-
PDF
- Split View
-
Views
-
Cite
Cite
Oscar Cano, Joaquín Osca, María-José Sancho-Tello, José Olagüe, José E. Castro, Antonio Salvador, Morbidity associated with three different antiplatelet regimens in patients undergoing implantation of cardiac rhythm management devices, EP Europace, Volume 13, Issue 3, March 2011, Pages 395–401, https://doi.org/10.1093/europace/euq431
Close - Share Icon Share
Abstract
Perioperative management of antiplatelet (AP) therapy in patients undergoing implantation of cardiac rhythm management devices (CRMD) remains an issue of concern that has not been prospectively evaluated in a large series. We sought to describe the morbidity associated with three different AP regimens in this setting.
We conducted a prospective observational study including 849 consecutive patients who were classified in three groups according to the presence of any AP treatment: Group 1 (n= 220): single AP therapy; Group 2 (n= 60): dual AP therapy; and Group 3 (n= 40): oral anticoagulant (OAC) + enoxaparin ‘bridging' + AP therapy. Two other groups served as controls: Group 4 (n= 375): no AP or OAC therapy; and Group 5 (n= 154): OAC + enoxaparin ‘bridging'. The incidence of pocket haematoma, pocket revisions, hospital stays duration, and unscheduled follow-up visits due to pocket-related complications were compared. Patients on Groups 2, 3 and 5 had significantly higher incidences of pocket haematoma (13.3, 15, and 14.9%, respectively) when compared with Groups 1 and 4 (3.2 and 2.4%, respectively), as well as longer hospital stays and more unscheduled follow-up visits. Of note, only patients on enoxaparin ‘bridging' required surgical revision of the pocket. Dual AP therapy (P< 0.001), enoxaparin ‘bridging' (P< 0.001) and renal insufficiency (P= 0.02) were independent predictors of pocket haematoma in multivariate analysis.
Dual AP therapy and OAC + AP therapy is strongly associated with a significant risk of pocket haematoma, longer hospital stays, and unscheduled follow-up visits. Importantly, surgical revision of the pocket was associated with enoxaparin ‘bridging' strategy but was never necessary in patients taking exclusively antiaggregant agents.
Introduction
Although perioperative management of oral anticoagulant (OAC) treatment has been widely studied, less is known about the effects of different novel antiplatelet (AP) regimens (dual AP and OAC + AP therapy) on pocket-related complications in patients undergoing implantation of cardiac rhythm management devices (CRMD).1–10 During the last years, indications for AP therapy as primary prevention of cardiovascular (CV) events have increased as well as the number of patients undergoing implantation of CRMD [pacemakers (PMs), implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices].11–15 A recent retrospective study has documented a significant increase in bleeding complications in patients on dual AP therapy undergoing pacemaker or ICD implantation, but only limited prospective data are currently available in the literature.16
The aim of our study was: (i) to describe the prevalence of three different AP regimens in a population of patients undergoing implantation of CRMD in a tertiary centre; and (ii) to evaluate the morbidity associated with each of these regimens in terms of pocket-related complications, hospital stays and unscheduled follow-up visits.
Methods
Population sample
We conducted a prospective observational study in a single tertiary centre with ≈400 device implantations per year. Consecutive patients undergoing CRMD procedures (new implants, generator replacements, and upgrading procedures) were included during a 2 year period from July 2008 to July 2010. Patients on AP treatment were divided in three different groups (Figure 1): Group 1: patients on single AP therapy, either aspirin or thienopiridine; Group 2: patients on dual AP therapy (aspirin + thienopiridin); and Group 3: patients on OAC + either single or dual AP therapy who were managed using an enoxaparin ‘bridging' strategy. Another two groups were used to establish comparisons: Group 4: patients without AP or OAC treatment; and Group 5: patients on OAC + enoxaparin ‘bridging'. The study was carried out following the Institutional Guidelines of the Hospital Universitario La Fe and all patients gave informed consent.
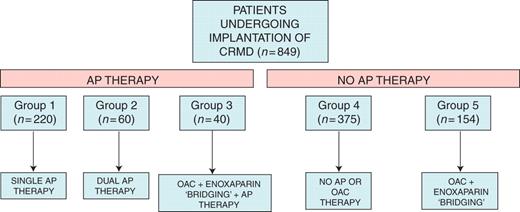
Flowchart showing the total number of patients in each subgroup. CRMD, cardiac rhythm management devices; OAC, oral anticoagulant; AP, antiplatelet.
Strategies for antiplatelet and oral anticoagulant therapy management
Management of oral AP therapy was left at the discretion of the referring physician. If discontinuation of AP therapy was decided a minimum of 7 days without taking the drug was demanded (either for aspirin or thienopiridin) and the patient was then considered as part of Group 4. Perioperative management of OAC in the enoxaparin ‘bridging' group consisted of interruption of acenocumarol 3–5 days before surgery and administration of subcutaneous low-molecular-weight heparin (enoxaparin, 1 mg/kg/12 h) at INR < 2 with the last dose administered 12 h before surgery. Subcutaneous enoxaparin was restarted at the same dose within 24 h until INR > 2 and acenocumarol was reinitiated the day after surgery except in cases of inadequate haemostasis in which reintroduction was delayed 48–72 h.
Surgical technique
All procedures were performed by three experienced implanters (O.C, J.O., and J.E.C.) under local anaesthesia and following the administration of intravenous prophylactic antibiotics and conscious sedation. For new implants leads were inserted using direct subclavian vein access under fluoroscopic guidance. All atrial and ventricular leads used in the study had an active fixation mechanism and the device was implanted in a subcutaneous pre-pectoral pocket using bipolar electrocautery in order to obtain haemostasis. In the case of generator replacements or upgradings, the same existing pocket was used without excision of the capsule. The previous pocket was not routinely expanded unless necessary. After surgery a pressure dressing was applied to the pocket for 24 h in all cases.
