-
PDF
- Split View
-
Views
-
Cite
Cite
Maren Tomaske, Jan Janousek, Vít Rázek, Roman A. Gebauer, Viktor Tomek, Gerd Hindricks, Walter Knirsch, Urs Bauersfeld, Adverse effects of Wolff–Parkinson–White syndrome with right septal or posteroseptal accessory pathways on cardiac function, EP Europace, Volume 10, Issue 2, February 2008, Pages 181–189, https://doi.org/10.1093/europace/eun005
Close - Share Icon Share
Abstract
Wolff–Parkinson–White syndrome with right septal or posteroseptal accessory pathways causes eccentric septal mechanical activation and may provoke left ventricular (LV) dyssynchrony and dysfunction. The aim of the study was to evaluate the effect of radiofrequency catheter ablation (RFA) of the accessory pathways on LV function.
Retrospectively, transthoracic echocardiography and electrocardiogram recordings were analysed in 34 patients (age: 14.2 ± 2.5 years) with right septal or posteroseptal accessory pathways prior and after (median: 1 day) successful RFA. Results prior to RFA, LV ejection fraction was decreased (<55%) in 19/34 patients (56%). After RFA, QRS duration was normalized (129 ± 23 vs. 90 ± 11, P < 0.0001), LV function improved (ejection fraction: 50 ± 10 vs. 56 ± 4%, P = 0.0005) and septal-to-posterior wall motion delay as a global measure for LV dyssynchrony decreased (110 ± 94 vs. 66 ± 53, P = 0.012). Longitudinal two-dimensional strain evaluated in five patients demonstrated a decrease of left intraventricular mechanical delay from 292 ± 125 to 118 ± 37 ms after RFA.
Wolff–Parkinson–White syndrome with right septal or posteroseptal accessory pathways may cause LV dyssynchrony and jeopardize global LV function. Radiofrequency catheter ablation resulted in normalized QRS duration, mechanical resynchronization, and improved LV function. Even in the absence of arrhythmias, RFA of right septal or posteroseptal pathways may be considered in patients with significantly decreased LV function.
Introduction
The prevalence of accessory pathways has been reported to be 0.1–0.3% 1 in the general population. Supraventricular tachycardia in manifest Wolff–Parkinson–White (WPW) syndrome is the most common arrhythmia. 2 In WPW syndrome, ventricles are electrically and mechanically pre-excited through an accessory pathway, which connects atria and ventricles. The eccentric ventricular activation via accessory pathway results in an asynchronous spread of ventricular depolarisation. In the mid-70s, echocardiographic observations displayed an abnormal interventricular septal motion in patients with WPW syndrome due to right septal or posteroseptal accessory pathways, whereas left-sided accessory pathways resulted in abnormal left ventricular (LV) posterior wall motion. 3–5 Especially the presence of abnormal interventricular septal wall motion may provoke LV dyssynchrony and dysfunction. 6 , 7
The introduction of electrophysiological mapping 8 enabled a precise location of accessory pathways. The current nomenclature for the different segments of the junctions was used to define the location of the accessory pathways. 9
Moreover, radiofrequency catheter ablation (RFA) of accessory pathways is established as a first line therapy of symptomatic accessory pathways in children beyond 5 years of age. 2 , 10
The purpose of this study was to evaluate the effect of RFA of right septal or posteroseptal accessory pathways on LV function in paediatric patients.
Methods
Study patients
Data from 43 consecutive children from three tertiary care centres who underwent RFA due to WPW syndrome with right septal or posteroseptal accessory pathways between January 2000 and December 2006 were analysed retrospectively. Patients with haemodynamically relevant heart disease or with concealed accessory pathways were excluded. Patient characteristics and demographic data were obtained from charts or electronic database reviews. In all patients, recurrent tachycardia was the indication for RFA. Prescribed antiarrhythmic drugs were ceased five halftimes prior to RFA.
Electrophysiological studies were performed in 43 children, with successful RFA of the accessory pathways in 39 children. In four patients, RFA was not attempted after detailed electrophysiological mapping due to high risk for complete AVB ( n = 2), long accessory pathway effective refractory period ( n = 1), or low weight ( n = 1). These four patients were excluded from further analysis. Furthermore, five patients with missing documentation of transthoracic echocardiography prior to or after RFA had to be excluded from further analysis. Thus, the study group consisted of a total of 34 children.
The study design was approved by the institutional Ethical Committee and written informed consent was obtained.
