-
PDF
- Split View
-
Views
-
Cite
Cite
Sebastian Spencker, Nalan Coban, Lydia Koch, Alexander Schirdewan, Dirk Mueller, Comparison of skin adhesive and absorbable intracutaneous suture for the implantation of cardiac rhythm devices, EP Europace, Volume 13, Issue 3, March 2011, Pages 416–420, https://doi.org/10.1093/europace/euq394
Close - Share Icon Share
Abstract
Wound healing is a major determent in the post-surgical course of patients (pts) after pacemaker (PM) and implantable cardioverter defibrillator (ICD) implantation. Insufficient closure may lead to serious complications with pocket infections leading to the device's explantation as the worst case scenario. In addition to the different types of suture and suture clips, a novel topical skin adhesive containing 2-octyl-cyanoacrylate is commercially available.
Over a period of 18 months, we prospectively assigned all cases of PM, ICD, and loop recorder implants either to skin adhesive (Group 1) or to absorbable intracutaneous polydioxanon suture (Group 2). Data were analysed with respect to operation time, wound infections, and healing disorders. One hundred and eighty-three pts were randomized into Group 1 [71 PMs, 60 ICD, 15 cardiac resynchronization therapy (CRT), 11 loop recorders, and 26 generator replacements]. One hundred and eighty-five pts were assigned to Group 2 (62 PMs, 70 ICD, 30 CRT, 7 loop recorders, and 16 generator replacements). There were no differences regarding sex, diabetes, renal insufficiency, corticosteroid therapy, oral anticoagulants, and acetylsalicylic asa/clopidogrel (P = n.s.). For the significantly higher amount of CRT devices (P < 0.05) in Group 2, the procedure times are given for surgeries except CRT. It was 49.1 ± 27.7min for Group 1 and 53.4 ± 31.9 min for Group 2 (P = n.s.).
Adverse events as insufficient closure, major and minor bleeding, pocket haematoma, erythema, incrustation, dehiscence, keloid, and explantation due to infection occurred significantly more often in the adhesive group (P = 0.02). The greatest impact on this result had early adverse events as insufficient closure, wound incrustation, and inflammation (9.3 vs. 6.0%; P = 0.02). We did not find any difference in long-term adverse events, infections in particular (2.7 vs. 1.6%; P = 0.47).
This study shows no benefit using skin adhesive in comparison to absorbable intracutaneous suture regarding surgery times for the implantation of cardiac rhythm devices. The rate of early adverse events after wound closure is higher after skin adhesive but no difference in long-term adverse events occurred.
Introduction
Wound closure after cardiac device implantation determines the aesthetic results after device surgery but, moreover, also has a decisive influence on the functional result. Insufficient wound healing opens the door for infections and cosmetically unfavourable scarring. The most commonly used procedure for wound closure is suturing. The use of continuous intracutaneous reabsorbable sutures is well-established and comfortable to the patients as stitches neither have to be removed nor leave suture tracks.
Using tissue adhesive for wound closure appears attractive as it is performed quickly, no specialized instruments are necessary and it does not carry a risk of needle stick for the surgeon. Moreover, no stitches remain within the wound, potentially leading to skin irritation or infectious foci. One prospective study showed significant advantages for the use of tissue adhesive (2-octyl-cyanoacrylate) regarding patient satisfaction, cost savings, and procedure times in breast surgery.1 However, no prospective and randomized data exist addressing the safety of skin adhesive compared with intracutaneous suture for wound closure after cardiac device implantation.
Methods
Over a period of 18 months, all consecutive patients scheduled for pacemaker (PM), implantable cardioverter defibrillator (ICD), and loop recorder implants and generator replacements were prospectively evaluated and followed over a period of 3 months. Two treatment groups were defined: skin closure either with topical adhesive with 2-octyl-cyanoacrylate (Group 1) or with absorbable intracutaneous polydioxanon suture (Group 2). In all patients (pts) subcuticular sutures had been applied before skin closure. Patients were unblinded assigned to either Group 1 or Group 2 according to the operation date. On even days suture with a conventional wound dressing was used and on uneven days wound closure was achieved by skin adhesive.
All implantations were performed by two specialized cardiologists (D.M. and S.S.) being well trained in the use of skin adhesive. Unipolar diathermy was used routinely to establish haemostasis. The pocket and the subcutis were closed by vicryl suture.