Definitions and follow-up schedule
Pocket haematoma was defined as a palpable mass that protruded >2 cm anterior to pulse generator as previously described.1,6 Criteria for surgical drainage of pocket haematoma included: progressive enlargement not expected to resolve with conservative treatment; the presence of tense swelling causing poor capillary perfusion, and haematoma causing severe pain to the patient. Inpatients were examined daily until hospital discharge and then after 7 days of implant. All pocket-related complications where evaluated and adjudicated by the same two investigators (O.C., M.J.S.T.). In case of disagreement, a third investigator (J.O.) was consulted. Outpatients were encouraged to report any changes in the pocket and were otherwise evaluated after 7 days of implant. When a pocket haematoma was discovered, the patient was followed weekly until total recovery was achieved or until re-intervention was scheduled. Minor complains including pocket pain or bruising were not taken into consideration in terms of pocket-related complications. Prolongation of hospital stay, presence of unscheduled follow-up visits both before and after the 1 week evaluation as well as the need of re-intervention due to pocket-related complications were assessed.
Statistical analysis
Results are reported as mean ± SD or using median as appropriate. Continuous variables were compared using Student's t-test and categorical variables were compared using the χ2 test or the exact method as appropriate. One-way analysis of variance was used to compare categorical variables with more than two categories. Binary logistic regression was used for multivariate analysis. A P-value of ≤0.05 was considered statistically significant.
Results
A total of 849 CRMD procedures (new implants, generator replacements, and upgradings) were performed at our institution from July 2008 to July 2010 and were included in the analysis. Baseline characteristics of the population sample are described in Table 1.
Baseline characteristics of the population sample according to the AP and or OAC treatment
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Age (years) | 74 ± 11 | 72 ± 8 | 70 ± 8 | 69 ± 18 | 70 ± 15 |
| Male (n, %) | 150 (68%) | 49 (83%) | 31 (78%) | 206 (55%) | 88 (57%) |
| Hypertension (n, %) | 151 (72%) | 43 (74%) | 33 (83%) | 179 (51%) | 98 (64%) |
| Diabetes (n, %) | 86 (41%) | 24 (41%) | 16 (40%) | 71 (20%) | 39 (26%) |
| Dyslipidaemia (n, %) | 101 (48%) | 35 (60%) | 29 (73%) | 75 (21%) | 57 (37%) |
| Obesity (n, %)a | 68 (34%) | 18 (32%) | 19 (50%) | 116 (34%) | 52 (36%) |
| Atrial fibrillation (n, %)b | 45 (21%) | 12 (20%) | 26 (65%) | 50 (14%) | 123 (81%) |
| Ischaemic heart disease (n, %) | 86 (40%) | 50 (83%) | 26 (65%) | 27 (7.4%) | 23 (15%) |
| Previous Stroke | 25 (12%) | 5 (8.3%) | 5 (13%) | 7 (2%) | 13 (9%) |
| Renal insufficiency (n, %)c | 21 (10%) | 6 (10%) | 9 (23%) | 23 (6.5%) | 14 (9%) |
| Device | |||||
| Pacemaker | 149 (68%) | 23 (38%) | 14 (35%) | 289 (77%) | 111 (72%) |
| ICD | 40 (18%) | 21 (36%) | 15 (38%) | 55 (15%) | 14 (9%) |
| CRT + ICD | 31 (14%) | 15 (25%) | 11 (27%) | 31 (8%) | 29 (19%) |
| Procedure | |||||
| New implant | 164 (75%) | 49 (82%) | 31 (78%) | 260 (69%) | 105 (68%) |
| Generator replacement | 50 (23%) | 9 (15%) | 5 (12%) | 105 (28%) | 37 (24%) |
| Upgrading | 6 (2%) | 2 (3%) | 4 (10%) | 10 (3%) | 12 (8%) |
| Platelet count (×109/L) (mean ± SD) | 205 ± 60 | 216 ± 77 | 207 ± 75 | 204 ± 80 | 205 ± 70 |
| INR (mean ± SD) | 1.07 ± 0.11 | 1.10 ± 0.13 | 1.11 ± 0.43 | 1.08 ± 0.11 | 1.09 ± 0.25 |
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Age (years) | 74 ± 11 | 72 ± 8 | 70 ± 8 | 69 ± 18 | 70 ± 15 |
| Male (n, %) | 150 (68%) | 49 (83%) | 31 (78%) | 206 (55%) | 88 (57%) |
| Hypertension (n, %) | 151 (72%) | 43 (74%) | 33 (83%) | 179 (51%) | 98 (64%) |
| Diabetes (n, %) | 86 (41%) | 24 (41%) | 16 (40%) | 71 (20%) | 39 (26%) |
| Dyslipidaemia (n, %) | 101 (48%) | 35 (60%) | 29 (73%) | 75 (21%) | 57 (37%) |
| Obesity (n, %)a | 68 (34%) | 18 (32%) | 19 (50%) | 116 (34%) | 52 (36%) |
| Atrial fibrillation (n, %)b | 45 (21%) | 12 (20%) | 26 (65%) | 50 (14%) | 123 (81%) |
| Ischaemic heart disease (n, %) | 86 (40%) | 50 (83%) | 26 (65%) | 27 (7.4%) | 23 (15%) |
| Previous Stroke | 25 (12%) | 5 (8.3%) | 5 (13%) | 7 (2%) | 13 (9%) |
| Renal insufficiency (n, %)c | 21 (10%) | 6 (10%) | 9 (23%) | 23 (6.5%) | 14 (9%) |
| Device | |||||
| Pacemaker | 149 (68%) | 23 (38%) | 14 (35%) | 289 (77%) | 111 (72%) |
| ICD | 40 (18%) | 21 (36%) | 15 (38%) | 55 (15%) | 14 (9%) |
| CRT + ICD | 31 (14%) | 15 (25%) | 11 (27%) | 31 (8%) | 29 (19%) |
| Procedure | |||||
| New implant | 164 (75%) | 49 (82%) | 31 (78%) | 260 (69%) | 105 (68%) |
| Generator replacement | 50 (23%) | 9 (15%) | 5 (12%) | 105 (28%) | 37 (24%) |
| Upgrading | 6 (2%) | 2 (3%) | 4 (10%) | 10 (3%) | 12 (8%) |
| Platelet count (×109/L) (mean ± SD) | 205 ± 60 | 216 ± 77 | 207 ± 75 | 204 ± 80 | 205 ± 70 |
| INR (mean ± SD) | 1.07 ± 0.11 | 1.10 ± 0.13 | 1.11 ± 0.43 | 1.08 ± 0.11 | 1.09 ± 0.25 |
aObesity is defined as a Body Mass Index ≥30.
bAny previous episode of atrial fibrillation.
cRenal insufficiency is defined as a glomerular filtration rate < 60 mL/min/1.73 m2.