Electrocardiogram
Prior and after RFA, all patients underwent a 12-lead electrocardiogram (ECG) during sinus rhythm using a Mac®5000 System (GE Medical Systems, Milwaukee, WI, USA). Measurements of QRS intervals were performed by computed electrocardiographic system analysis of intervals or measured manually as the longest QRS duration in any lead.
Echocardiography
Echocardiography was obtained from standard precordial views with appropriate transducers (5.0, 3.5, and 2.5 MHz) on either Sonos 5500 or 7500 (Philips, Eindhoven, the Netherlands), or the Vivid 7 (GE Vingmed Ultrasound, Horton, Norway). Mono- and two-dimensional transthoracic echocardiography and Doppler evaluation were performed according to recent guidelines. 11 Echocardiographic data stored on VHS videotapes or digitized acquisitions were analysed retrospectively. To assess global function, end-diastolic and end-systolic LV diameters were determined from parasternal long-axis M-mode for fractional shortening calculation. Moreover, end-diastolic and end-systolic LV volumes were determined from the apical four-chamber view using the monoplane Simpson's rule to calculate ejection fraction (LVEF). Where feasible, the intra- and interventricular dyssynchrony indices were evaluated. As a measure of intraventricular dyssynchrony, the delay between peak septal and left posterior wall systolic motion (septal-to-posterior wall motion delay, SPWMD) was determined. 12 This was accomplished by taking into account the maximum wall thickening as expression of active contraction. 13 Furthermore, the interval between the onset of electrical systole (delta wave or QRS complex) and the opening of the pulmonic and aortic valves was recorded. The difference between the left and right ventricular pre-ejection periods (interventricular mechanical delay, IVMD) was calculated and regarded as a measure of interventricular dyssynchrony. 14
Additionally, when advanced techniques became available in two centres, two-dimensional strain analysis was performed. A total of five patients underwent echocardiography based on frame-to-frame tracking of acoustic tissue pixels within two-dimensional echocardiographic images, as described in detail previously. 15–17 In the apical four-chamber view, longitudinal strain was evaluated in a total of eight myocardial segments (anterior, posterior, septal, lateral) at the basal and mid-ventricular level. In the parasternal short-axis view at the mitral valve level a six-segmental LV model was used and circumferential strain was analysed. Analysis was performed offline using the Echopac version 4.0.2 (GE Vingmed Ultrasound, Horton, Norway). Frame rates were 60–120 frames/s. Visual control of tracking quality was performed to ensure accurate automatic tracking. Negative deflections of strain were interpreted as segmental contraction and positive deflections as stretch, respectively. For each segment, the time from the QRS complex to maximal peak negative longitudinal and circumferential strain was determined. To define severity of LV dyssynchrony, the maximum difference of measured timing between any two segments for each longitudinal and circumferential strain (ΔTime 2Dstrain) prior and after RFA was reported.
To assess interobserver variability, a second experienced paediatric cardiologist who was blinded whether echocardiography was prior or after RFA, performed independent measurements of the echocardiographic data.
Radiofrequency catheter ablation procedure
Three quadripolar electrode catheters were inserted percutanously and advanced to the right atrium, right ventricular apex, and His bundle position. A decapolar catheter was advanced into the coronary sinus. The location of the accessory pathway was confirmed using standard electrophysiological manoeuvres. Electrophysiological mapping and RFA of accessory pathways were performed with six or seven French thermistor ablation catheters. The RFA procedure was considered successful if neither antegrade nor retrograde accessory pathway conduction did recur for at least 30 min after RFA. Long-term success of RFA was defined as absence of tachycardia recurrence and pre-excitation pattern on surface ECG, respectively.
Statistical analysis
Data are expressed as either median (range) or mean (± standard deviation) depending on distribution pattern of the data evaluated by the Kolmogorov–Smirnov test. Differences in continuous variables among groups of patients were evaluated by two-tailed t -test or by Mann–Whitney rank sum test as appropriate, differences in categorical variables by Fisher's exact test. Using the paired t -test, intra-individual changes in continuous variables between the two follow-up intervals were evaluated. Correlations between non-parametric variables were measured by Spearman's correlation. A P -value <0.05 is considered statistically significant. For echocardiographic measurements, the inter-observer agreement ( r -value) was assessed by Pearson product moment correlation. Statistical analysis was performed using the statistical software package PRISM for Windows, Version 4.0 (GraphPad Software, San Diego, CA, USA).