Adverse events were recorded for the wound closure perioperatively, during the days before discharge and at an outpatient appointment 3 months post-operatively. We defined early adverse events as occurring perioperatively or before discharge: We defined late adverse events as follows: The combined primary endpoint was the occurrence of any adverse event. Secondary endpoint was the operation time.
insufficient closure,
major bleeding with need for transfusion,
minor bleeding,
pocket haematoma with need for operative revision,
wound irritation,
incrustation.
pocket infection,
wound dehiscence
keloid formation.
Patients were included in the study if they had given informed consent. Eighteen of the screened pts refused to give informed consent and could not be included into the register. The study was approved by the local institutional review board.
Statistics were performed using the SPSS 17.0 for Windows software (SPSS Institute, Chicago, IL, USA). Qualitative data are presented as mean value ± SD. They are compared using the Student's t-test or the Mann–Whitney U test if they had no normal-distribution. Quantitative data are presented as frequencies and compared by the Pearson χ2 test. A P-value of <0.05 was considered as statistically significant.
Results
From June 2006 until December 2007 pts were included into the study. One hundred and eighty-three pts were allocated to Group 1 [71 PMs, 60 ICD, 15 cardiac resynchronization therapy (CRT), 11 loop recorders, and 26 generator replacements]. One hundred and eighty-five pts were assigned to Group 2 (62 PMs, 70 ICD, 30 CRT, 7 loop recorders, and 16 generator replacements). The baseline characteristics are displayed in Table 1 and the procedural details are shown in Table 2. The patient groups differed in two important points:
The pts in Group 1 were significantly older (70.4 ± 11.7 vs. 66.3 ± 15.0 years, P = 0.01).
Significantly more ICD and CRT-D devices were assigned into Group 2.
| Baseline characteristics . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Male sex (%) | 116 (63.4) | 135 (72.9) | 0.05 |
| Age (±SEM) | 70.4 ± 11.7 | 66.3 ± 15.0 | 0.01* |
| Coronary artery disease | 105 (57.4) | 99 (53.5) | 0.75 |
| Dilated cardiomyopathy | 28 (15.3) | 37 (20.0) | 0.24 |
| Hypertensive heartdiesease | 29 (15.7) | 19 (10.3) | 0.12 |
| Other underlying disease | 21 (11.6) | 30 (16.2) | 0.19 |
| Ejection fraction (±SEM) | 48 ± 19 | 46 ± 21 | 0.26 |
| LVEDD | 55 ± 11 | 56 ± 11 | 0.22 |
| Diabetes (%) | 45 (24.6) | 45 (24.3) | 0.93 |
| Renal disease (Creatinin >1.2) | 50 (27.3) | 51 (27.6) | 0.91 |
| Chron. steroid use | 10 (5.5) | 9 (4.9) | 0.59 |
| ASA | 98 (55.6) | 101 (54.6) | 0.84 |
| Clopidogrel | 38 (20.8) | 46 (24.9) | 0.35 |
| ASA and Clopidogrel | 28 (15.3) | 35 (18.9) | 0.63 |
| Warfarin | 65 (35.6) | 53 (29.2) | 0.16 |
| Triple anticoagulation | 6 (3.3) | 8 (4.3) | 0.13 |
| Baseline characteristics . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Male sex (%) | 116 (63.4) | 135 (72.9) | 0.05 |
| Age (±SEM) | 70.4 ± 11.7 | 66.3 ± 15.0 | 0.01* |
| Coronary artery disease | 105 (57.4) | 99 (53.5) | 0.75 |
| Dilated cardiomyopathy | 28 (15.3) | 37 (20.0) | 0.24 |
| Hypertensive heartdiesease | 29 (15.7) | 19 (10.3) | 0.12 |
| Other underlying disease | 21 (11.6) | 30 (16.2) | 0.19 |
| Ejection fraction (±SEM) | 48 ± 19 | 46 ± 21 | 0.26 |
| LVEDD | 55 ± 11 | 56 ± 11 | 0.22 |
| Diabetes (%) | 45 (24.6) | 45 (24.3) | 0.93 |
| Renal disease (Creatinin >1.2) | 50 (27.3) | 51 (27.6) | 0.91 |
| Chron. steroid use | 10 (5.5) | 9 (4.9) | 0.59 |
| ASA | 98 (55.6) | 101 (54.6) | 0.84 |
| Clopidogrel | 38 (20.8) | 46 (24.9) | 0.35 |
| ASA and Clopidogrel | 28 (15.3) | 35 (18.9) | 0.63 |
| Warfarin | 65 (35.6) | 53 (29.2) | 0.16 |
| Triple anticoagulation | 6 (3.3) | 8 (4.3) | 0.13 |
LVEDD, left ventricular end diastolic diameter.