Baseline characteristics of the population sample according to the AP and or OAC treatment
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Age (years) | 74 ± 11 | 72 ± 8 | 70 ± 8 | 69 ± 18 | 70 ± 15 |
| Male (n, %) | 150 (68%) | 49 (83%) | 31 (78%) | 206 (55%) | 88 (57%) |
| Hypertension (n, %) | 151 (72%) | 43 (74%) | 33 (83%) | 179 (51%) | 98 (64%) |
| Diabetes (n, %) | 86 (41%) | 24 (41%) | 16 (40%) | 71 (20%) | 39 (26%) |
| Dyslipidaemia (n, %) | 101 (48%) | 35 (60%) | 29 (73%) | 75 (21%) | 57 (37%) |
| Obesity (n, %)a | 68 (34%) | 18 (32%) | 19 (50%) | 116 (34%) | 52 (36%) |
| Atrial fibrillation (n, %)b | 45 (21%) | 12 (20%) | 26 (65%) | 50 (14%) | 123 (81%) |
| Ischaemic heart disease (n, %) | 86 (40%) | 50 (83%) | 26 (65%) | 27 (7.4%) | 23 (15%) |
| Previous Stroke | 25 (12%) | 5 (8.3%) | 5 (13%) | 7 (2%) | 13 (9%) |
| Renal insufficiency (n, %)c | 21 (10%) | 6 (10%) | 9 (23%) | 23 (6.5%) | 14 (9%) |
| Device | |||||
| Pacemaker | 149 (68%) | 23 (38%) | 14 (35%) | 289 (77%) | 111 (72%) |
| ICD | 40 (18%) | 21 (36%) | 15 (38%) | 55 (15%) | 14 (9%) |
| CRT + ICD | 31 (14%) | 15 (25%) | 11 (27%) | 31 (8%) | 29 (19%) |
| Procedure | |||||
| New implant | 164 (75%) | 49 (82%) | 31 (78%) | 260 (69%) | 105 (68%) |
| Generator replacement | 50 (23%) | 9 (15%) | 5 (12%) | 105 (28%) | 37 (24%) |
| Upgrading | 6 (2%) | 2 (3%) | 4 (10%) | 10 (3%) | 12 (8%) |
| Platelet count (×109/L) (mean ± SD) | 205 ± 60 | 216 ± 77 | 207 ± 75 | 204 ± 80 | 205 ± 70 |
| INR (mean ± SD) | 1.07 ± 0.11 | 1.10 ± 0.13 | 1.11 ± 0.43 | 1.08 ± 0.11 | 1.09 ± 0.25 |
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Age (years) | 74 ± 11 | 72 ± 8 | 70 ± 8 | 69 ± 18 | 70 ± 15 |
| Male (n, %) | 150 (68%) | 49 (83%) | 31 (78%) | 206 (55%) | 88 (57%) |
| Hypertension (n, %) | 151 (72%) | 43 (74%) | 33 (83%) | 179 (51%) | 98 (64%) |
| Diabetes (n, %) | 86 (41%) | 24 (41%) | 16 (40%) | 71 (20%) | 39 (26%) |
| Dyslipidaemia (n, %) | 101 (48%) | 35 (60%) | 29 (73%) | 75 (21%) | 57 (37%) |
| Obesity (n, %)a | 68 (34%) | 18 (32%) | 19 (50%) | 116 (34%) | 52 (36%) |
| Atrial fibrillation (n, %)b | 45 (21%) | 12 (20%) | 26 (65%) | 50 (14%) | 123 (81%) |
| Ischaemic heart disease (n, %) | 86 (40%) | 50 (83%) | 26 (65%) | 27 (7.4%) | 23 (15%) |
| Previous Stroke | 25 (12%) | 5 (8.3%) | 5 (13%) | 7 (2%) | 13 (9%) |
| Renal insufficiency (n, %)c | 21 (10%) | 6 (10%) | 9 (23%) | 23 (6.5%) | 14 (9%) |
| Device | |||||
| Pacemaker | 149 (68%) | 23 (38%) | 14 (35%) | 289 (77%) | 111 (72%) |
| ICD | 40 (18%) | 21 (36%) | 15 (38%) | 55 (15%) | 14 (9%) |
| CRT + ICD | 31 (14%) | 15 (25%) | 11 (27%) | 31 (8%) | 29 (19%) |
| Procedure | |||||
| New implant | 164 (75%) | 49 (82%) | 31 (78%) | 260 (69%) | 105 (68%) |
| Generator replacement | 50 (23%) | 9 (15%) | 5 (12%) | 105 (28%) | 37 (24%) |
| Upgrading | 6 (2%) | 2 (3%) | 4 (10%) | 10 (3%) | 12 (8%) |
| Platelet count (×109/L) (mean ± SD) | 205 ± 60 | 216 ± 77 | 207 ± 75 | 204 ± 80 | 205 ± 70 |
| INR (mean ± SD) | 1.07 ± 0.11 | 1.10 ± 0.13 | 1.11 ± 0.43 | 1.08 ± 0.11 | 1.09 ± 0.25 |
aObesity is defined as a Body Mass Index ≥30.
bAny previous episode of atrial fibrillation.
cRenal insufficiency is defined as a glomerular filtration rate < 60 mL/min/1.73 m2.