Results
Demographic data and clinical characteristics
The study population included a total of 34 study patients. None of the patients was in supraventricular tachycardia or suffered from incessant tachycardia immediately before the echocardiographic study or ablation. Demographic data and clinical characteristics at the time of RFA procedure are depicted in Table 1 . Three of 4 (12%) patients with symptoms of congestive heart failure prior to RFA were treated with angiotensin-converting enzyme inhibitors. None of the patients of the study cohort suffered from incessant tachycardia, rapid conduction of atrial fibrillation via accessory pathway, or other arrhythmias that might have contributed to the reduced LV function. All RFA procedures were performed without complications.
Baseline characteristics of the 34 study patients at the time of radiofrequency ablation
| . | Total ( n = 34) . |
|---|---|
| Characteristics | |
| Male/female | 19 / 15 |
| Age (years) a | 14.2 ± 2.5 |
| Weight (kg) a | 58.5 ± 20.2 |
| Body surface area (m 2 ) a | 1.63 ± 0.34 |
| Echocardiographic assessment after RFA b | 1 day (1 day–10.7 months) |
| Right accessory pathway location: n (%) | |
| Midseptal | 6 (18) |
| Parahisian | 6 (18) |
| Posteroseptal | 22 (65) |
| . | Total ( n = 34) . |
|---|---|
| Characteristics | |
| Male/female | 19 / 15 |
| Age (years) a | 14.2 ± 2.5 |
| Weight (kg) a | 58.5 ± 20.2 |
| Body surface area (m 2 ) a | 1.63 ± 0.34 |
| Echocardiographic assessment after RFA b | 1 day (1 day–10.7 months) |
| Right accessory pathway location: n (%) | |
| Midseptal | 6 (18) |
| Parahisian | 6 (18) |
| Posteroseptal | 22 (65) |
a Data are given as means ± SD.
b Data is given as median (range).
Baseline characteristics of the 34 study patients at the time of radiofrequency ablation
| . | Total ( n = 34) . |
|---|---|
| Characteristics | |
| Male/female | 19 / 15 |
| Age (years) a | 14.2 ± 2.5 |
| Weight (kg) a | 58.5 ± 20.2 |
| Body surface area (m 2 ) a | 1.63 ± 0.34 |
| Echocardiographic assessment after RFA b | 1 day (1 day–10.7 months) |
| Right accessory pathway location: n (%) | |
| Midseptal | 6 (18) |
| Parahisian | 6 (18) |
| Posteroseptal | 22 (65) |
| . | Total ( n = 34) . |
|---|---|
| Characteristics | |
| Male/female | 19 / 15 |
| Age (years) a | 14.2 ± 2.5 |
| Weight (kg) a | 58.5 ± 20.2 |
| Body surface area (m 2 ) a | 1.63 ± 0.34 |
| Echocardiographic assessment after RFA b | 1 day (1 day–10.7 months) |
| Right accessory pathway location: n (%) | |
| Midseptal | 6 (18) |
| Parahisian | 6 (18) |
| Posteroseptal | 22 (65) |
a Data are given as means ± SD.
b Data is given as median (range).
Median time for post-interventional echocardiographic assessment was 1 day (range: 1 day–11 months). In three patients with moderately reduced LV function (LVEF ≤ 41%) and symptoms of congestive heart failure prior to RFA, re-evaluation was performed at prolonged follow-up.
Electrocardiogram
Surface 12-lead ECG displayed normalized QRS duration with disappearance of the delta wave in all 34 patients after successful RFA procedure ( Table 2 ). Moreover, long-term success of RFA was achieved in all 34 patients.
Comparison of pre- and post-radiofrequency ablation measurements in the 34 study patients
| Parameter . | Number of patients ( n = ) . | Pre-RFA a . | Post-RFA a . | P -value . |
|---|---|---|---|---|
| QRS duration (ms) | 34 | 129 ± 23 | 90 ± 11 | <0.0001 |
| Fractional shortening (%) | 34 | 33 ± 6 | 38 ± 5 | <0.0001 |
| LVEF(%) | 25 | 50 ± 10 | 56 ± 4 | 0.0005 |
| IVMD (ms) | 6 | 29 ± 25 | 25 ± 20 | 0.063 NS |
| SPWMD (ms) | 22 | 110 ± 94 | 66 ± 53 | 0.012 |
| Parameter . | Number of patients ( n = ) . | Pre-RFA a . | Post-RFA a . | P -value . |
|---|---|---|---|---|
| QRS duration (ms) | 34 | 129 ± 23 | 90 ± 11 | <0.0001 |
| Fractional shortening (%) | 34 | 33 ± 6 | 38 ± 5 | <0.0001 |
| LVEF(%) | 25 | 50 ± 10 | 56 ± 4 | 0.0005 |
| IVMD (ms) | 6 | 29 ± 25 | 25 ± 20 | 0.063 NS |
| SPWMD (ms) | 22 | 110 ± 94 | 66 ± 53 | 0.012 |
a Data are given as mean ± SD. P -value comparing pre- and post-RFA findings.
