-
PDF
- Split View
-
Views
-
Cite
Cite
Markus Roos, Richard Kobza, Peiman Jamshidi, Peter Bauer, Thérèse Resink, Reinhard Schlaepfer, Peter Stulz, Michel Zuber, Paul Erne, Improved cardiac performance through pacing-induced diaphragmatic stimulation: a novel electrophysiological approach in heart failure management?, EP Europace, Volume 11, Issue 2, February 2009, Pages 191–199, https://doi.org/10.1093/europace/eun377
Close - Share Icon Share
Abstract
Pharmacological conditioning of the phrenic nerve can positively influence systolic performance, and diaphragm activation improves ventilatory function. Here we investigate whether pacing-induced diaphragmatic stimulation (PIDS) may improve left ventricular (LV) systolic function.
We studied a total of 35 patients (4 females, mean age 67 ± 9 years, ejection fraction 61 ± 14%) within 7 days following open heart surgery. The haemodynamic impact of different PIDS and ventricular pacing configurations and coupling intervals was tested in 132 episodes. Success of PIDS was assessed using fluoroscopy and palpation. Left ventricular systolic performance was recorded using the electromechanical activation time (EMAT) obtained through acoustic cardiography. Eighteen subjects were tested in the catheter laboratory and 17 in the intensive care unit. For both groups, EMAT significantly improved when the diaphragm was stimulated 20 ms after the onset of ventricular pacing. In all instances, PIDS could be induced with or without causing patient symptoms, and LV systolic performance improvement was comparable in symptomatic and asymptomatic modes. No desensitization of the diaphragm was observed following PIDS delivery 4–6 and 24 h following open heart surgery.
Pacing-induced diaphragmatic stimulation, if synchronized to the onset of ventricular contraction with a fixed, non-zero coupling delay, can improve LV systolic function reproducibly for at least 1 h without causing patient symptoms. The absence of diaphragm desensitization further underscores the potential of PIDS as a practical therapeutic approach in device-based heart failure management.
Introduction
Patients with severe congestive heart failure can become refractory to standard medical therapy. A number of device-based therapies have been developed to prevent the progression of heart failure and to improve systolic function in such patients. One of the most prominent examples is cardiac resynchronization therapy (CRT), 1 which has proven to be an effective technique for decreasing morbidity and mortality and increasing quality of life in patients with severe and moderate heart failure and mechanical dyssynchrony. Implantation of a biventricular (BiV) pacemaker in such patients leads to a more synchronous contraction pattern of the right ventricle (RV) and left ventricle (LV). Assuming good placement of the ventricular pacing leads and optimized pacemaker settings, there is improvement in systolic performance and reverse remodelling of the heart. This effect can also be achieved by other methods, depending on the underlying root cause for the severe systolic dysfunction and the benefit offered in treating specific root causes. Examples include LV assist devices 2 for patients with severely failing hearts awaiting heart transplantation, cardiac contractility modulation, 3 and external counter pulsation therapy 4 and for patients with very low systolic strength and no dyssynchrony.
Although CRT has been proven to effectively improve systolic function, electrical stimulation of the heart through endocardial leads can also cause unwanted stimulation of skeletal muscle. The left phrenic nerve, which provides innervation for the diaphragm, arises from the cervical spine and descends to the diaphragm through the mediastinum where the heart is situated. As the left phrenic nerve passes the heart, it courses along the pericardium, superficial to the left atrium and LV. When in close in proximity to pacing leads, the nerve can be stimulated by a pacing pulse, 5 leading to involuntary contractions of the diaphragm. Phrenic nerve stimulation has been reported in as many as 24% of patients with implanted CRT devices 6 and can also occur in patients with regular pacemakers. 7 The induced diaphragmatic contraction is mostly symptomatic, so it will either be felt by the patient or is easy to palpate during physical examination. Nevertheless, there appears to be a range of stimulation voltages in which patients are asymptomatic, despite the presence of diaphragmatic pacing (M. Zuber, unpublished results, 2007).
Pacing-induced diaphragmatic stimulation (PIDS) is an effective tool for controlling respiration and has utility for breathing disorders in spite of its potential to cause pain and symptoms. 8 As the apex and the LV of the heart are in contact with the diaphragm, diaphragmatic movements may affect LV systolic function through a well-timed stimulation of the diaphragm. Improvement of cardiac performance through controlled and asymptomatic stimulation of the diaphragm has potential in assisting patients with severe and drug-refractory systolic heart failure.
Patients and methods
This study is registered with the US National Institute of Health, registration no. NCT00541541. The study was approved by the local Ethics Committee and conducted with the informed consent of all subjects.
Subjects
We studied a total of 35 patients within 7 days following open heart surgery. Patient demographics and other characteristics are shown in Table 1 . Patients enrolled in the study had to have a bypass operation, which allowed access to the left dorsal location of the diaphragm such that the temporary stimulation lead to the diaphragm could be attached in addition to other temporary leads. Patients excluded from the study were those without ideal access to the left dorsal location of the diaphragm and/or implanted with a permanent pacemaker, a fast changing need for vasoactive therapy, or a loss of capture in any of the temporary electrodes.