Prevalence of the different antiplatelet treatment regimens in patients undergoing implantation of cardiac rhythm management devices
A total of 234 patients (27.5%) had indication for single AP treatment when they were referred for device implantation (aspirin n= 213, thienopiridine n= 25) while 68 patients (8%) had indication for dual AP therapy (aspirin + thienopiridin). Following their referring physician preferences, device implantation was finally carried out without interruption of single and dual AP therapy in 220 (26%) and 60 patients (7%), respectively. In 14 patients on single AP therapy and 8 patients on dual AP the referring physician decided to discontinue AP treatment at least 7 days before surgery and these patients were considered as part of the control Group 4. Finally, 46 patients (5.4% of the population sample) were on OAC + single (n= 38) or dual AP therapy (n= 8) before implantation of which 40 underwent implantation without interrupting AP agents (32 were on single AP therapy and 8 on dual AP therapy). Indications for AP therapy in each group were as follows: Group 1: primary prevention of CV events (50%); secondary prevention of coronary heart disease (40%) and secondary prevention of stroke (10%); Group 2: secondary prevention of coronary heart disease (95%) and secondary prevention of stroke (5%); and Group 3: secondary prevention of coronary heart disease (70%); presence of a prosthetic heart valve (23%) and secondary prevention of recurrent stroke in the presence of atrial fibrillation (7%). Primary prevention of CV events included those patients without clinical evidence of CV disease who had an estimated 10 year cardiac heart disease risk ≥20%.
Incidence of pocket haematoma
A total of 53 pocket haematomas were documented in 849 patients (6.2%), with only 8 haematomas requiring pocket revision. Univariate analysis identified nine variables associated with a higher risk of developing pocket haematoma (Table 2). Multivariate analysis showed that dual AP therapy, enoxaparin ‘bridging', and chronic renal insufficiency were independent predictors of pocket haematoma, while obesity acted as a protective factor for the development of pocket-related complications (Table 3). The mean time for haematoma formation in the entire sample was 3.54 days (minimum 1 day and maximum 12 days) with up to 87% of haematomas appearing within the first five postoperative days. Of note, the mean time for the appearance of the pocket haematoma was longer in patients on OAC who received enoxaparin ‘bridging' (Groups 3 and 5) when compared with pocket haematomas developed in patients taking either single or dual AP therapy (Groups 1 and 2; mean time 4.4 days ± 2.9 days vs. 2.3 ± 1.3 days, P = 0.003; Figure 2).
Univariate analysis of predictors of pocket haematoma in the entire population sample
| Variable . | No pocket haematoma (n= 796) . | Pocket haematoma (n= 53) . | P-value . |
|---|---|---|---|
| Age (years) | 71 ± 15 | 70 ± 15 | 0.6 |
| Male (%) | 492 (61.8%) | 32 (60.4%) | 0.8 |
| Obesity (%)a | 263 (33%) | 10 (19.6%) | 0.04 |
| Renal insufficiency (%)b | 63 (7.9%) | 10 (18.9%) | 0.025 |
| Mechanical Prosthesis (%) | 51 (6.4%) | 12 (22.6%) | <0.001 |
| Structural heart disease (%)c | 341 (42.8%) | 36 (67.9%) | <0.001 |
| Implant duration (min) | 56 ± 28 | 68 ± 41 | 0.05 |
| Platelet count (×109/L) | 208 ± 75 | 202 ± 77 | 0.6 |
| INR | 1.35 ± 0.4 | 1.21 ± 0.21 | 0.8 |
| New implants (%) | |||
| Pacemakers | 389 (69%) | 19 (46.4%) | 0.003 |
| ICD | 100 (17.6%) | 11 (26.8%) | |
| ICD + CRT | 78 (13.4%) | 11 (26.8%) | |
| Generator exchange (%) | |||
| Pacemakers | 109 (82%) | 7 (87.5%) | 0.002 |
| ICD | 20 (15%) | 1 (12.5%) | |
| ICD + CRT | 4 (3%) | 0 | |
| Upgradings (%) | |||
| Pacemakers | 11 (35.5%) | 1 (33.3%) | 0.7 |
| ICD | 4 (12.9%) | 0 | |
| ICD + CRT | 16 (51.6%) | 2 (66.7%) | |
| Dual AP Therapy (%) | 48 (6%) | 12 (22.6%) | <0.001 |
| Enoxaparin ‘bridging' (%) | 149 (18.7%) | 28 (52.8%) | <0.001 |
| Variable . | No pocket haematoma (n= 796) . | Pocket haematoma (n= 53) . | P-value . |
|---|---|---|---|
| Age (years) | 71 ± 15 | 70 ± 15 | 0.6 |
| Male (%) | 492 (61.8%) | 32 (60.4%) | 0.8 |
| Obesity (%)a | 263 (33%) | 10 (19.6%) | 0.04 |
| Renal insufficiency (%)b | 63 (7.9%) | 10 (18.9%) | 0.025 |
| Mechanical Prosthesis (%) | 51 (6.4%) | 12 (22.6%) | <0.001 |
| Structural heart disease (%)c | 341 (42.8%) | 36 (67.9%) | <0.001 |
| Implant duration (min) | 56 ± 28 | 68 ± 41 | 0.05 |
| Platelet count (×109/L) | 208 ± 75 | 202 ± 77 | 0.6 |
| INR | 1.35 ± 0.4 | 1.21 ± 0.21 | 0.8 |
| New implants (%) | |||
| Pacemakers | 389 (69%) | 19 (46.4%) | 0.003 |
| ICD | 100 (17.6%) | 11 (26.8%) | |
| ICD + CRT | 78 (13.4%) | 11 (26.8%) | |
| Generator exchange (%) | |||
| Pacemakers | 109 (82%) | 7 (87.5%) | 0.002 |
| ICD | 20 (15%) | 1 (12.5%) | |
| ICD + CRT | 4 (3%) | 0 | |
| Upgradings (%) | |||
| Pacemakers | 11 (35.5%) | 1 (33.3%) | 0.7 |
| ICD | 4 (12.9%) | 0 | |
| ICD + CRT | 16 (51.6%) | 2 (66.7%) | |
| Dual AP Therapy (%) | 48 (6%) | 12 (22.6%) | <0.001 |
| Enoxaparin ‘bridging' (%) | 149 (18.7%) | 28 (52.8%) | <0.001 |
aObesity is defined as a Body Mass Index ≥30.
bRenal insufficiency is defined as a glomerular filtration rate <60 mL/min/1.73 m2.
cStructural heart disease is defined as left ventricular hypertrophy >15 mm, left ventricular ejection fraction <50%, moderate or greater degrees of valvulopathy, prior myocardial infarction, significant coronary artery disease or the presence of primary myocardial diseases.