IVMD, interventricular mechanical delay; LVEF, left ventricular ejection fraction; NS, not significant; RFA, radiofrequency ablation; SPWMD, septal-to-posterior wall motion delay.
Comparison of pre- and post-radiofrequency ablation measurements in the 34 study patients
| Parameter . | Number of patients ( n = ) . | Pre-RFA a . | Post-RFA a . | P -value . |
|---|---|---|---|---|
| QRS duration (ms) | 34 | 129 ± 23 | 90 ± 11 | <0.0001 |
| Fractional shortening (%) | 34 | 33 ± 6 | 38 ± 5 | <0.0001 |
| LVEF(%) | 25 | 50 ± 10 | 56 ± 4 | 0.0005 |
| IVMD (ms) | 6 | 29 ± 25 | 25 ± 20 | 0.063 NS |
| SPWMD (ms) | 22 | 110 ± 94 | 66 ± 53 | 0.012 |
| Parameter . | Number of patients ( n = ) . | Pre-RFA a . | Post-RFA a . | P -value . |
|---|---|---|---|---|
| QRS duration (ms) | 34 | 129 ± 23 | 90 ± 11 | <0.0001 |
| Fractional shortening (%) | 34 | 33 ± 6 | 38 ± 5 | <0.0001 |
| LVEF(%) | 25 | 50 ± 10 | 56 ± 4 | 0.0005 |
| IVMD (ms) | 6 | 29 ± 25 | 25 ± 20 | 0.063 NS |
| SPWMD (ms) | 22 | 110 ± 94 | 66 ± 53 | 0.012 |
a Data are given as mean ± SD. P -value comparing pre- and post-RFA findings.
IVMD, interventricular mechanical delay; LVEF, left ventricular ejection fraction; NS, not significant; RFA, radiofrequency ablation; SPWMD, septal-to-posterior wall motion delay.
Echocardiographic findings
Interobserver agreement 18 of measured parameter was excellent for fractional shortening ( r = 0.81), SPWMD ( r = 0.91), and IVMD ( r = 0.81), respectively. For LVEF, inter-observer variability was good ( r = 0.74).
Global left ventricular function
Pre- and post-RFA findings are depicted in Table 2 . Left ventricular ejection fraction was lower than 55% in 19/34 (56%) prior and 8/34 (24%) of patients after successful RFA ( P = 0.008) ( Figure 1 ). Global LV function improved significantly after successful RFA as measured by fractional shortening ( P < 0.0001) and LVEF ( P = 0.0005), respectively. A subanalysis of pre-RFA findings in the 12 patients with midseptal and parahisian pathways revealed lower LVEF when compared with those with posteroseptal pathway location (LVEF: 45 ± 13 vs. 53 ± 8%, P = 0.022). In 8 (67%) patients of this subgroup, LVEF was lower than 55%.
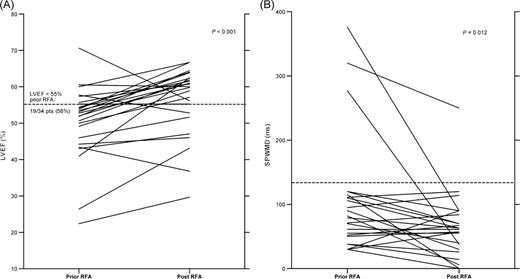
Pre- and post-radiofrequency ablation findings for lower left ventricular ejection fraction and septal-to-posterior wall motion delay. Findings prior to radiofrequency ablation revealed significantly lower left ventricular ejection fraction ( n = 25) ( A ) and prolonged septal-to-posterior wall motion delay ( n = 22) ( B ) when compared with post-radiofrequency ablation findings. Pts, patients.