Patient demographics and pacing configuration/coupling intervals used to test the effect of pacing-induced diaphragmatic stimulation on cardiac performance
| . | All . | Phase I (4–7d post op) . | Phase II (4–6h post op) . | Phase III (24h post op) . |
|---|---|---|---|---|
| General | ||||
| Patients ( n ) | 35 | 18 | 7 | 10 |
| Females ( n ) | 4 | 2 | 1 | 1 |
| Age (years) | 67 ± 9 | 67 ± 8 | 68 ± 8 | 65 ± 11 |
| EF (%) | 61 ± 14 | 62 ± 13 | 61 ± 18 | 61 ± 15 |
| Baseline heart rate (bpm) | 84 ± 10 | 82 ± 12 | 82 ± 10 | 84 ± 8 |
| Temporary pacing leads | ||||
| Right atrium | 35 | 18 | 7 | 10 |
| Right ventricle | 7 | 2 | 2 | 4 |
| Left ventricle | 31 | 16 | 6 | 9 |
| Left diaphragm | 35 | 18 | 7 | 10 |
| Pacing modes tested with PIDS | ||||
| DDD (RA/RV) | 4 | 2 | 1 | 1 |
| DDD (RA/LV) | 28 | 16 | 6 | 6 |
| DDD (RA/BiV) | 3 | — | — | 3 |
| PIDs coupling delays tested | ||||
| Vp to PIDS 30 ms | 11 | 11 | — | — |
| Vp to PIDS 20 ms | 35 | 18 | 7 | 10 |
| Vp to PIDS 10 ms | 11 | 11 | — | — |
| Vp only | 35 | 18 | 7 | 10 |
| PIDS to Vp 10 ms | 11 | 11 | — | — |
| PIDS to Vp 20 ms | 18 | 18 | — | — |
| PIDS to Vp 30 ms | 11 | 16 | — | — |
| . | All . | Phase I (4–7d post op) . | Phase II (4–6h post op) . | Phase III (24h post op) . |
|---|---|---|---|---|
| General | ||||
| Patients ( n ) | 35 | 18 | 7 | 10 |
| Females ( n ) | 4 | 2 | 1 | 1 |
| Age (years) | 67 ± 9 | 67 ± 8 | 68 ± 8 | 65 ± 11 |
| EF (%) | 61 ± 14 | 62 ± 13 | 61 ± 18 | 61 ± 15 |
| Baseline heart rate (bpm) | 84 ± 10 | 82 ± 12 | 82 ± 10 | 84 ± 8 |
| Temporary pacing leads | ||||
| Right atrium | 35 | 18 | 7 | 10 |
| Right ventricle | 7 | 2 | 2 | 4 |
| Left ventricle | 31 | 16 | 6 | 9 |
| Left diaphragm | 35 | 18 | 7 | 10 |
| Pacing modes tested with PIDS | ||||
| DDD (RA/RV) | 4 | 2 | 1 | 1 |
| DDD (RA/LV) | 28 | 16 | 6 | 6 |
| DDD (RA/BiV) | 3 | — | — | 3 |
| PIDs coupling delays tested | ||||
| Vp to PIDS 30 ms | 11 | 11 | — | — |
| Vp to PIDS 20 ms | 35 | 18 | 7 | 10 |
| Vp to PIDS 10 ms | 11 | 11 | — | — |
| Vp only | 35 | 18 | 7 | 10 |
| PIDS to Vp 10 ms | 11 | 11 | — | — |
| PIDS to Vp 20 ms | 18 | 18 | — | — |
| PIDS to Vp 30 ms | 11 | 16 | — | — |
Where appropriate data are given as mean ± SD.
BiV, biventricular; DDD, atrioventricular synchronous pacing mode; EF, ejection fraction; LV, left ventricle; PIDS, pacing-induced diaphragmatic stimulation; RA, right atrium; RV, right ventricle; Vp, ventricular pacing pulse; post op, post-operation.