*Statistically significant with P < 0.05.
| Baseline characteristics . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Male sex (%) | 116 (63.4) | 135 (72.9) | 0.05 |
| Age (±SEM) | 70.4 ± 11.7 | 66.3 ± 15.0 | 0.01* |
| Coronary artery disease | 105 (57.4) | 99 (53.5) | 0.75 |
| Dilated cardiomyopathy | 28 (15.3) | 37 (20.0) | 0.24 |
| Hypertensive heartdiesease | 29 (15.7) | 19 (10.3) | 0.12 |
| Other underlying disease | 21 (11.6) | 30 (16.2) | 0.19 |
| Ejection fraction (±SEM) | 48 ± 19 | 46 ± 21 | 0.26 |
| LVEDD | 55 ± 11 | 56 ± 11 | 0.22 |
| Diabetes (%) | 45 (24.6) | 45 (24.3) | 0.93 |
| Renal disease (Creatinin >1.2) | 50 (27.3) | 51 (27.6) | 0.91 |
| Chron. steroid use | 10 (5.5) | 9 (4.9) | 0.59 |
| ASA | 98 (55.6) | 101 (54.6) | 0.84 |
| Clopidogrel | 38 (20.8) | 46 (24.9) | 0.35 |
| ASA and Clopidogrel | 28 (15.3) | 35 (18.9) | 0.63 |
| Warfarin | 65 (35.6) | 53 (29.2) | 0.16 |
| Triple anticoagulation | 6 (3.3) | 8 (4.3) | 0.13 |
| Baseline characteristics . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Male sex (%) | 116 (63.4) | 135 (72.9) | 0.05 |
| Age (±SEM) | 70.4 ± 11.7 | 66.3 ± 15.0 | 0.01* |
| Coronary artery disease | 105 (57.4) | 99 (53.5) | 0.75 |
| Dilated cardiomyopathy | 28 (15.3) | 37 (20.0) | 0.24 |
| Hypertensive heartdiesease | 29 (15.7) | 19 (10.3) | 0.12 |
| Other underlying disease | 21 (11.6) | 30 (16.2) | 0.19 |
| Ejection fraction (±SEM) | 48 ± 19 | 46 ± 21 | 0.26 |
| LVEDD | 55 ± 11 | 56 ± 11 | 0.22 |
| Diabetes (%) | 45 (24.6) | 45 (24.3) | 0.93 |
| Renal disease (Creatinin >1.2) | 50 (27.3) | 51 (27.6) | 0.91 |
| Chron. steroid use | 10 (5.5) | 9 (4.9) | 0.59 |
| ASA | 98 (55.6) | 101 (54.6) | 0.84 |
| Clopidogrel | 38 (20.8) | 46 (24.9) | 0.35 |
| ASA and Clopidogrel | 28 (15.3) | 35 (18.9) | 0.63 |
| Warfarin | 65 (35.6) | 53 (29.2) | 0.16 |
| Triple anticoagulation | 6 (3.3) | 8 (4.3) | 0.13 |
LVEDD, left ventricular end diastolic diameter.