Eccentric cardiac activation
Interventricular dyssynchrony did not differ from pre-RFA findings (IVMD, P = 0.063). Intraventricular LV synchrony improved, demonstrated by a significant decrease in SPWMD with a delayed onset of the systolic septal motion ( P = 0.012) ( Table 2 , Figure 1 ). After exclusion of the three patients with extremely prolonged SPWMD, the decrease in SPWMD still was significant ( P = 0.039). A subanalysis of pre-RFA findings in the 12 patients with midseptal and parahisian pathways demonstrated a prolonged SPWMD when compared with those with posteroseptal pathway location (SPWPD: 144 ± 73 vs. 94 ± 67 ms, P = 0.043).
Accordingly, the difference between the segments with earliest and latest negative longitudinal and circumferential strain evaluated in five patients decreased after RFA (ΔTime 2Dstrain longitudinal: 292 ± 125 vs. 118 ± 37 ms; ΔTime 2Dstrain circumferential: 191 ± 37 vs. 116 ± 21 ms). Analysis of mechanical dyssynchrony revealed an early mechanical activation of the basal interventricular septum representing the site of accessory pathway insertion. This was accompanied by a stretch of the LV free wall and followed by septal relaxation concurrent with free-wall contraction ( Figures 2–4 ). Detailed findings of the two-dimensional strain analysis for each of the five patients prior and after RFA are given in Table 3 .
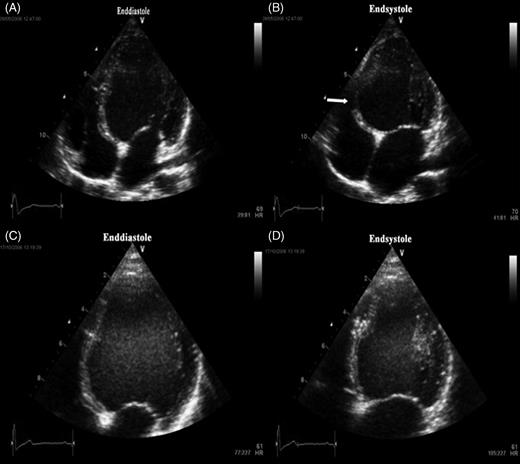
Apical four-chamber view. End-diastolic ( A ) and end-systolic ( B ) frame in the apical four-chamber view prior to radiofrequency ablation of a parahisian pathway in a 10-year-old boy. Typical bulging of basal septum in end-systole ( B , white arrow) is seen along with significantly decreased left ventricular ejection fraction of 26%. End-diastolic ( C ) and end-systolic ( D ) frame in the apical four-chamber view 3 months after ablation. Paradoxical septal motion is abolished and ejection fraction has increased to 43%.
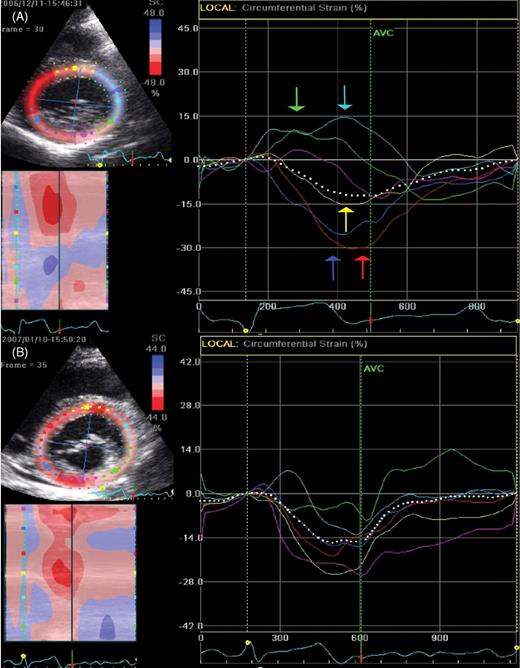
Circumferential two-dimensional strain in the parasternal short-axis view at the level of the mitral valve is displayed. Colour coding of the strain curves refers to the respective left ventricular segments. Prior to radiofrequency ablation of a right septal pathway ( A ), circumferential two-dimensional strain curves at the level of the mitral valve display an inhomogenous beginning of peak negative circumferential strain. Furthermore, in early systole, anterior and lateral segments display stretch while septal, anterioseptal, and inferior segments display contraction. One month after ablation ( B ), circumferential two-dimensional strain curves display an almost homogenous beginning of peak negative circumferential strain.