Univariate analysis of predictors of pocket haematoma in the entire population sample
| Variable . | No pocket haematoma (n= 796) . | Pocket haematoma (n= 53) . | P-value . |
|---|---|---|---|
| Age (years) | 71 ± 15 | 70 ± 15 | 0.6 |
| Male (%) | 492 (61.8%) | 32 (60.4%) | 0.8 |
| Obesity (%)a | 263 (33%) | 10 (19.6%) | 0.04 |
| Renal insufficiency (%)b | 63 (7.9%) | 10 (18.9%) | 0.025 |
| Mechanical Prosthesis (%) | 51 (6.4%) | 12 (22.6%) | <0.001 |
| Structural heart disease (%)c | 341 (42.8%) | 36 (67.9%) | <0.001 |
| Implant duration (min) | 56 ± 28 | 68 ± 41 | 0.05 |
| Platelet count (×109/L) | 208 ± 75 | 202 ± 77 | 0.6 |
| INR | 1.35 ± 0.4 | 1.21 ± 0.21 | 0.8 |
| New implants (%) | |||
| Pacemakers | 389 (69%) | 19 (46.4%) | 0.003 |
| ICD | 100 (17.6%) | 11 (26.8%) | |
| ICD + CRT | 78 (13.4%) | 11 (26.8%) | |
| Generator exchange (%) | |||
| Pacemakers | 109 (82%) | 7 (87.5%) | 0.002 |
| ICD | 20 (15%) | 1 (12.5%) | |
| ICD + CRT | 4 (3%) | 0 | |
| Upgradings (%) | |||
| Pacemakers | 11 (35.5%) | 1 (33.3%) | 0.7 |
| ICD | 4 (12.9%) | 0 | |
| ICD + CRT | 16 (51.6%) | 2 (66.7%) | |
| Dual AP Therapy (%) | 48 (6%) | 12 (22.6%) | <0.001 |
| Enoxaparin ‘bridging' (%) | 149 (18.7%) | 28 (52.8%) | <0.001 |
| Variable . | No pocket haematoma (n= 796) . | Pocket haematoma (n= 53) . | P-value . |
|---|---|---|---|
| Age (years) | 71 ± 15 | 70 ± 15 | 0.6 |
| Male (%) | 492 (61.8%) | 32 (60.4%) | 0.8 |
| Obesity (%)a | 263 (33%) | 10 (19.6%) | 0.04 |
| Renal insufficiency (%)b | 63 (7.9%) | 10 (18.9%) | 0.025 |
| Mechanical Prosthesis (%) | 51 (6.4%) | 12 (22.6%) | <0.001 |
| Structural heart disease (%)c | 341 (42.8%) | 36 (67.9%) | <0.001 |
| Implant duration (min) | 56 ± 28 | 68 ± 41 | 0.05 |
| Platelet count (×109/L) | 208 ± 75 | 202 ± 77 | 0.6 |
| INR | 1.35 ± 0.4 | 1.21 ± 0.21 | 0.8 |
| New implants (%) | |||
| Pacemakers | 389 (69%) | 19 (46.4%) | 0.003 |
| ICD | 100 (17.6%) | 11 (26.8%) | |
| ICD + CRT | 78 (13.4%) | 11 (26.8%) | |
| Generator exchange (%) | |||
| Pacemakers | 109 (82%) | 7 (87.5%) | 0.002 |
| ICD | 20 (15%) | 1 (12.5%) | |
| ICD + CRT | 4 (3%) | 0 | |
| Upgradings (%) | |||
| Pacemakers | 11 (35.5%) | 1 (33.3%) | 0.7 |
| ICD | 4 (12.9%) | 0 | |
| ICD + CRT | 16 (51.6%) | 2 (66.7%) | |
| Dual AP Therapy (%) | 48 (6%) | 12 (22.6%) | <0.001 |
| Enoxaparin ‘bridging' (%) | 149 (18.7%) | 28 (52.8%) | <0.001 |
aObesity is defined as a Body Mass Index ≥30.
bRenal insufficiency is defined as a glomerular filtration rate <60 mL/min/1.73 m2.
cStructural heart disease is defined as left ventricular hypertrophy >15 mm, left ventricular ejection fraction <50%, moderate or greater degrees of valvulopathy, prior myocardial infarction, significant coronary artery disease or the presence of primary myocardial diseases.
Multivariate analysis of predictors of pocket haematoma in the entire population sample
| Variable . | Odds ratio . | P-value . | 95% CI . |
|---|---|---|---|
| Obesity | 0.38 | 0.007 | 0.18–0.76 |
| Renal insufficiency | 2.44 | 0.027 | 1.11–5.36 |
| Mechanical prosthesis | 1.47 | 0.430 | 0.56–3.90 |
| Structural heart disease | 0.98 | 0.977 | 0.42–2.27 |
| Implant duration | 1.01 | 0.302 | 0.99–1.01 |
| Device type (PM vs. ICD ± CRT) | 1.84 | 0.212 | 0.70–4.83 |
| Dual AP therapy | 8.27 | <0.001 | 3.25–21.1 |
| Enoxaparin ‘bridging' | 7.10 | <0.001 | 3.34–15.09 |
| Variable . | Odds ratio . | P-value . | 95% CI . |
|---|---|---|---|
| Obesity | 0.38 | 0.007 | 0.18–0.76 |
| Renal insufficiency | 2.44 | 0.027 | 1.11–5.36 |
| Mechanical prosthesis | 1.47 | 0.430 | 0.56–3.90 |
| Structural heart disease | 0.98 | 0.977 | 0.42–2.27 |
| Implant duration | 1.01 | 0.302 | 0.99–1.01 |
| Device type (PM vs. ICD ± CRT) | 1.84 | 0.212 | 0.70–4.83 |
| Dual AP therapy | 8.27 | <0.001 | 3.25–21.1 |
| Enoxaparin ‘bridging' | 7.10 | <0.001 | 3.34–15.09 |
PM, pacemaker; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy.