Patient demographics and pacing configuration/coupling intervals used to test the effect of pacing-induced diaphragmatic stimulation on cardiac performance
| . | All . | Phase I (4–7d post op) . | Phase II (4–6h post op) . | Phase III (24h post op) . |
|---|---|---|---|---|
| General | ||||
| Patients ( n ) | 35 | 18 | 7 | 10 |
| Females ( n ) | 4 | 2 | 1 | 1 |
| Age (years) | 67 ± 9 | 67 ± 8 | 68 ± 8 | 65 ± 11 |
| EF (%) | 61 ± 14 | 62 ± 13 | 61 ± 18 | 61 ± 15 |
| Baseline heart rate (bpm) | 84 ± 10 | 82 ± 12 | 82 ± 10 | 84 ± 8 |
| Temporary pacing leads | ||||
| Right atrium | 35 | 18 | 7 | 10 |
| Right ventricle | 7 | 2 | 2 | 4 |
| Left ventricle | 31 | 16 | 6 | 9 |
| Left diaphragm | 35 | 18 | 7 | 10 |
| Pacing modes tested with PIDS | ||||
| DDD (RA/RV) | 4 | 2 | 1 | 1 |
| DDD (RA/LV) | 28 | 16 | 6 | 6 |
| DDD (RA/BiV) | 3 | — | — | 3 |
| PIDs coupling delays tested | ||||
| Vp to PIDS 30 ms | 11 | 11 | — | — |
| Vp to PIDS 20 ms | 35 | 18 | 7 | 10 |
| Vp to PIDS 10 ms | 11 | 11 | — | — |
| Vp only | 35 | 18 | 7 | 10 |
| PIDS to Vp 10 ms | 11 | 11 | — | — |
| PIDS to Vp 20 ms | 18 | 18 | — | — |
| PIDS to Vp 30 ms | 11 | 16 | — | — |
| . | All . | Phase I (4–7d post op) . | Phase II (4–6h post op) . | Phase III (24h post op) . |
|---|---|---|---|---|
| General | ||||
| Patients ( n ) | 35 | 18 | 7 | 10 |
| Females ( n ) | 4 | 2 | 1 | 1 |
| Age (years) | 67 ± 9 | 67 ± 8 | 68 ± 8 | 65 ± 11 |
| EF (%) | 61 ± 14 | 62 ± 13 | 61 ± 18 | 61 ± 15 |
| Baseline heart rate (bpm) | 84 ± 10 | 82 ± 12 | 82 ± 10 | 84 ± 8 |
| Temporary pacing leads | ||||
| Right atrium | 35 | 18 | 7 | 10 |
| Right ventricle | 7 | 2 | 2 | 4 |
| Left ventricle | 31 | 16 | 6 | 9 |
| Left diaphragm | 35 | 18 | 7 | 10 |
| Pacing modes tested with PIDS | ||||
| DDD (RA/RV) | 4 | 2 | 1 | 1 |
| DDD (RA/LV) | 28 | 16 | 6 | 6 |
| DDD (RA/BiV) | 3 | — | — | 3 |
| PIDs coupling delays tested | ||||
| Vp to PIDS 30 ms | 11 | 11 | — | — |
| Vp to PIDS 20 ms | 35 | 18 | 7 | 10 |
| Vp to PIDS 10 ms | 11 | 11 | — | — |
| Vp only | 35 | 18 | 7 | 10 |
| PIDS to Vp 10 ms | 11 | 11 | — | — |
| PIDS to Vp 20 ms | 18 | 18 | — | — |
| PIDS to Vp 30 ms | 11 | 16 | — | — |
Where appropriate data are given as mean ± SD.
BiV, biventricular; DDD, atrioventricular synchronous pacing mode; EF, ejection fraction; LV, left ventricle; PIDS, pacing-induced diaphragmatic stimulation; RA, right atrium; RV, right ventricle; Vp, ventricular pacing pulse; post op, post-operation.
Haemodynamic monitoring
Although monitoring the effect of PIDS on LV function either invasively through an LV catheter or non-invasively through Doppler echocardiography would have been desirable, neither method is practical and justifiable for monitoring in the post-operative environment. As an alternative, we used acoustic cardiography, a technique that allows continuous recording of simultaneous sound and ECG data over short, intermediate, and long periods. 9
Acoustic cardiography
All acoustic cardiography recordings were obtained using an Audicor ® device (Inovise Medical, Inc., Portland, OR, USA). Using the standard array of 12-lead ECG electrodes, this device employs unique dual-purpose sensors in the V3 and V4 positions. These sensors simultaneously acquire V3 and V4 ECG signals and cardiac acoustic data from the region of the LV apex. The Audicor ® system produces a variety of computerized diagnostic parameters, including the electromechanical activation time (EMAT), which is the interval in milliseconds from the onset of the QRS to the mitral component of the S1. Electromechanical activation time measures the length of time that the LV requires to generate sufficient force to close the mitral valve, constituting the initial portion of the pre-ejection period. In patients with systolic dysfunction (i.e. systolic heart failure patients), the LV requires more time to increase the force required to close the mitral valve. Electromechanical activation time is prolonged, reaching abnormal values in those patients. 10 , 11 Ejection fraction (EF) is more widely used as a measure of systolic function, but unlike EMAT, it fails to measure the time required for LV contraction. Recent work has shown that EMAT is more closely related to LV d P /d t maximum (d P /d t max) than is LVEF, 12 trending well with d P /d t max during pre-load changes (P. Jamshidi, unpublished results, 2007). Given this d P /d t max correlation, EMAT provides clinical utility in the diagnosis of heart failure patients, augmenting other diagnostic tests 13 and also guiding heart failure therapy to improve exercise tolerance and therefore quality of life. 14
Detection of pacing-induced diaphragmatic stimulation with acoustic cardiography
The presence of PIDS manifests as a specific ‘fingerprint’ in the sound trace and the corresponding time frequency of the sound trace. 15 As illustrated in Figure 1 A , a high frequency deflection follows the ventricular pacing spike by no more than 30 ms in the simultaneously recorded ECG. This deflection is not present in the absence of PIDS ( Figure 1 B ). The PIDS-induced artefact in the sound recording is probably produced through the mechanical impact of the diaphragm on the chest wall, where it is detected by the acoustic sensor.