Measurement of left ventricular dyssynchrony prior and after radiofrequency ablation in the five patients enrolled
| Patient (age in years) . | Prior RFA . | After RFA . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . | Time since RFA (months) . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . |
| 1 (10.6) | 135 | 22 | 418 | n.a. | 0.95 | 70 | 30 | 170 | n.a. |
| 2 (11.6) | 150 | 26 | 419 | n.a. | 2.7 | 80 | 43 | 143 | n.a. |
| 5 (15.0) | 92 | 52 | 237 | 255 | 0.95 | 86 | 61 | 82 | 125 |
| 6 (15.7) | 142 | 58 | 131 | 95 | 0.2 | 84 | 53 | 100 | 92 |
| 7 (12.1) | 198 | 56 | 254 | 224 | 0.53 | 120 | 62 | 96 | 131 |
| Patient (age in years) . | Prior RFA . | After RFA . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . | Time since RFA (months) . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . |
| 1 (10.6) | 135 | 22 | 418 | n.a. | 0.95 | 70 | 30 | 170 | n.a. |
| 2 (11.6) | 150 | 26 | 419 | n.a. | 2.7 | 80 | 43 | 143 | n.a. |
| 5 (15.0) | 92 | 52 | 237 | 255 | 0.95 | 86 | 61 | 82 | 125 |
| 6 (15.7) | 142 | 58 | 131 | 95 | 0.2 | 84 | 53 | 100 | 92 |
| 7 (12.1) | 198 | 56 | 254 | 224 | 0.53 | 120 | 62 | 96 | 131 |
n.a., not available; ΔTime 2Dstrain, maximum difference of measured timing between any two segments for each longitudinal and circumferential strain; LVEF, left ventricular ejection fraction; RFA, radiofrequency ablation.
Measurement of left ventricular dyssynchrony prior and after radiofrequency ablation in the five patients enrolled
| Patient (age in years) . | Prior RFA . | After RFA . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . | Time since RFA (months) . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . |
| 1 (10.6) | 135 | 22 | 418 | n.a. | 0.95 | 70 | 30 | 170 | n.a. |
| 2 (11.6) | 150 | 26 | 419 | n.a. | 2.7 | 80 | 43 | 143 | n.a. |
| 5 (15.0) | 92 | 52 | 237 | 255 | 0.95 | 86 | 61 | 82 | 125 |
| 6 (15.7) | 142 | 58 | 131 | 95 | 0.2 | 84 | 53 | 100 | 92 |
| 7 (12.1) | 198 | 56 | 254 | 224 | 0.53 | 120 | 62 | 96 | 131 |
| Patient (age in years) . | Prior RFA . | After RFA . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . | Time since RFA (months) . | QRS duration (ms) . | LVEF (%) . | ΔTime 2Dstrain longitudinal (ms) . | ΔTime 2Dstrain circumferential (ms) . |
| 1 (10.6) | 135 | 22 | 418 | n.a. | 0.95 | 70 | 30 | 170 | n.a. |
| 2 (11.6) | 150 | 26 | 419 | n.a. | 2.7 | 80 | 43 | 143 | n.a. |
| 5 (15.0) | 92 | 52 | 237 | 255 | 0.95 | 86 | 61 | 82 | 125 |
| 6 (15.7) | 142 | 58 | 131 | 95 | 0.2 | 84 | 53 | 100 | 92 |
| 7 (12.1) | 198 | 56 | 254 | 224 | 0.53 | 120 | 62 | 96 | 131 |
n.a., not available; ΔTime 2Dstrain, maximum difference of measured timing between any two segments for each longitudinal and circumferential strain; LVEF, left ventricular ejection fraction; RFA, radiofrequency ablation.
Correlations
There was no correlation between initial QRS duration and initial LVEF ( P = 0.51) or initial SPWMD ( P = 0.08). Moreover, there was no correlation between the decrease in QRS duration and LVEF improvement ( P = 0.86) or SPWMD shortening ( P = 0.53).
