | 546 ± 109 | 18.2 ± 1.3 | 12.3 ± 1.6 |
| LIPV ILMC | −46 ± 7 | 16 | 4 | 19 | 11 | 67 ± 23 | 516 ± 88 | 18.3 ± 1.4 | 12.6 ± 1.6 |
| LIPV CMC | −47 ± 7 | 15 | 5 | 18 | – | – | 576 ± 121 | 18.1 ± 1.2 | 12.1 ± 1.5* |
| RSPV total | −51 ± 6 | 38 | 2 | 40 | – | – | 486 ± 66 | 21 ± 1.4 | 17.8 ± 1.4 |
| RSPV ILMC | −51 ± 6 | 19 | 1 | 20 | 13 | 60 ± 24 | 480 ± 0 | 20.4 ± 1.5 | 17.8 ± 1.6 |
| RSPV CMC | −50 ± 6 | 19 | 1 | 20 | – | – | 492 ± 95 | 21.4 ± 1.1 | 17.8 ± 1.1* |
| RIPV total | −45 ± 6 | 22 | 18 | 35 | – | – | 564 ± 128 | 18.0 ± 1.7 | 16.0 ± 1.7 |
| RIPV ILMC | −46 ± 6** | 12 | 8 | 17 | 6 | 76 ± 31*** | 588 ± 145** | 17.8 ± 1.6** | 15.8 ± 1.5** |
| RIPV CMC | −44 ± 6** | 10 | 10 | 18 | – | – | 540 ± 107** | 18.3 ± 1.7** | 16.1 ± 1.8*,** |
Values are expressed as mean ± standard deviation or numbers.
Isol, isolation; NS, not significant.
*P = NS for all parameters as compared with the ILMC group.
**P < 0.0001 by analysis of variance for repeated measures within the ILMC or CMC group,
***P < 0.05 by analysis of variance for repeated measures within the ILMC.
| . | °C . | Occlusion grade 4 (n) . | Occlusion grade 3 (n) . | Isolation (n) . | Isol live (s) . | Time to isol (s) . | Total time (mm) . | Big diameter (mm) . | Small diameter (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| LSPV total | −51 ± 7 | 36 | 4 | 38 | – | – | 486 ± 38 | 20.5 ± 1.8 | 14.6 ± 1.9 |
| LSPV ILMC | −51 ± 6 | 19 | 1 | 19 | 14 | 61 ± 26 | 480 ± 0 | 20.4 ± 2.2 | 14.7 ± 2.2 |
| LSPV CMC | −51 ± 9 | 17 | 3 | 19 | – | – | 492 ± 54 | 20.6 ± 1.5 | 14.5 ± 1.7* |
| LIPV total | −46 ± 7 | 31 | 9 | 37 | – | – | 546 ± 109 | 18.2 ± 1.3 | 12.3 ± 1.6 |
| LIPV ILMC | −46 ± 7 | 16 | 4 | 19 | 11 | 67 ± 23 | 516 ± 88 | 18.3 ± 1.4 | 12.6 ± 1.6 |
| LIPV CMC | −47 ± 7 | 15 | 5 | 18 | – | – | 576 ± 121 | 18.1 ± 1.2 | 12.1 ± 1.5* |
| RSPV total | −51 ± 6 | 38 | 2 | 40 | – | – | 486 ± 66 | 21 ± 1.4 | 17.8 ± 1.4 |
| RSPV ILMC | −51 ± 6 | 19 | 1 | 20 | 13 | 60 ± 24 | 480 ± 0 | 20.4 ± 1.5 | 17.8 ± 1.6 |
| RSPV CMC | −50 ± 6 | 19 | 1 | 20 | – | – | 492 ± 95 | 21.4 ± 1.1 | 17.8 ± 1.1* |
| RIPV total | −45 ± 6 | 22 | 18 | 35 | – | – | 564 ± 128 | 18.0 ± 1.7 | 16.0 ± 1.7 |
| RIPV ILMC | −46 ± 6** | 12 | 8 | 17 | 6 | 76 ± 31*** | 588 ± 145** | 17.8 ± 1.6** | 15.8 ± 1.5** |
| RIPV CMC | −44 ± 6** | 10 | 10 | 18 | – | – | 540 ± 107** | 18.3 ± 1.7** | 16.1 ± 1.8*,** |
| . | °C . | Occlusion grade 4 (n) . | Occlusion grade 3 (n) . | Isolation (n) . | Isol live (s) . | Time to isol (s) . | Total time (mm) . | Big diameter (mm) . | Small diameter (mm) . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | . |
| LSPV total | −51 ± 7 | 36 | 4 | 38 | – | – | 486 ± 38 | 20.5 ± 1.8 | 14.6 ± 1.9 |