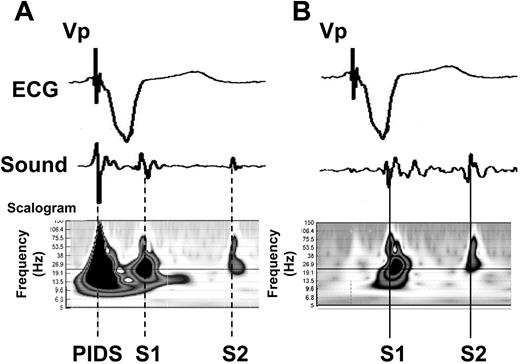
Representative time domain (ECG, heart sound) as well as scalogram illustrations (heart sound only) for cardiac conditions with and without the presence of pacing-induced diaphragmatic stimulation (PIDS) and the corresponding sound artefacts. ( A ) PIDS induced as a side effect of ventricular pacing and ( B ) ventricular pacing without PIDS. ECG, electrocardiogram; S1, first heart sound; S2, second heart sound; PIDS, pacing-induced diaphragmatic stimulation; Vp, ventricular pacing pulse. Scalogram=squared magnitude of the wavelet transform, showing the amount of signal energy as a function of time and frequency.
Confirmation of pacing-induced diaphragmatic movements
Fluoroscopy (Integris H 3500, Philips, The Netherlands) was used in phase I of the study to confirm pacing-induced diaphragmatic movements with the lowest pacing output capable of stimulating the diaphragm without causing symptoms. Asymptomatic PIDS was defined as diaphragmatic movements detectable on the fluoroscopic screen or by palpation without recognition by the patient. Based on the findings of phase I, in phase II and III, asymptomatic PIDS was defined as palpable diaphragmatic movements at the lowest possible pacing output (in the case of ventilated patients) or without recognition by the patient.
Electrode placement and pacing equipment
During surgery, all 35 patients received temporary bipolar electrodes (distance between contacts ∼2 cm) attached to the free right atrial wall and to the left posterior diaphragm, both distant to the phrenic nerve. In seven patients, a temporary RV epicardial electrode was placed on the anterior wall. In 31 patients, a temporary LV epicardial electrode was placed on the antero-lateral wall. Three patients received both RV and LV epicardial electrodes. All temporary electrodes were removed 1 day prior to discharge.
All pacing electrodes were connected and controlled by an external pacing analyzer module (CS 3000, Biotronik GmbH, Berlin, Germany). This module supports three stimulation/sensing leads independently. For the three patients with electrodes in the RV and LV, both electrodes were connected to the same channel through a Y-cable connector.
Study design and pacing stimulation protocols
The study comprised three phases ( Figure 2 ). Phase I evaluated the haemodynamic impact of different PIDS-ventricular pacing configurations and different coupling intervals between the ventricular pacing and PIDS in both symptomatic and asymptomatic settings. Phase II monitored the short-term (up to 1 h) consistency of the haemodynamic effect of PIDS 4–6 h following open heart surgery. Phase III examined the long-term reproducibility (24 h after the initial PIDS episode) of the haemodynamic effect of PIDS. The different pacing configurations and modes tested in the three study phases are presented in Table 1 .

Study design and stimulation protocols. Phase I-tested pacing-induced diaphragmatic stimulation (PIDS)-ventricular pacing configurations and different coupling intervals between the ventricular pacing and PIDS in both symptomatic and asymptomatic settings. Phase II monitored the consistency of the haemodynamic effect of PIDS for up to 1 h. Phase III followed the phase II protocol together with a repeated stimulation after 24 h to examine reproducibility of the haemodynamic effect of PIDS. AV, atrioventricular; DDD, atrioventricular synchronous pacing mode; EMAT, electromechanical activation time; PIDS, pacing-induced diaphragmatic stimulation; PR, ECG interval from P wave to R wave; Vp, ventricular pulse.
Phase I: acute haemodynamic effect of pacing-induced diaphragmatic stimulation timing on patients 4–7 days following open heart surgery (Patients 1–18)
After assessing baseline haemodynamics without pacing, the pacing threshold for asymptomatic PIDS was determined. Various pacing configurations were initiated ( Figure 2 and Table 1 ), and the haemodynamic changes were recorded with acoustic cardiography. Pacing configurations of greatest focus were those in which the timing relationship between the ventricular activation and PIDS was altered. We tested the effect of PIDS on ventricular function in the atrioventricular (AV) synchronous pacing mode (DDD mode), with a constant AV delay close to the intrinsic PR interval throughout the various PIDS coupling intervals in symptomatic and asymptomatic PIDS configurations.
Phase II: consistency of the haemodynamic effect of pacing-induced diaphragmatic stimulation evaluated on patients in the intensive care unit 4–6 h post-operation (Patients 19–25)
On the basis of the results obtained from the first 18 patients tested in phase I, we determined the PIDS-ventricular pacing configuration and coupling interval that elicited the best haemodynamic condition/response. Phase II compared this best PIDS-ventricular pacing configuration/coupling interval with the corresponding non-PIDS DDD pacing mode in a further seven patients by continuously recording EMAT in each mode for up to 60 min.
Phase III: stability of haemodynamic effect of pacing-induced diaphragmatic stimulation evaluated on patients 24 h post-operation (Patients 26–35)
Phase III followed the same protocol as phase II, but with further EMAT recordings 24 h after the initial PIDS episode in the baseline, non-PIDS DDD pacing modes, and in the best PIDS-ventricular-coupled pacing mode to investigate the long-term effects and stability of PIDS. Three of the 10 patients in this phase were also tested in the BiV mode with and without PIDS. In the BiV mode, the delay between the RV and LV pacing pulse was 0 ms, such that both ventricles were stimulated simultaneously over a Y-cable connector.