Discussion
Manifest WPW syndrome causes premature electrical and mechanical activation of myocardial segments close to the ventricular insertion of the accessory pathway. The extent of the pre-excited ventricular myocardium depends on the relative timing of normal and eccentric ventricular activation. Larger pre-excited areas may lead to significant dyssynchrony in the ventricular motion and thereby cause ventricular dysfunction. In this study, we have restricted the evaluation of LV function to right septal and posteroseptal accessory pathways for the following reasons: in right free-wall pathways, LV activation is almost synchronous over the normal conduction system, with a precontraction area being limited to the RV free wall; in left free-wall pathways, the amount of pre-excited myocardium is rather small. A long conduction time to the atrial pathway insertion is in competition with the AV node conduction, thus again most of the LV is activated normally. Conventional echocardiographic studies in left-sided accessory pathways demonstrated abnormal LV posterior wall motion, 5 , 19 with normal LV function determined by Doppler indices. 20 In contrast to left free-wall pathways, septal accessory connections are activated early due to short conduction time from the sinus node to the atrial pathway insertion. Thus, they may cause an early pre-excitation of a critical part of the interventricular septum inducing significant LV dyssynchrony and thereby reduced LV function. 6 , 7
An early activation of the basal septum will induce segmental contraction which is unopposed by the activation of the remaining LV myocardium. This asynchronous septal motion has been confirmed by conventional and advanced technique echocardiography 5 , 19 , 21 , 22 and was also documented in our patients ( Figures 2–4 ). Low local preload imposed on the early contracting segments will lead to decreased myocardial work and hypotrophy, as described in experimental studies on cardiac pacing. 23 , 24 Hypotrophy is probably an additional reason for extreme systolic bulging of the pre-excited segment towards the right ventricle as documented in Figure 2 and 3 . However, there is little data, whether the eccentric septal mechanical activation may provoke significant LV dysfunction. 6 , 7 , 25
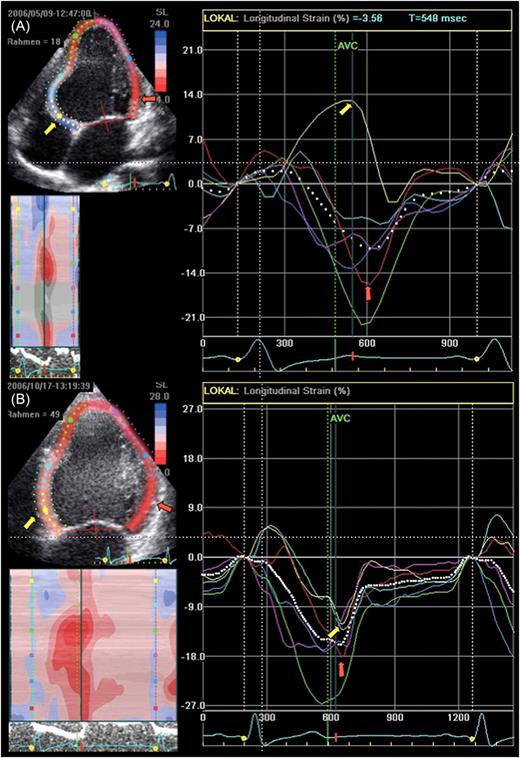
Longitudinal two-dimensional strain in the apical four-chamber view of the same patient as shown in Figure 2 is displayed. Colour coding of the strain curves refers to the respective left ventricular segments. The longitudinal two-dimensional strain in the apical four-chamber prior to radiofrequency ablation of the parahisian pathway ( A ): early activated basal septum (yellow arrow and curve) shows relaxation and stretch (positive strain) throughout most of the whole systole caused by contraction (negative strain) of normally timed left ventricular segments (i.e. basal lateral wall, red arrow). Early contraction of the unloaded pre-excited segment could not be detected in this analysis. Three months after ablation ( B ), there is an almost complete normalization of the segmental contraction timing with good alignment of septal (yellow arrow) and lateral wall (red arrow) peak negative strain.
In the present study, a total of 34 children with right septal or posteroseptal accessory pathways were evaluated prior and after successful catheter ablation. The main findings were, that interventricular synchrony was restored at a median of 1 day after RFA. This is in accordance to former findings, indicating an immediate resolution of the abnormal ventricular depolarization after successful catheter ablation. 21 , 22 Moreover, reduced LV function (<55%) prior to RFA was observed in more than half of our patient cohort prior to RFA; and was more pronounced in patients with midseptal and parahisian pathways when compared with posteroseptal pathways. However, in 7 of 34 (21%) patients, LV function was still reduced after successful RFA. As some of the post-interventional echocardiographic evaluations were done shortly after cardiac catheterization, fluid shifts, stress, and altered peripheral vascular resistances might have adversely influenced LVEF. Interestingly, in the three patients with the lowest LV function (LVEF ≤ 41%) and symptoms of congestive heart failure prior to RFA, re-evaluation at prolonged follow-up indicated considerably improved LV function. The septal hypotrophy observed in these patients is theoretically acting like a functional aneurysm ( Figure 2A – D ) and may induce pathological ventricular remodelling with progressive dysfunction, as previously described by Fazio et al . 7 Reverse remodelling of the heart in patients with this phenomena will take some months to recover.