| LSPV ILMC | −51 ± 6 | 19 | 1 | 19 | 14 | 61 ± 26 | 480 ± 0 | 20.4 ± 2.2 | 14.7 ± 2.2 |
| LSPV CMC | −51 ± 9 | 17 | 3 | 19 | – | – | 492 ± 54 | 20.6 ± 1.5 | 14.5 ± 1.7* |
| LIPV total | −46 ± 7 | 31 | 9 | 37 | – | – | 546 ± 109 | 18.2 ± 1.3 | 12.3 ± 1.6 |
| LIPV ILMC | −46 ± 7 | 16 | 4 | 19 | 11 | 67 ± 23 | 516 ± 88 | 18.3 ± 1.4 | 12.6 ± 1.6 |
| LIPV CMC | −47 ± 7 | 15 | 5 | 18 | – | – | 576 ± 121 | 18.1 ± 1.2 | 12.1 ± 1.5* |
| RSPV total | −51 ± 6 | 38 | 2 | 40 | – | – | 486 ± 66 | 21 ± 1.4 | 17.8 ± 1.4 |
| RSPV ILMC | −51 ± 6 | 19 | 1 | 20 | 13 | 60 ± 24 | 480 ± 0 | 20.4 ± 1.5 | 17.8 ± 1.6 |
| RSPV CMC | −50 ± 6 | 19 | 1 | 20 | – | – | 492 ± 95 | 21.4 ± 1.1 | 17.8 ± 1.1* |
| RIPV total | −45 ± 6 | 22 | 18 | 35 | – | – | 564 ± 128 | 18.0 ± 1.7 | 16.0 ± 1.7 |
| RIPV ILMC | −46 ± 6** | 12 | 8 | 17 | 6 | 76 ± 31*** | 588 ± 145** | 17.8 ± 1.6** | 15.8 ± 1.5** |
| RIPV CMC | −44 ± 6** | 10 | 10 | 18 | – | – | 540 ± 107** | 18.3 ± 1.7** | 16.1 ± 1.8*,** |
Values are expressed as mean ± standard deviation or numbers.
Isol, isolation; NS, not significant.
*P = NS for all parameters as compared with the ILMC group.
**P < 0.0001 by analysis of variance for repeated measures within the ILMC or CMC group,
***P < 0.05 by analysis of variance for repeated measures within the ILMC.
Temperature
Mean minimal temperatures of the two groups were significantly higher in the inferior as compared with the superior veins (P < 0.0001, Table 2 and 3) when compared within each group. There was no statistical difference in terms of lower temperature achieved during the freeze when comparing homologous veins between the two groups.
Temperature measures according to different grades of occlusion and isolation
| . | Occlusion grade 3 . | Occlusion grade 4 . | P value . |
|---|---|---|---|
| Mean °C all | −41 ± 3 | −50 ± 6 | <0.0001 |
| Mean °C ILMC | −43 ± 2 | −50 ± 6 | <0.001 |
| Mean °C CMC | −40 ± 3* | −51 ± 6* | <0.0001 |
| Isolation YES | Isolation NO | P value | |
| Mean °C all | −49 ± 7 | −40 ± 4 | <0.0001 |
| Mean °C ILMC | −49 ± 7 | −43 ± 2 | <0.05 |
| Mean °C CMC | −49 ± 7* | −37 ± 4** | <0.001 |
| . | Occlusion grade 3 . | Occlusion grade 4 . | P value . |
|---|---|---|---|
| Mean °C all | −41 ± 3 | −50 ± 6 | <0.0001 |
| Mean °C ILMC | −43 ± 2 | −50 ± 6 | <0.001 |
| Mean °C CMC | −40 ± 3* | −51 ± 6* | <0.0001 |
| Isolation YES | Isolation NO | P value | |
| Mean °C all | −49 ± 7 | −40 ± 4 | <0.0001 |
| Mean °C ILMC | −49 ± 7 | −43 ± 2 | <0.05 |
| Mean °C CMC | −49 ± 7* | −37 ± 4** | <0.001 |
Values are expressed as mean ± standard deviation, ILMC indicates inner lumen mapping catheter, CMC circular mapping catheter,