Multivariate analysis of predictors of pocket haematoma in the entire population sample
| Variable . | Odds ratio . | P-value . | 95% CI . |
|---|---|---|---|
| Obesity | 0.38 | 0.007 | 0.18–0.76 |
| Renal insufficiency | 2.44 | 0.027 | 1.11–5.36 |
| Mechanical prosthesis | 1.47 | 0.430 | 0.56–3.90 |
| Structural heart disease | 0.98 | 0.977 | 0.42–2.27 |
| Implant duration | 1.01 | 0.302 | 0.99–1.01 |
| Device type (PM vs. ICD ± CRT) | 1.84 | 0.212 | 0.70–4.83 |
| Dual AP therapy | 8.27 | <0.001 | 3.25–21.1 |
| Enoxaparin ‘bridging' | 7.10 | <0.001 | 3.34–15.09 |
| Variable . | Odds ratio . | P-value . | 95% CI . |
|---|---|---|---|
| Obesity | 0.38 | 0.007 | 0.18–0.76 |
| Renal insufficiency | 2.44 | 0.027 | 1.11–5.36 |
| Mechanical prosthesis | 1.47 | 0.430 | 0.56–3.90 |
| Structural heart disease | 0.98 | 0.977 | 0.42–2.27 |
| Implant duration | 1.01 | 0.302 | 0.99–1.01 |
| Device type (PM vs. ICD ± CRT) | 1.84 | 0.212 | 0.70–4.83 |
| Dual AP therapy | 8.27 | <0.001 | 3.25–21.1 |
| Enoxaparin ‘bridging' | 7.10 | <0.001 | 3.34–15.09 |
PM, pacemaker; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy.
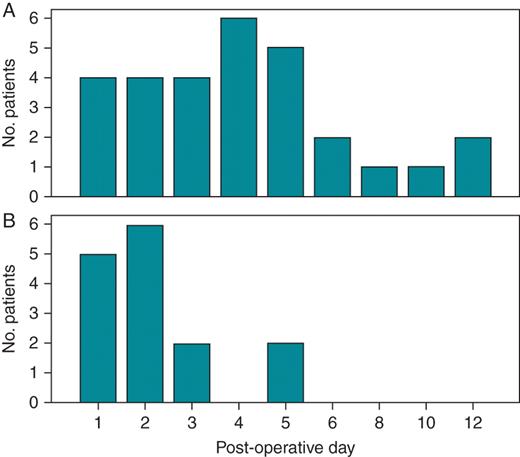
Time course of pocket haematoma formation in patients receiving OAC (A, Groups 3 and 5) and in patients receiving either single or dual AP therapy (B, Groups 1 and 2). See text for details. No., number of patients.
The incidence of pocket haematoma in the different groups is represented in Table 4. Patients on single AP therapy had comparable incidence of pocket haematoma than patients in the control Group 4 (3.2% for single AP vs. 2.4% for Group 4, P = NS). Three subgroups of patients exhibited significantly higher incidence of pocket haematoma when compared with patients not taking AP or anticoagulant agents (Group 4): 1. patients on dual AP therapy (P= 0.007); 2. patients on OAC + enoxaparin ‘bridging' + AP therapy (P= 0.015); and 3. patients on OAC + enoxaparin ‘bridging' (P< 0.001). Significant differences in the incidence of pocket haematoma were also present in the same three subgroups when compared with patients on single AP therapy (P= 0.026, P= 0.04, and P< 0.001, for Groups 2, 3, and 5, respectively). Of note, the highest incidence of pocket haematoma (15%) was registered in patients on combined treatment with OAC + enoxaparin ‘bridging' + AP therapy. The incidence of pocket haematoma in the eight patients of this subgroup who were on OAC + enoxaparin ‘bridging' + dual AP therapy was up to 38% (3 of 8 patients). Surgical revision of the pocket was only required in patients using enoxaparin ‘bridging' (33% of haematomas in Group 3 and 26% of haematomas in Group 5 required pocket revision) while none of the patients with pocket haematoma in the single and dual AP treatment groups required pocket revision.
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Pocket haematoma (n, %) | 7 (3.2%)*,†,‡ | 8 (13.3%)*,§ | 6 (15%)†,¶ | 9 (2.4%)§,¶,# | 23 (14.9%)‡,# |
| Pocket revision (n, total) | 0/7 | 0/8 | 2/6 | 0/9 | 6/23 |
| Pericardial effusion requiring pericardiocentesis (n, %) | 1 (0.5%) | 0 | 0 | 1 (0.3%) | 0 |
| Hemothorax (n, %) | 0 | 0 | 0 | 2 (0.6%) | 0 |
| Hospital Stay (days) (mean ± SD) | 2.3 ± 3.6†,‡ | 3.8 ± 4.3 | 5.1 ± 5.8†,¶ | 2.2 ± 4.9¶,# | 4.8 ± 6‡,# |
| No. Unscheduled follow-up visits (mean, range) | 0.09 (0–4) | 0.46 (0–7)§ | 0.29 (0–6) | 0.10 (0–6)§,# | 0.38 (0–7)# |
| Haematoma prolongs hospital stay (n, %) | 3 (1.4%) | 2 (3.3%) | 3 (7.5%) | 5 (1.3%)# | 9 (5.8%)# |
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Pocket haematoma (n, %) | 7 (3.2%)*,†,‡ | 8 (13.3%)*,§ | 6 (15%)†,¶ | 9 (2.4%)§,¶,# | 23 (14.9%)‡,# |
| Pocket revision (n, total) | 0/7 | 0/8 | 2/6 | 0/9 | 6/23 |
| Pericardial effusion requiring pericardiocentesis (n, %) | 1 (0.5%) | 0 | 0 | 1 (0.3%) | 0 |
| Hemothorax (n, %) | 0 | 0 | 0 | 2 (0.6%) | 0 |
| Hospital Stay (days) (mean ± SD) | 2.3 ± 3.6†,‡ | 3.8 ± 4.3 | 5.1 ± 5.8†,¶ | 2.2 ± 4.9¶,# | 4.8 ± 6‡,# |
| No. Unscheduled follow-up visits (mean, range) | 0.09 (0–4) | 0.46 (0–7)§ | 0.29 (0–6) | 0.10 (0–6)§,# | 0.38 (0–7)# |
| Haematoma prolongs hospital stay (n, %) | 3 (1.4%) | 2 (3.3%) | 3 (7.5%) | 5 (1.3%)# | 9 (5.8%)# |
*P< 0.05 for the comparison between Group 1 and Group 2.