*Statistically significant with P < 0.05.
| Devices (%) . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Pacemaker | |||
| Single chamber | 30 (16.4) | 17 (9.2) | 0.06 |
| Dual chamber | 41 (22.4) | 45 (24.3) | 0.85 |
| CRT | 0 | 3 (1.6) | 0.32 |
| Replacement | 13 (7.1) | 4 (2.2) | 0.09 |
| All | 71 (38.8) | 65 (35.1) | 0.47 |
| Loop recorder | 11 (6.0) | 7 (3.8) | 0.32 |
| ICD | |||
| Single chamber | 40 (21.9) | 50 (27.0) | 0.39 |
| Dual chamber | 20 (11.0) | 20 (10.8) | 0.85 |
| CRT | 15 (8.1) | 27 (14.6) | 0.02* |
| Replacement | 13 (7.1) | 12 (6.5) | 0.92 |
| All | 75 (41.0) | 97 (52.4) | 0.03* |
| Procedure time (±SEM) | |||
| Without CRT | 53 ± 31min | 59 ± 36 min | 0.26 |
| CRT only | 146 ± 47 min | 137 ± 41 min | 0.1 |
| Submuscular generator | 6 (3.3) | 6 (3.3) | 0.99 |
| Devices (%) . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Pacemaker | |||
| Single chamber | 30 (16.4) | 17 (9.2) | 0.06 |
| Dual chamber | 41 (22.4) | 45 (24.3) | 0.85 |
| CRT | 0 | 3 (1.6) | 0.32 |
| Replacement | 13 (7.1) | 4 (2.2) | 0.09 |
| All | 71 (38.8) | 65 (35.1) | 0.47 |
| Loop recorder | 11 (6.0) | 7 (3.8) | 0.32 |
| ICD | |||
| Single chamber | 40 (21.9) | 50 (27.0) | 0.39 |
| Dual chamber | 20 (11.0) | 20 (10.8) | 0.85 |
| CRT | 15 (8.1) | 27 (14.6) | 0.02* |
| Replacement | 13 (7.1) | 12 (6.5) | 0.92 |
| All | 75 (41.0) | 97 (52.4) | 0.03* |
| Procedure time (±SEM) | |||
| Without CRT | 53 ± 31min | 59 ± 36 min | 0.26 |
| CRT only | 146 ± 47 min | 137 ± 41 min | 0.1 |
| Submuscular generator | 6 (3.3) | 6 (3.3) | 0.99 |
*Statistically significant with P < 0.05.
| Devices (%) . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Pacemaker | |||
| Single chamber | 30 (16.4) | 17 (9.2) | 0.06 |
| Dual chamber | 41 (22.4) | 45 (24.3) | 0.85 |
| CRT | 0 | 3 (1.6) | 0.32 |
| Replacement | 13 (7.1) | 4 (2.2) | 0.09 |
| All | 71 (38.8) | 65 (35.1) | 0.47 |
| Loop recorder | 11 (6.0) | 7 (3.8) | 0.32 |
| ICD | |||
| Single chamber | 40 (21.9) | 50 (27.0) | 0.39 |
| Dual chamber | 20 (11.0) | 20 (10.8) | 0.85 |
| CRT | 15 (8.1) | 27 (14.6) | 0.02* |
| Replacement | 13 (7.1) | 12 (6.5) | 0.92 |
| All | 75 (41.0) | 97 (52.4) | 0.03* |
| Procedure time (±SEM) | |||
| Without CRT | 53 ± 31min | 59 ± 36 min | 0.26 |
| CRT only | 146 ± 47 min | 137 ± 41 min | 0.1 |
| Submuscular generator | 6 (3.3) | 6 (3.3) | 0.99 |
| Devices (%) . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Pacemaker | |||
| Single chamber | 30 (16.4) | 17 (9.2) | 0.06 |
| Dual chamber | 41 (22.4) | 45 (24.3) | 0.85 |
| CRT | 0 | 3 (1.6) | 0.32 |
| Replacement | 13 (7.1) | 4 (2.2) | 0.09 |
| All | 71 (38.8) | 65 (35.1) | 0.47 |
| Loop recorder | 11 (6.0) | 7 (3.8) | 0.32 |
| ICD | |||
| Single chamber | 40 (21.9) | 50 (27.0) | 0.39 |
| Dual chamber | 20 (11.0) | 20 (10.8) | 0.85 |
| CRT | 15 (8.1) | 27 (14.6) | 0.02* |
| Replacement | 13 (7.1) | 12 (6.5) | 0.92 |
| All | 75 (41.0) | 97 (52.4) | 0.03* |
| Procedure time (±SEM) | |||
| Without CRT | 53 ± 31min | 59 ± 36 min | 0.26 |
| CRT only | 146 ± 47 min | 137 ± 41 min | 0.1 |
| Submuscular generator | 6 (3.3) | 6 (3.3) | 0.99 |
*Statistically significant with P < 0.05.