*P = ns as compared with the ILMC group,
**P < 0.05 as compared with the ILMC group.
Temperature measures according to different grades of occlusion and isolation
| . | Occlusion grade 3 . | Occlusion grade 4 . | P value . |
|---|---|---|---|
| Mean °C all | −41 ± 3 | −50 ± 6 | <0.0001 |
| Mean °C ILMC | −43 ± 2 | −50 ± 6 | <0.001 |
| Mean °C CMC | −40 ± 3* | −51 ± 6* | <0.0001 |
| Isolation YES | Isolation NO | P value | |
| Mean °C all | −49 ± 7 | −40 ± 4 | <0.0001 |
| Mean °C ILMC | −49 ± 7 | −43 ± 2 | <0.05 |
| Mean °C CMC | −49 ± 7* | −37 ± 4** | <0.001 |
| . | Occlusion grade 3 . | Occlusion grade 4 . | P value . |
|---|---|---|---|
| Mean °C all | −41 ± 3 | −50 ± 6 | <0.0001 |
| Mean °C ILMC | −43 ± 2 | −50 ± 6 | <0.001 |
| Mean °C CMC | −40 ± 3* | −51 ± 6* | <0.0001 |
| Isolation YES | Isolation NO | P value | |
| Mean °C all | −49 ± 7 | −40 ± 4 | <0.0001 |
| Mean °C ILMC | −49 ± 7 | −43 ± 2 | <0.05 |
| Mean °C CMC | −49 ± 7* | −37 ± 4** | <0.001 |
Values are expressed as mean ± standard deviation, ILMC indicates inner lumen mapping catheter, CMC circular mapping catheter,
*P = ns as compared with the ILMC group,
**P < 0.05 as compared with the ILMC group.
Pulmonary vein isolation
At the end of all procedures all 160 PVs (100%) were isolated. A total 151 veins (94%) could be isolated solely with the CB. In the remaining nine veins (6%) (4 RIPVs, 2 left inferior pulmonary veins (LIPVs), 2 LSPVs, and 1 right superior pulmonary vein (RSPV)), the residual PVPs could be eliminated with a focal radiofrequency (RF) catheter. The conductions gaps could be observed systematically in the inferior portions of the inferior veins, in the anterior region in the LSPVs and the RSPVs. All these veins were relatively large in dimensions and had been initially approached with a 28 mm balloon. The choice of ablating the conduction gaps with a focal tip catheter instead of a smaller balloon was based on the aim to obtain a lesion as proximal as possible. Hypothetically, the 23 mm CB might have created a more distal level of isolation in these large diameter PVs. There were no differences in terms of isolation in both groups.
Isolation with the inner lumen mapping catheter
Real-time isolation recording could be observed in 44 (55%) of the 80 PVs in the ILMC group. Mean time to isolation was 65 ± 23 s. Mean time to isolation was significantly shorter when comparing the superior to the inferior veins (P < 0.05, Table 2). Furthermore, RT isolation recording was observed more frequently in the superior veins (14 and 13 vs. 11 and 6); however, the difference was not statistically significant. In 2 veins of the remaining 36 veins, cryoenergy application resulted in PVP delay. The ILMC was then positioned deeper in another branch of the same vein resulting in better occlusion and in final documentation of isolation. Of the remaining 34 veins, 4 needed a ‘focal’ RF touch-up to achieve isolation. In one large LSPV (24 of 18 mm) a CMC catheter was introduced in order to verify isolation as the ILMC diameter was considered too small for this vein. The CMC could confirm successful isolation. All the other PVs exhibited electrical isolation at a more proximal verification.