Statistical analysis
We tested the null hypothesis for any differences in EMAT between different episodes of pacing using the paired t -test. We chose a two-tailed alpha <0.05 to be statistically significant.
Results
Acute haemodynamic effect of pacing-induced diaphragmatic stimulation timing on patients 4–7 days following open heart surgery (phase I)
Using fluoroscopy and simultaneous observation of the presence or absence of patient symptoms, we found that in all patients it was possible to stimulate the diaphragm at voltages that resulted in either symptomatic or asymptomatic diaphragmatic movements. Asymptomatic diaphragmatic stimulation voltages were typically below 2.5 V at 0.4 mA. Therefore, we examined the effects of various coupling intervals between ventricular pacing and PIDS on EMAT and also evaluated the difference in EMAT between asymptomatic and symptomatic pacing amplitudes for each coupling interval. In the first test series and in 18 patients, a coupling interval of 20 ms was applied. In a second test series, coupling intervals of 10, 20, and 30 ms were applied in 11 of these 18 patients. Electromechanical activation time in the DDD-only (no PIDS) mode was documented in every session.
Table 2 summarizes the values for EMAT obtained in the different PIDS-ventricular pacing configurations with a 20 ms coupling interval in both asymptomatic and symptomatic settings. In both settings, EMAT was significantly improved when the PIDS pulse was delivered 20 ms after the ventricular pacing pulse when compared with the DDD-only mode. Electromechanical activation time was not significantly different between the DDD-only mode and the mode in which the PIDS pulse was delivered 20 ms before the ventricular pacing pulse.
Influence of the pacing-induced diaphragmatic stimulation-left ventricular pacing configurations in time-synchronous DDD mode and symptomatic and asymptomatic settings on electromechanical activation time
| . | EMAT (ms) . | . | ΔEMAT (ms) [vs. DDD (no PIDS)] . | |
|---|---|---|---|---|
| Configuration | Symptomatic ( n = 18) | Asymptomatic ( n = 18) | Symptomatic ( n = 18) | Asymptomatic ( n = 18) |
| Vp-PIDS 20 ms | 106 ± 22* | 104 ± 21* | −18 ± 17 | −20 ± 19 |
| DDD (no PIDS) | 124 ± 21 | 124 ± 21 | ||
| PIDS-Vp 20 ms | 120 ± 24 | 118 ± 27 | 4 ± 18 | 6 ± 15 |
| . | EMAT (ms) . | . | ΔEMAT (ms) [vs. DDD (no PIDS)] . | |
|---|---|---|---|---|
| Configuration | Symptomatic ( n = 18) | Asymptomatic ( n = 18) | Symptomatic ( n = 18) | Asymptomatic ( n = 18) |
| Vp-PIDS 20 ms | 106 ± 22* | 104 ± 21* | −18 ± 17 | −20 ± 19 |
| DDD (no PIDS) | 124 ± 21 | 124 ± 21 | ||
| PIDS-Vp 20 ms | 120 ± 24 | 118 ± 27 | 4 ± 18 | 6 ± 15 |
ΔEMAT represents difference in EMAT between the non-PIDS mode and the Vp-PIDS or PIDS-Vp modes.
Data for EMAT and ΔEMAT are presented as mean ± SD.
* P < 0.01 indicates a significant difference from DDD (no PIDS) configuration. DDD (no PIDS), DDD mode without diaphragmatic stimulation; PIDS-Vp 20 ms, ventricle stimulated 20 ms after diaphragmatic stimulation; Vp, ventricular pacing pulse; Vp-PIDS 20 ms, diaphragm stimulated 20 ms after ventricular stimulation.
Influence of the pacing-induced diaphragmatic stimulation-left ventricular pacing configurations in time-synchronous DDD mode and symptomatic and asymptomatic settings on electromechanical activation time
| . | EMAT (ms) . | . | ΔEMAT (ms) [vs. DDD (no PIDS)] . | |
|---|---|---|---|---|
| Configuration | Symptomatic ( n = 18) | Asymptomatic ( n = 18) | Symptomatic ( n = 18) | Asymptomatic ( n = 18) |
| Vp-PIDS 20 ms | 106 ± 22* | 104 ± 21* | −18 ± 17 | −20 ± 19 |
| DDD (no PIDS) | 124 ± 21 | 124 ± 21 | ||
| PIDS-Vp 20 ms | 120 ± 24 | 118 ± 27 | 4 ± 18 | 6 ± 15 |
| . | EMAT (ms) . | . | ΔEMAT (ms) [vs. DDD (no PIDS)] . | |
|---|---|---|---|---|
| Configuration | Symptomatic ( n = 18) | Asymptomatic ( n = 18) | Symptomatic ( n = 18) | Asymptomatic ( n = 18) |
| Vp-PIDS 20 ms | 106 ± 22* | 104 ± 21* | −18 ± 17 | −20 ± 19 |
| DDD (no PIDS) | 124 ± 21 | 124 ± 21 | ||
| PIDS-Vp 20 ms | 120 ± 24 | 118 ± 27 | 4 ± 18 | 6 ± 15 |
ΔEMAT represents difference in EMAT between the non-PIDS mode and the Vp-PIDS or PIDS-Vp modes.