†P< 0.05 for the comparison between Group 1 and Group 3.
‡P< 0.05 for the comparison between Group 1 and Group 5.
§P< 0.05 for the comparison between Group 4 and Group 2.
¶P< 0.05 for the comparison between Group 4 and Group 3.
#P< 0.05 for the comparison between Group 4 and Group 5.
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Pocket haematoma (n, %) | 7 (3.2%)*,†,‡ | 8 (13.3%)*,§ | 6 (15%)†,¶ | 9 (2.4%)§,¶,# | 23 (14.9%)‡,# |
| Pocket revision (n, total) | 0/7 | 0/8 | 2/6 | 0/9 | 6/23 |
| Pericardial effusion requiring pericardiocentesis (n, %) | 1 (0.5%) | 0 | 0 | 1 (0.3%) | 0 |
| Hemothorax (n, %) | 0 | 0 | 0 | 2 (0.6%) | 0 |
| Hospital Stay (days) (mean ± SD) | 2.3 ± 3.6†,‡ | 3.8 ± 4.3 | 5.1 ± 5.8†,¶ | 2.2 ± 4.9¶,# | 4.8 ± 6‡,# |
| No. Unscheduled follow-up visits (mean, range) | 0.09 (0–4) | 0.46 (0–7)§ | 0.29 (0–6) | 0.10 (0–6)§,# | 0.38 (0–7)# |
| Haematoma prolongs hospital stay (n, %) | 3 (1.4%) | 2 (3.3%) | 3 (7.5%) | 5 (1.3%)# | 9 (5.8%)# |
| . | Group 1 (n= 220) . | Group 2 (n= 60) . | Group 3 (n= 40) . | Group 4 (n= 375) . | Group 5 (n= 154) . |
|---|---|---|---|---|---|
| Pocket haematoma (n, %) | 7 (3.2%)*,†,‡ | 8 (13.3%)*,§ | 6 (15%)†,¶ | 9 (2.4%)§,¶,# | 23 (14.9%)‡,# |
| Pocket revision (n, total) | 0/7 | 0/8 | 2/6 | 0/9 | 6/23 |
| Pericardial effusion requiring pericardiocentesis (n, %) | 1 (0.5%) | 0 | 0 | 1 (0.3%) | 0 |
| Hemothorax (n, %) | 0 | 0 | 0 | 2 (0.6%) | 0 |
| Hospital Stay (days) (mean ± SD) | 2.3 ± 3.6†,‡ | 3.8 ± 4.3 | 5.1 ± 5.8†,¶ | 2.2 ± 4.9¶,# | 4.8 ± 6‡,# |
| No. Unscheduled follow-up visits (mean, range) | 0.09 (0–4) | 0.46 (0–7)§ | 0.29 (0–6) | 0.10 (0–6)§,# | 0.38 (0–7)# |
| Haematoma prolongs hospital stay (n, %) | 3 (1.4%) | 2 (3.3%) | 3 (7.5%) | 5 (1.3%)# | 9 (5.8%)# |
*P< 0.05 for the comparison between Group 1 and Group 2.
†P< 0.05 for the comparison between Group 1 and Group 3.
‡P< 0.05 for the comparison between Group 1 and Group 5.
§P< 0.05 for the comparison between Group 4 and Group 2.
¶P< 0.05 for the comparison between Group 4 and Group 3.
#P< 0.05 for the comparison between Group 4 and Group 5.
On the other hand, none of the 32 patients in which AP therapy was interrupted before surgery developed either pocket haematoma or thrombo-embolic events. One patient in Group 4 suffered an ischaemic stroke 24 h after an upgrading procedure (from a VVI pacemaker to a biventricular ICD). No other thrombo-embolic complications were registered.
Hospital stays and unscheduled follow-up visits
Patients with pocket haematoma had significantly longer hospital stays than those without pocket-related complications (mean hospital stay 7.9 ± 7.3 days for patients with pocket haematoma vs. 2.6 ± 4.5 days for patients without pocket haematoma, P< 0.001; Table 4). Likewise, patients on OAC + enoxaparin ‘bridging' and patients on OAC + AP therapy had significantly longer hospital stays (4.8 ± 6 days and 5.1 ± 5.8 days, respectively) when compared with patients in the control group or those on single AP therapy (2.2 ± 4.9 days and 2.3 ± 3.6 days, P< 0.001 and P= 0.016, respectively). The mean hospital stay for patients on dual AP therapy also tended to be longer (3.8 ± 4.3 days) but did not reach statistical significance. Unscheduled follow-up visits due to pocket-related complications were significantly more frequent in patients on dual AP therapy and those on OAC + enoxaparin ‘bridging' (P= 0.018 and P= 0.004, respectively, compared with patients with no AP or OAC treatment).
Discussion
This is the first prospective study systematically reporting the effects of three different AP treatment regimens (single, dual, and OAC + either single or dual AP therapy) on the appearance of pocket-related complications in patients undergoing implantation of CRMD. The presence of anticoagulant or antiaggregant therapy in this setting has become an important issue considering that in our series up to 40% of patients were taking AP therapy and ≈23% were under OAC treatment. The management of OAC in patients receiving CRMD has been widely studied. Traditional heparin ‘bridging' strategy has been usually associated with a high incidence of pocket haematoma ranging from 7.5 to 25%.1,2,5–10 More recently, some observational and two small randomized studies have suggested that device implantation without stopping OAC is safe and that heparin ‘bridging' may itself be associated with more pocket-related complications.6,7,9,10 Of note, an increasing number of patients (5.4%) are currently under combined OAC + AP treatment. Secondary prevention of coronary heart disease represented the principal indication for dual AP therapy (95% of patients) and OAC + AP therapy (70%) in our population sample. The effects of these two AP regimens in this setting have not been widely investigated. To date no prospective studies specifically designed to evaluate the morbidity associated with dual AP treatment are present in the literature and, since very recently, only indirect data extracted from studies designed to assess the effect of different OAC strategies was available.2
Michaud et al.1 described 73 patients taking aspirin at the time of implantation of pacemaker/ICD in a study designed to evaluate the effect of initiation of heparin 6 vs. 24 h after implantation of the device. They reported no single pocket haematoma in this population, which only included three patients with dual AP treatment. Wiegand et al.2 studied the influence of different variables on the appearance of pocket haematoma after pacemaker/ICD implantation. Antiplatelet therapy with aspirin was present in 1275 patients with an incidence of pocket haematoma/bleeding of 3.1%. Only 23 patients were on dual AP therapy (aspirin + thienopiridin) and the incidence of pocket haematoma/bleeding in this subgroup was 21.7%, three-fold higher than patients on OAC (7.6%).