Early reconnection
Early reconnection occurred in five (6%) of veins in the Achieve group. This phenomenon was more frequently observed in the inferior PVs. In four veins (3 RIPV and 1 RSPV), LA-PV reconnection occurred immediately after the first energy application. Time to isolation was, respectively, 98, 88, and 133 s in the RIPVs and 123 s in the RSPV (mean: 110 ± 21 s). Twenty minutes after the second application, these veins were electrically isolated. Interestingly, RT isolation occurred significantly later in these veins if compared to PVs that did not exhibit reconnection (111 ± 18 s vs. 57 ± 16 s; P < 0.0001). Furthermore, as calculated by receiver operating curve analysis, occurrence of RT isolation ≥85 s was associated with early recurrence (sensitivity 100%, specificity 94% at this cut-off value, P < 0.005, area under the curve 0.97).
Only one RIPV presented electrical reconnection at 20 min following CB. In this vein, RT recordings had not been documented. A focal touch-up application abolished the recovered PVP.
Isolation with the circular mapping catheter
Following CB ablation, isolation could be documented in 75 PVs. A ‘focal’ touch-up with an RF catheter was needed in the remaining five veins (2 RIPVs, 1 LSPV and 2 LIPVs).
Early reconnection
Twenty minutes after ablation, reconnection occurred in three (4%) veins (1 LSPV and 2 RIPV). Focal applications lead to isolation of the latter.
Follow-up
At a mean follow-up of 10 ± 3 months, 38 (70%) patients did not experience arrhythmic recurrence. There was no significant difference in terms of recurrence between both groups: five and seven patients in the ILMC and in the CMC group, respectively (P = 0,7). On these, five individuals underwent a repeat ablation and were asymptomatic during further follow-up.
Complications
No serious adverse events occurred in any procedure. Transient phrenic nerve palsy was observed in two patients in the ILMC group while ablating with a 23 mm CB in the RSPV. Diaphragmactic contraction completely recovered in both cases before the termination of the procedure.
Discussion
To our knowledge, this is the first study comparing the novel Achieve ILMC to the classical CMC in terms of mapping PVP in the setting of a single TSP CB ablation. Our main findings are that (i) PV isolation with the CB using the Achieve is feasible in nearly all veins, (ii) RT recordings during CB application can be observed in roughly 55% of the veins, and (iii) CB ablation with the Achieve ILMC is significantly faster and associated to shorter fluoroscopy times.
In our study, the Achieve catheter demonstrated to be a reliable substitute to a guidewire permitting a grade 4 occlusion in 66 (82.5%) veins. In the remaining 14 veins, a ‘hockey stick’ or ‘pull down’ technique7 in conjunction with the ILMC resulted in successful isolation of nine (11%) veins. In only one patient the ILMC had to be exchanged with a guidewire because of CB instability in a RIPV. In a recent publication, Tang et al.5 describe their first experience using an ILMC (ProMap, Prorhythm, Ronkokoma, USA) in the setting of PVI with the CB. In their series, of 84 veins 8 (10%) veins could not be occluded with the CB. The authors' explanation to this finding was that all the eight veins exhibited early branching therefore creating an impediment to successful occlusion. Chun et al.8 in a recent observation conducted on 18 patients undergoing CB ablation with RT recordings from the PVs, concluded that the ILMC utilized in their study could not be used in all PVs instead of a stiff guidewire. In fact, in up to 46% of veins the ILMC had to be exchanged with a regular stiff guidewire in order to achieve occlusion. The discrepancy between our findings and the abovementioned might, in part, lay on the structural differences between the two ILMCs (Achieve and ProMap). Although the ProMap exhibits a 0.35 Fr caliber on the total catheters' length with a very soft distal tip, the Achieve's shaft is composed of three different calibers, thus guaranteeing better stability of the CB in the ostium. In fact, the ProMap catheter was originally conceived for PV mapping in conjunction with the high-intensity focused ultrasound balloon, which does not require direct balloon–tissue contact. Conversely, the CB's efficacy relies on optimal adhesion with the endocardium. Thus, the novel Achieve might provide more mechanical support when wedging the CB in the PV ostium due to its stiffer tip. Furthermore, in our series, early branching was observed in a minority of veins in the Achieve group. In all of these, occlusion could be successfully obtained by inserting the ILMC in one or the other branch.