There were no differences regarding the underlying heart disease or risk factors for impaired wound healing as diabetes, renal insufficiency, or corticosteroid therapy. Moreover, no differences exist for the distribution of submuscular pockets, oral anticoagulants, and/or acetylsalicylic aci (ASA)/Clopidogrel (P = n.s.). The operation times did not differ significantly between the two groups.
Early endpoints were reached significantly more often in the adhesive group compared with the suture group (9.3 vs. 6.0%; P = 0.02). The differentiation of the occurring events is displayed in Table 3. As some pts suffered more than one isolated wound problem the total number of adverse events exaggerates the number of 25 and 12, respectively. Example given, all nine pts in Group 1 presenting with wound incrustation suffered marked irritation and erythema leading to a closer follow-up.
| Early Wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Insufficient closure (%) | 7 (3.8) | 0 | 0.01* |
| Minor bleeding (%) | 4 (2.2) | 3 (1.6) | 0.69 |
| Major bleeding (%) | 0 | 1 (0.5) | 0.99 |
| Pocket haematoma (%) | 4 (2.2) | 5 (2.7) | 0.75 |
| Wound irritation (%) | 13 (6.0) | 7 (3.8) | 0.16 |
| Wound excoriation (%) | 9 (4.9) | 0 | 0.02* |
| Early Wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Insufficient closure (%) | 7 (3.8) | 0 | 0.01* |
| Minor bleeding (%) | 4 (2.2) | 3 (1.6) | 0.69 |
| Major bleeding (%) | 0 | 1 (0.5) | 0.99 |
| Pocket haematoma (%) | 4 (2.2) | 5 (2.7) | 0.75 |
| Wound irritation (%) | 13 (6.0) | 7 (3.8) | 0.16 |
| Wound excoriation (%) | 9 (4.9) | 0 | 0.02* |
*Statistically significant.
| Early Wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Insufficient closure (%) | 7 (3.8) | 0 | 0.01* |
| Minor bleeding (%) | 4 (2.2) | 3 (1.6) | 0.69 |
| Major bleeding (%) | 0 | 1 (0.5) | 0.99 |
| Pocket haematoma (%) | 4 (2.2) | 5 (2.7) | 0.75 |
| Wound irritation (%) | 13 (6.0) | 7 (3.8) | 0.16 |
| Wound excoriation (%) | 9 (4.9) | 0 | 0.02* |
| Early Wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Insufficient closure (%) | 7 (3.8) | 0 | 0.01* |
| Minor bleeding (%) | 4 (2.2) | 3 (1.6) | 0.69 |
| Major bleeding (%) | 0 | 1 (0.5) | 0.99 |
| Pocket haematoma (%) | 4 (2.2) | 5 (2.7) | 0.75 |
| Wound irritation (%) | 13 (6.0) | 7 (3.8) | 0.16 |
| Wound excoriation (%) | 9 (4.9) | 0 | 0.02* |
*Statistically significant.
A complete wound closure was not achievable in seven pts with glue, thus making an additional suture necessary. We did not observe any difference in the occurrence of minor or major bleedings or pocket haematoma. Wound irritations as erythema and incrustation of the scar occurred more often in the adhesive group.
The early adverse events, especially insufficient wound closure and healing problems as incrustation and irritations, obviously have major impact on the significant difference in adverse events observed.
We did not find any difference in the occurrences of late wound problems being favourable low in both groups (2.7 vs. 1.6%; P = 0.47). The differentiation of late endpoints is displayed in Table 4. All endpoints are shown in Figure 1.