Of importance, none of our patients was in supraventricular tachycardia before RFA or suffered from incessant tachycardia, rapid conduction of atrial fibrillation via accessory pathway, or other arrhythmias that might have contributed to the reduced LV function. Thus, tachycardia-induced cardiomyopathy as origin for the reduced LV function prior to RFA is unlikely in our study group.
Though septal motion abnormality in right septal and posteroseptal accessory pathways appears to be similar to that of a left bundle brunch block or RV pacing, there are some important differences. Left bundle branch block and chronic RV pacing in complete AV block result in an impaired LV systolic function due to global dyssynchronous mechanical activation. 26–29 Right septal and posteroseptal accessory pathways induce an early activation in the basal septum with a relatively synchronous activation of the rest of the LV myocardium. This fact probably explains, why LV function was reduced in 56% of our study cohort while the remainder of the patients simply exhibited eccentric ventricular activation; and why septal pre-excitation did not cause a major disarrangement of interventricular synchrony. However, our results confirm previous reports demonstrating a reduced LV function in patients with sinus rhythm, improving after successful RFA of right posteroseptal accessory pathways. 6 , 20 , 25
Potential clinical implications
Right septal and posteroseptal pathways were successfully ablated as demonstrated by others. 30 , 31 The decision to advise an invasive study and catheter ablation of accessory pathways in asymptomatic patients with a WPW pattern is still controversial. 32 Besides the potential risk for episodes of rapidly conducted atrial fibrillation, 33 severe LV dysfunction and septal hypotrophy due to septal pre-excitation may be further primary preventive indications for RFA in selected patients. Echocardiography should be performed in all patients with pre-excitation on surface ECG. In asymptomatic patients, RFA may be restricted to those who develop severe LV dysfunction due to septal hypotrophy and paradoxical motion on echocardiography. The avoidance of progression or even recovery of septal hypotrophy should be considered.
Study limitations
A main limitation of this study is its retrospective design with regard to completeness and reproducibility of the collected variables. In order to avoid potential selection bias, every patient with structurally normal heart undergoing RFA due to right septal or posteroseptal accessory pathways in the three institutions, was enrolled. Nevertheless, five patients with missing documentation of transthoracic echocardiography prior or after RFA had to be excluded from further analysis.
In order to obtain objective measurements, a second experienced paediatric cardiologist obtained independent measurements of the global LV function as well as inter- and intraventricular dyssynchrony. Measurements of time intervals were based on a 1-channel surface ECG displayed on the monitor during echocardiography with difficult discrimination of the onset of ventricular activation in some patients. However, interobserver agreement was good, indicating reproducible measurements and a low probability for misclassification.
The measurement of shortening fraction and LVEF to assess global LV function may overestimate the scale of the LV impairment. The use of two- or three-dimensional-based parameters would improve accuracy. To prove the potential clinical relevant extent of reduction in LV function, pre- and post-RFA exercise testing may be an option.
Moreover, a restricted number of patients with midseptal and parahisian accessory pathways limits the statistical validity of a conclusive statement concerning the difference between midseptal/parahisian or posteroseptal accessory pathways.
Conclusion
Wolff–Parkinson–White syndrome with right septal or posteroseptal accessory pathways causes LV dyssynchrony and jeopardizes global LV function. Septal hypotrophy as a result of early unopposed septal activation can be observed in some patients. Radiofrequency ablation of accessory pathways resulted in normalized QRS duration as well as mechanical resynchronization and improved LV function. Recovery of septal hypotrophy was observed in all affected patients. Even in the absence of arrhythmias, decreased LV function and septal hypotrophy may imply premature RFA procedure in patients with right septal or posteroseptal pathways. Prospective studies with comparative measurements of LV dyssynchrony of either septal or right/left free-wall accessory pathways and their impact on LV function are required.
Conflict of interest : none declared.
Funding
M.T. receives grant support from Guidant/Boston Scientific, but unrelated to this study. R.A.G. was supported by the Research project No. 64203 of the University Hospital Motol, Prague, Czech Republic.
References
- cardiac arrhythmia
- electrocardiogram
- ventricular function, left
- wolff-parkinson-white syndrome
- echocardiography
- atrioventricular accessory pathway
- radiofrequency catheter ablation
- left ventricle
- echocardiography, transthoracic
- cardiac function
- ejection fraction
- radiofrequency ablation
- qrs complex duration