Data for EMAT and ΔEMAT are presented as mean ± SD.
* P < 0.01 indicates a significant difference from DDD (no PIDS) configuration. DDD (no PIDS), DDD mode without diaphragmatic stimulation; PIDS-Vp 20 ms, ventricle stimulated 20 ms after diaphragmatic stimulation; Vp, ventricular pacing pulse; Vp-PIDS 20 ms, diaphragm stimulated 20 ms after ventricular stimulation.
Individual and summarized values for EMAT in the different PIDS-ventricular pacing coupling intervals of 10, 20, and 30 ms in the asymptomatic setting are given in Figure 3 . These data demonstrate that when compared with EMAT in the DDD-only mode, only the coupling configuration of delivery of the PIDS pulse 20 ms after the ventricular pacing pulse (Vp) significantly improved EMAT. The outcome in asymptomatic and symptomatic settings was comparable (data not shown).
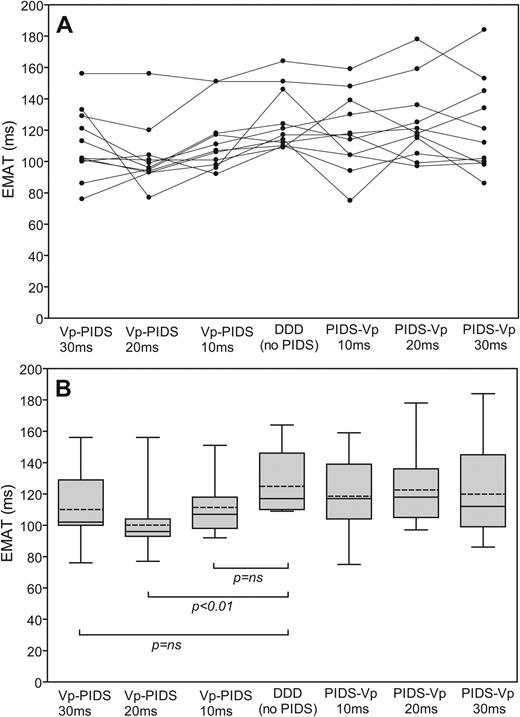
Individual ( A ) and summarized ( B ) values for mean electromechanical activation time (EMAT) during diaphragmatic stimulation for time-synchronous DDD (right atrium-left ventricle)-diaphragmatic pacing with different coupling intervals ( n = 11). The boundaries of the boxes indicate the 25th and 75th percentiles, the whiskers indicate minimum and maximum values, and the horizontal line marks the median value. DDD (no PIDS), DDD mode without diaphragm stimulation; EMAT, electromechanical activation time; PIDS-Vp 10 ms (or 20 or 30 ms), ventricle stimulation 10 ms (or 20 or 30 ms) after diaphragmatic stimulation; Vp-PIDS 10 ms (or 20 or 30 ms), diaphragmatic stimulation 10 ms (or 20 or 30 ms) after ventricle stimulation.
Figure 4 presents individual and summarized values for EMAT in all the non-PIDS modes and the best PIDS-ventricular pacing coupling configuration and interval (i.e. Vp-PIDS 20 ms). In all patients, EMAT was best in the intrinsic mode (no pacing). Atrial overdrive pacing (AAI) did not significantly increase EMAT above that in the intrinsic mode. Compared with intrinsic and AAI modes, ventricular-based pacing modes (VVI, DDD, and Vp-PIDS) all significantly increased EMAT, with Vp-PIDS having the least negative impact. Electromechanical activation time was significantly better for the Vp-PIDS configuration in comparison with the non-PIDS mode.
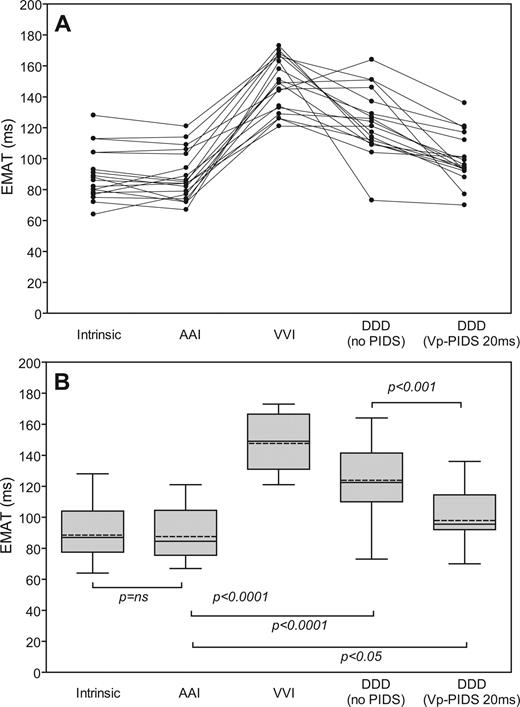
Individual ( A ) and summarized ( B ) values for mean electromechanical activation time (EMAT) in intrinsic rhythm, single- and dual-chamber pacing modes and in the Vp-PIDS 20 ms pacing mode ( n = 18). For description of the box plot design, see legend to Figure 3 . AAI, single-chamber pacemaker mode with lead in the atrium; DDD (no PIDS), DDD mode without diaphragmatic stimulation; DDD (Vp-PIDS 20 ms), DDD mode with diaphragmatic stimulation 20 ms after ventricle stimulation; VVI, single-chamber pacemaker mode with lead in the ventricle.