| Late wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Infection (%) | 1 (0.5) | 2 (1.1) | 0.57 |
| Dehiscence (%) | 3 (1.6) | 1 (0.5) | 0.31 |
| Keloid (%) | 1 (0.5) | 0 | 0.31 |
| Late wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Infection (%) | 1 (0.5) | 2 (1.1) | 0.57 |
| Dehiscence (%) | 3 (1.6) | 1 (0.5) | 0.31 |
| Keloid (%) | 1 (0.5) | 0 | 0.31 |
| Late wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Infection (%) | 1 (0.5) | 2 (1.1) | 0.57 |
| Dehiscence (%) | 3 (1.6) | 1 (0.5) | 0.31 |
| Keloid (%) | 1 (0.5) | 0 | 0.31 |
| Late wound problems . | Group 1: skin adhesive (n = 183) . | Group 2: suture (n = 185) . | P-value . |
|---|---|---|---|
| Infection (%) | 1 (0.5) | 2 (1.1) | 0.57 |
| Dehiscence (%) | 3 (1.6) | 1 (0.5) | 0.31 |
| Keloid (%) | 1 (0.5) | 0 | 0.31 |
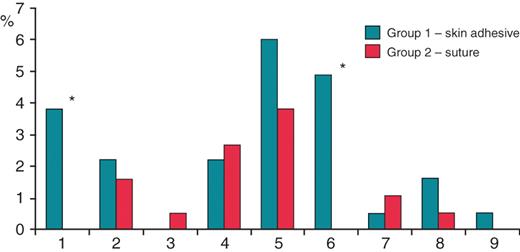
Study endpoints. Columns 1–6: early wound problems. 1, Insufficient closure; 2, Minor bleeding; 3, Major bleeding; 4, Pocket haematoma; 5, Wound irritation; 6, Wound incrustation. Columns 7–9: late wound problems. 7, Infection; 8, Dehiscence; 9, Keloid. Significant differences (P < 0.05) are marked by *.
During the 3 months observation period 25 pts (13.7%, Group 1) vs. 12 pts (6.5%, Group 2) suffered any adverse event as defined above. Thus, the combined primary endpoint was reached significantly (P = 0.02) more often in the skin adhesive group (Figure 2). Statistical analysis could not show any prognostic factors for the appearance of adverse events. Most important, we did not find a difference in adverse events for the age of the patients, for the implantation of CRT devices or for longer operation times.
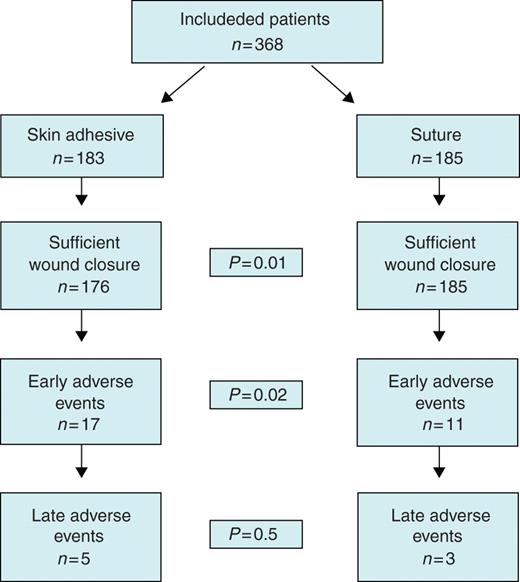
Study flow chart showing the distribution of clinical endpoints. Of note: insufficient wound closure in seven patients has to be added to 17 early adverse events in group one for a significant difference of P = 0.02 for all early adverse events.
The secondary endpoint of the study was addressing the operation time. All operations were performed by two experienced cardiologists (S.S. and D.M.) and we did not record a significant difference in the operation time (Table 2).
Discussion
This prospective register was conducted to perform a head-to-head comparison of two different strategies of wound closure in device therapy. The worst complication in these procedures is the infection of the pocket as usually leads to a device explantation followed by new implantation contra laterally.
The 2-octyl-cyanoacrylate skin adhesive has to be applied on a wound secured by subcuticular suture and perfectly adapted skin in two thin layers. Dry wound surrounding and accurate haemostasis are of outstanding importance for a complete and reliable wound closure. Otherwise, the dressing might not be tight and, as no dressing shall be attached on the skin adhesive, there is danger of infection.
When used in a correct fashion, the use of 2-octyl-cyanoacrylate provides a persistent wound closure with a tensile strength comparable with multilayer suture.2
Regarding the early adverse events we observed insufficient wound closure in 3.8% in the pts assigned to skin adhesive. If a tiny bleeding flows off the wound, skin closure with glue is insecure regarding the danger of infections. These patients were treated with suture for security reasons. In pts of the suture group with ongoing tiny bleed proper wound dressing was applied and left for 2 days.