More recently, two studies have directly addressed the issue of oral AP treatment in patients undergoing implantation of CRMD. In a retrospective study Thal et al.17 reported the incidence of pocket haematoma in patients undergoing CRMD implantation without interruption of warfarin (n= 58) or with single (n= 135) or dual (n= 20) AP treatment. Patients under dual AP therapy had a significantly higher incidence of pocket haematoma (25%) compared with patients on warfarin (3.4%) or single AP therapy (0%). Dreger et al.18 studied a cohort of 109 patients with dual AP therapy (38 patients recruited prospectively and 71 retrospectively) who were compared with 318 controls with single or no AP treatment. In this study, all patients received vacuum drainage systems during surgery. The incidence of pocket haematoma was extremely low and comparable between the two groups (0.9% for dual AP therapy vs. 0.9% for controls, P= 0.581) but fluid losses via drainage systems were significantly higher among dual AP treatment patients. The authors report a 5.2 and 14.6% prevalence of dual AP treatment in patients undergoing pacemaker and ICD implantation, respectively. Finally, in a large retrospective study Tompkins et al.16 describe a significant increase in bleeding complications among patients on dual AP therapy (n= 155) compared with patients not taking AP agents (7.2 vs. 1.6%, P= 0.004), whereas a trend without achieving statistical significance was found in patients on single AP treatment vs. controls (3.9 vs. 1.6%, P= 0.078).
Nine different variables were associated with pocket haematoma in univariate analysis (Table 2) although only obesity, chronic renal insufficiency, dual AP therapy and enoxaparin ‘bridging' remained significant after multivariate analysis (Table 3). We also investigated if mechanical valves were themselves predictive of pocket haematoma. Considering that all of these patients were part of either Group 3 or 5 it was not unexpected that this effect could have disappeared in multivariate analysis. However, there were no differences in the risk of haematoma in patients with or without mechanical valves when we analysed data in Groups 3 and 5 excluding the rest of the patients. Multivariate analysis showed that obesity acted as a protecting factor against the development of pocket haematoma (10 of 273 obese patients with pocket haematoma, 3.7%). The presence of a greater amount of subcutaneous tissue in this population could just have facilitated redistribution of the bleeding thus hampering the identification of pocket haematoma according to our pre-specified criteria. Anyway, the fact that none of the obese patients with pocket haematoma required surgical revision reinforces the idea that obesity may protect at least against clinically significant haematomas. Although all devices in our study were implanted using direct subclavian puncture, we do not consider that implant technique could have significantly influenced in the risk of pocket haematoma formation. Previous data suggest that either cephalic vein cutdown or direct subclavian puncture have comparable incidences of pocket haematoma.19,20 Another interesting finding is the fact that pocket haematomas developed in patients under OAC who received enoxaparin ‘bridging' appeared significantly later compared with haematomas associated with AP therapy. The presence of some sort of summative anticoagulant effect of enoxaparin coexisting with the initial effects of the restarted acenocumarol at postoperative Days 3–4 could be the explanation for this observation.
According to the results of the present study and others, single AP therapy does not increase the incidence of pocket haematoma in patients undergoing CRMD surgery. However, dual AP therapy significantly increases the risks of pocket haematoma and is also associated with longer hospital stays and unscheduled follow-up visits. Of note, and in contradistinction with pocket haematomas associated with OAC + enoxaparin ‘bridging', no single patient on the dual AP therapy arm who developed pocket haematoma required surgical revision while this was necessary in up to 26% of the pocket haematomas in the former group. This is a very relevant finding considering that discontinuation of AP therapy in patients with recent percutaneous coronary interventions is associated with stent thrombosis.21–23 Our results show that patients on dual AP therapy have significant risk of developing pocket bleeding complications but, importantly, these complications can be easily managed with conservative measures. Finally, we present a third subgroup of patients which have not yet been taken into consideration in the available literature, those with OAC + enoxaparin ‘bridging' + either single or dual AP therapy. This subgroup had the highest incidence of pocket haematoma (15%). Of note, patients on this subgroup who were on OAC + single AP therapy had a similar behaviour than patients on Group 5 with comparable incidence of pocket haematoma (3 of 32, 9.4%) whereas those who were on OAC + dual AP therapy had the highest incidence of pocket haematoma (3 of 8, 35%). These findings are consistent with the previous observations revealing that single AP therapy does not add any additional risk in this scenario while dual AP therapy clearly increases the risk of pocket-related complications with a summative effect to the inherent risk associated with traditional OAC + heparin ‘bridging' strategy.
Limitations
This study represents a single-centre experience. The sample size is relatively small, especially in the dual AP and OAC + AP therapy subgroups, so definitive conclusions regarding these groups should be taken with caution. Although investigators were not blinded to the antiaggregant or anticoagulant state of the patient, all of them were strongly encouraged to avoid this information during evaluation of pocket-related complications so we consider that investigator bias is unlikely. All patients receiving OAC in our study were treated with acenocumarol and subcutaneous enoxaparin. Whether similar results would have been observed with warfarin and different types of heparin is uncertain.
Conclusion
In summary, the incidence of pocket haematoma during device implantation is significantly higher in patients on dual AP therapy and those on OAC + enoxaparin ‘bridging' + AP therapy when compared with patients not taking antiaggregants or anticoagulants. However, surgical revision of the pocket was only necessary when enoxaparin ‘bridging' was used and not in haematomas occurring in patients treated exclusively with antiaggregant agents. Considering that almost 40% of patients referred for device implantation are currently under AP therapy, evidenced-based practise guidelines are necessary in order to guide the management of antiaggregant therapy in this setting.
Conflict of interest: none declared.