Consistency of the haemodynamic effect of pacing-induced diaphragmatic stimulation (phases II and III)
In all 17 patients included in phases II and III of the study, continuous acoustic cardiography recordings in the DDD mode and in the asymptomatic Vp-PIDS 20 ms pacing mode were taken for up to 60 min. Figure 5 provides an example of the temporal stability of EMAT in either mode for a single patient. Individual and summarized values for EMAT in DDD and Vp-PIDS 20 ms pacing modes for all patients enrolled in phases II and III are given in Figure 6 . As observed in phase I, EMAT in the Vp-PIDS 20 ms pacing mode was significantly lower than that in the no PIDS mode.
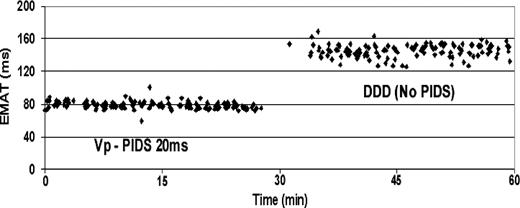
Variability of electromechanical activation time (EMAT) during diaphragmatic stimulation for 1 h in various time-synchronous asymptomatic left ventricular diaphragmatic pacing modes. Each data point in the figure corresponds to the result of a 10 s acoustic cardiography analysis. DDD (no PIDS), DDD mode without diaphragm stimulation; Vp-PIDS 20 ms, DDD mode with diaphragmatic stimulation 20 ms after ventricle stimulation.
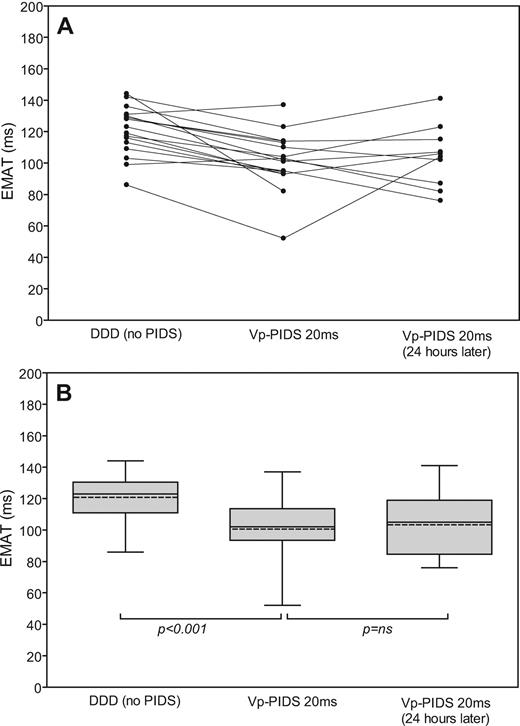
Individual ( A ) and summarized ( B ) values for mean electromechanical activation time (EMAT) during temporary diaphragmatic stimulation for time-synchronous DDD-asymptomatic diaphragmatic pacing for up to 60 min in the intensive care unit ( n = 17) and up to 30 min 24 h later ( n = 10). For description of the box plot design, see legend to Figure 3 . DDD, fixed-rate dual-chamber pacing; DDD (no PIDS), DDD mode without diaphragmatic stimulation; Vp-PIDS 20 ms, diaphragmatic stimulation 20 ms after ventricle stimulation.
Stability of the haemodynamic effect of pacing-induced diaphragmatic stimulation after 24 h (phase III)
In all of the 10 patients enrolled in study phase III, continuous acoustic cardiography recordings in the asymptomatic Vp-PIDS 20 ms pacing mode were collected 24 h after phase II measurements for up to 30 min. Individual and summarized values for EMAT recorded at the two times did not differ significantly ( Figure 6 ), illustrating that the haemodynamic effects of PIDS are reproducible, at least within a period of 24 h.
Discussion
Previous studies on PIDS 16–18 have focused on patients with respiratory conditions, in which PIDS was found to be an effective and reliable therapeutic approach. Respiratory weakness also commonly occurs in patients with heart failure. 17 Conditioning of the phrenic nerve and diaphragm activation with theophylline have been shown to not only improve ventilatory function, 19 but also positively influence systolic performance. 20 This study sought to determine whether the diaphragm, through PIDS, could be utilized as an accessory mechanical aid to improve the systolic performance in patients with heart disease. To the best of our knowledge, this is the first study that systematically evaluates the haemodynamic effect of various coupling configurations and intervals between PIDS and the onset of ventricular contraction.
As illustrated in Figure 1 , PIDS manifests itself in acoustic cardiography recordings as a sound artefact of finite duration within 20–30 ms after initiation of PIDS. The artefact in the sound recording is probably caused by the mechanical impact of the diaphragm against the chest wall. Due to the proximity of the ventricular apex to the diaphragm, PIDS will also mechanically impact the heart and therefore change the ventricular activation energy at the onset of systole. Thus in practical terms, PIDS could potentially act as a mechanical aid for the adjacent LV. As shown in all phases of the study, there are definable coupling intervals between the onset of electrical systole and PIDS, which yielded an improvement of LV systolic function.