Minor and major bleeding is a known complication of any surgical procedure and is in most cases not related with the type of skin closure. Theoretically, a difference in superficial bleedings is probable as suture sets the underlying tissue under pressure and might cease minor bleeding in that way. On the other hand, the superficial stitches might be a source of bleeding. However, in this trial we did not observe differences in minor or in major bleedings.
Regarding the wound healing in the first couple of days, we noticed a different healing pattern with more erythema and more incrustation and fibrin coating under the skin adhesive. Up to some amount this might be a physiologic reaction as the wound is tight by the glue. In the mentioned patients the amount of erythema and incrustation was that extensive, that the treatment plan had to be changes either to a prophylactic antibiotic therapy or to closer follow-up visits with leucocyte and C-reactive protein control, thus recording an adverse event.
The study clearly shows no difference in late adverse event as infections, keloid formation, and wound dehiscence when comparing sutures with the skin adhesive.
Experiences with 2-octyl-cyanoacrylate for wound closure after cardiac device implants are rare as only one retrospective study has been published so far.3 Herein, 460 implants between 1993 and 2001 were reviewed. In the earlier years suture was used in 335 pts and in the younger years skin adhesive in 125 pts. No differences in the baseline characteristics and the devices used were recorded. Endpoints were allergy, cellulites, explants due to infection, and total adverse events. There was a trend that allergy, cellulites, and total adverse events (2.9 vs. 0.8%; n.s.) occurred more often in the suture group. No difference was found in the most important endpoint: explants due to infection (0.3 vs. 0.8%; n.s.).
Gennari et al. prospectively compared skin closure after breast surgery in 133 pts. The patients were randomized to either 2-octyl-cyanoacrylate or to monofilament suture.1 During the 1 year follow-up period the authors did not find any significant difference in the wound healing pattern, dehiscence, and haematoma. Particularly, infections were substantially low in both groups. Moreover, the study showed a superiority of the skin adhesive regarding patient satisfaction and therapy costs. In the study, costs were reduced despite the higher price of skin adhesive (20.3 ± 0.8 vs. 10 ± 0.4€) because no dressing was used and no bandage changed in the early post-operative days were required. Both issues have not been tested in our trial but give important additional information for the decision to use glue or suture. In difference to the data shown above, inflammation and erythematic response occurred more often (but not significantly) in the suture group.
This study also showed a significant advantage for the adhesive regarding the operation time measured by the time needed for wound closure (19.9 ± 2 vs. 145 ± 20.6 s). This time has to be set in relation with the wound length being 85.6 ± 5.8 vs. 103.9 ± 9.3 mm (n.s.). Obviously, the longer the wound, the more time saving the skin adhesive may become. The average length of an ICD or PM wound may be around 40 mm which might be too short to have an impact on the operation time as a whole. The time for wound closure was not measured in the current study as it seemed a detail not being really relevant for examination.
Similar effectiveness and no difference in complication rates have been found in a prospective and randomized study comparing 2-octyl-cyanoacrylate and suture for the closure of laparoscopic cholecystectomy incisions.4 In other studies, the skin adhesive has been reported to be comparable or even superior for traumatic lacerations, after plastic facial surgery as well as for wound closure after head and neck surgery.5–7 It has also been tested to be effective for the use in the field by the US army.8
The studies mentioned did neither show any substantial benefit nor any substantial drawback for the use of 2-octyl-cyanoacrylate for wound closure. The data provided above display an inferiority of skin adhesive compared with intracutaneous suture in the early days after operation which do not translate into an increase of long-term adverse events. In particular, no increase in severe infections leading to device explants or wound revisions was observed. Nevertheless, meticulous attention has to be paid to make sure that no blood is emerges the wound as it defines insufficient closure.
Limitations
This study was conducted as a prospective register with randomization according to the operation date. The mode of assignment to the treatment group may have an influence which might be overcome by a proper randomization. Another major limitation is the relatively short observation period of only 3 months as pocket infections often appear after several months often years post-implantation. A controlled prospective and randomized trial with longer follow-up period would be useful for a final conclusion.
Conclusion
This study shows no benefit using skin adhesive in comparison to absorbable intracutaneous suture regarding surgery times for the implantation of cardiac rhythm devices. The rate of early adverse events after wound closure is higher after skin adhesive but no difference in long-term adverse events occurred.
Conflict of interest: none declared.