In our study, PV RT recordings during CB ablation could be observed in 55% of veins, which is in line with earlier reports4,5,8,9 and can be explained by the fact that often the ILMC catheter has to be positioned distally to the PV sleeve in order to guarantee sufficient stability of the CB in the ostium. Furthermore, RT recordings were more frequently observed in the superior veins rather than in the inferior ones. This might be in part explained by the fact that the PV sleeves extension is typically shorter in the inferior veins as compared with the superior ones.10,11 Moreover, due to their orientation, the inferior veins are often more difficult to occlude with the CB bringing the operator to place the ILMC more distally in these veins to ensure balloon stability. In our observation, if RT isolation recording occurs after 85 s, the likelihood of early LA-PV reconnection is significantly higher. This is perfectly in line with what has been recently published.9 Furthermore, as previously described in RF studies, shorter times to PV conduction block might be associated with sustained isolation.12 Therefore, the operator might decide to stop the application and reposition the balloon with another orientation in the PV ostium in case of persistence of LA-PV conduction after 85 s. Finally, most LA-PV reconnections occurred in inferior PVs. This finding might be supported by the fact that in these veins time to isolation was longer if compared to the superior ones. However, in our opinion, the above-mentioned considerations should not be generalized based only on our findings, due to the small number of reconnected veins observed in our study.
The Achieve has been specifically designed for PVI procedures in conjunction with the CB. In our comparison, the CB procedure utilizing this novel catheter for PV mapping proved significantly faster and associated to lower fluoroscopy times. This might be explained by the fact that no exchange between mapping catheter and CB has to occur during the ILMC procedure. The sequential insertion of the CMC in each PV ostium to gather baseline electrical information, the subsequent exchange with the CB and the final re-exchange with the mapping catheter is certainly a longer procedure than RT isolation recording or immediate retraction to a more proximal site following CB.
In our study, despite the efficacy of the Achieve catheter in stabilizing the CB in the PV ostium, there was no difference observed in terms of number of isolated PVs when compared with the CMC group. A possible explanation might be that the patients in the CMC group represented our mature experience with CB ablation with a regular guidewire. Conversely, the ILMC group consisted in our very early procedures with the Achieve catheter.
Although in our center operators perform a single TSP in the setting of PVI with the CB, other electrophysiologists prefer a double puncture when using a CMC. Obviously, double TS access offers the advantage of not having to exchange catheters in the same sheath, reducing the risk of air embolism. However, although procedural times might have been slightly shorter in comparison to single TS CB ablation with a CMC, the risk of an additional TS puncture should never be underestimated.13–15 Furthermore, a very recent publication by Chan et al.16 reports a relatively high rate of iatrogenic patency of the foramen ovale following CB ablation. Although no related clinical symptoms occurred, 9 months after a CB ablation with single TS puncture, up to 31% of patients presented this finding at the TEE. Therefore, adding another TS access to the already 15 Fr needed for the CB might provoke further unnecessary trauma to the interatrial septum. In this respect, CB ablation in conjunction with the ILMC might not only overcome these issues but also reduce the risk of air embolization, as the Achieve is inserted inside the CB lumen and no catheter exchange is required. Although most veins were isolated after the first freeze in the Achieve group, we proceeded to at least two complete freezes in each PV according to our standard protocol. The reason lays in the physiological properties of ablating with cryothermal energy. In fact, repeated freeze–thaw cycles are traditionally associated to more effective lesions when compared with a single application.17,18 However, if future studies will determine a cut-off in terms of time to isolation and associated to freedom of AF on long-term follow-up with a single application, the current strategy might evolve and lead to even faster procedures.19.
Conclusion
Cryoballoon ablation in conjunction with the novel Achieve ILMC is feasible, safe, and affords PVI in nearly all veins in similar proportions to the approach with the traditional guidewire. However, RT recordings can be appreciated in only 55% of veins. Finally, when compared with the procedure with the CMC, CB ablation with the Achieve is significantly faster and associated to shorter fluoroscopy times.
Limitations
The main limitation might be due to the small sample size in our study. Future larger randomized studies are needed to confirm our findings.
Conflict of interest: G.B.C. has received compensation for teaching and proctoring services for AF solutions as well as speaker fees. A.S. and Cd.A. have received compensation for teaching as well as speaker fees for AF solutions Medtronic.
References
Author notes
The first two authors contributed equally to the study.