A PIDS pulse 20 ms after the ventricular pacing pulse seems generally beneficial. However, there is likely to be some patient specificity with respect to the ideal non-zero coupling interval. The explanation that an excitation of the diaphragm after ventricular pacing was the best coupling might be complex. The diaphragm contracts rather slowly; the first movement after the excitation is caudal and only secondarily cranial. A well-synchronized timing after the ventricular pacing could initially benefit the diastole of the subsequent heart beat and then, during the upward movement, provide mechanical assistance for the consecutive systole. However, the reduction in EMAT for the Vp-PIDS 20 ms pacing configuration supports the hypothesis that the induced diaphragmatic movement acts as a mechanical aid and lowers the activation energy for LV contraction.
Open heart surgery patients were selected for evaluating the effect of PIDS on LV systolic function as placement of temporary pacing leads in such patients is standard practice. Their underlying heart condition is different from the population potentially targeted for mechanical assist therapy (see Study limitations section). The mean EF of our study population was 61%. Therefore, it is not surprising that LV systolic performance was best in the non-pacing (intrinsic) and AAI modes and that, due to a more inefficient depolarization pattern in the ventricle, any ventricular pacing reduced LV systolic performance when compared with that in the intrinsic condition. Nevertheless, with a well-synchronized PIDS mode, the LV systolic performance approached that in the intrinsic condition. Importantly, Vp-PIDS 20 ms in either asymptomatic or symptomatic PIDS settings yielded an EMAT that was significantly shortened compared with that in the non-PIDS mode (ΔEMAT = −18 and −20 ms, respectively). In this study, we documented an 18–20 ms reduction of EMAT, which in invasive studies was associated with a significant improvement in d P /d t max (P. Jamshidi, unpublished results, 2007).
Pacing-induced diaphragmatic stimulation could be applied for up to an hour without any patient symptoms and provide the same haemodynamic benefit as symptomatic PIDS. Therefore, the technique has potential as a mechanical assist therapy in patients with severe, drug-refractory heart failure, who do not fulfil the criteria for CRT. Through use of a commercially available three-chamber device with LV/RV electrodes coupled over a Y-cable connector to the RV lead output and the diaphragm electrode to the LV lead output, PIDS may also be a useful adjunct therapy for CRT patients or bridging aid to transplantation in severe heart failure patients. Long-term stability of PIDS would be an important requisite for successful therapy in patients with heart disease. Previous studies have suggested that a permanent high-frequency stimulation of the diaphragm might induce diaphragmatic fatigue and, occasionally, irreversible damage to the lower motor neuron. 21 , 22 Generation of diaphragm fatigue may be due to activation of the fast-twitch, fatigue muscle fibres. 23 The low-frequency stimulations (slightly above 1 Hz) applied over 1 h in this study did not lead to diaphragm fatigue or desensitization. We documented that PIDS and its positive impact on LV systolic function can be reproduced 24 h after the initial PIDS episode.
Although the results of our study need to be verified and PIDS should be tested in subjects with heart failure, the evidence presented herein support the potential of PIDS as a viable device-based therapeutic approach for improving ventricular performance. Pacing-induced diaphragmatic stimulation can be applied without causing symptoms for the patients while achieving the same haemodynamic benefit. The beneficial effect of asymptomatic PIDS on ventricular performance is not simply an acute effect, but is consistent for up to an hour and can be fully reproduced at least 24 h after the initial PIDS event. Possibly, the optimal coupling interval between the onset of ventricular systole and PIDS will be patient-specific and, as for CRT, 24 influenced by body position, heart rate, and pre-load conditions. Therefore, PIDS as a device-based therapy would need to be applied in a closed-loop fashion using a reliable haemodynamic monitoring parameter such as acoustic cardiography.
Study limitations
This study was designed to explore the effect of PIDS on LV systolic function. The patients selected following open heart surgery were neither pacemaker-dependent nor in heart failure. The number of patients in the various study phases is small, and the results should be confirmed in a larger group of patients. Continuous PIDS was limited to a maximum of 1 h in the supine position, and the haemodynamic effect of PIDS in various positions over days and weeks needs to be investigated in further studies.
Conclusions
Pacing-induced diaphragmatic stimulation, if synchronized to the onset of ventricular contraction with a fixed, non-zero coupling delay, can improve LV systolic function reproducibly for up to 1 h without causing patient symptoms. The improvements are significant even in a group of patients with preserved EF. The associated LV systolic function is significantly better than regular VVI and DDD pacing modes. The absence of diaphragm desensitization enhances the potential of PIDS as a practical therapeutic approach in device-based heart failure management.
Conflict of interest: One author, P.B., works for Inovise Medical, Inc., the company that provided the acoustic cardiography technology for haemodynamic monitoring. He contributed to the data analysis and manuscript preparation. All other authors have no conflict of interest to disclose.
Funding
Inovise Medical, Inc. provided the equipment and acoustic sensors for the study. Otherwise no financial support from grants, contracts, or industries was provided.



