-
PDF
- Split View
-
Views
-
Cite
Cite
Tinka J van Trier, Marjolein Snaterse, Steven H J Hageman, Nienke ter Hoeve, Madoka Sunamura, Eric P Moll van Charante, Henrike Galenkamp, Jaap W Deckers, Fabrice M A C Martens, Frank L J Visseren, Wilma J M Scholte op Reimer, Ron J G Peters, Harald T Jørstad, Unexploited potential of risk factor treatment in patients with atherosclerotic cardiovascular disease, European Journal of Preventive Cardiology, Volume 30, Issue 7, May 2023, Pages 601–610, https://doi.org/10.1093/eurjpc/zwad038
Close - Share Icon Share
Abstract
Most patients with atherosclerotic cardiovascular disease remain at (very) high risk for recurrent events due to suboptimal risk factor control.
This study aimed to quantify the potential of maximal risk factor treatment on 10-year and lifetime risk of recurrent atherosclerotic cardiovascular events in patients 1 year after a coronary event.
Pooled data from six studies are as follows: RESPONSE 1, RESPONSE 2, OPTICARE, EUROASPIRE IV, EUROASPIRE V, and HELIUS. Patients aged ≥45 years at ≥6 months after coronary event were included. The SMART-REACH score was used to estimate 10-year and lifetime risk of recurrent atherosclerotic cardiovascular events with current treatment and potential risk reduction and gains in event-free years with maximal treatment (lifestyle and pharmacological). In 3230 atherosclerotic cardiovascular disease patients (24% women), at median interquartile range (IQR) 1.1 years (1.0–1.8) after index event, 10-year risk was median (IQR) 20% (15–27%) and lifetime risk 54% (47–63%). Whereas 70% used conventional medication, 82% had ≥1 drug-modifiable risk factor not on target. Furthermore, 91% had ≥1 lifestyle-related risk factor not on target. Maximizing therapy was associated with a potential reduction of median (IQR) 10-year risk to 6% (4–8%) and of lifetime risk to 20% (15–27%) and a median (IQR) gain of 7.3 (5.4–10.4) atherosclerotic cardiovascular disease event-free years.
Amongst patients with atherosclerotic cardiovascular disease, maximizing current, guideline-based preventive therapy has the potential to mitigate a large part of their risk of recurrent events and to add a clinically important number of event-free years to their lifetime.
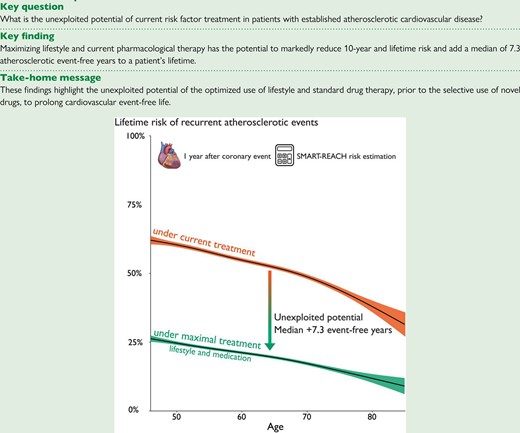
Lay Summary
Patients with heart disease are at high risk of new cardiac events. This study amongst 3230 patients who had a heart attack or received a stent or bypass surgery shows missed potential for healthy life after a heart attack. The average age of study patients was 61 years, and 24% were women. At 1 year after the cardiac event, nearly one in three (30%) continued smoking, 79% were overweight, 45% reported insufficient physical activity, 40% had high blood pressure, and 65% had a too high LDL (‘bad’) cholesterol. We calculated that adherence to lifestyle advice and medications could on average halve the risk for another heart attack and add over 7 healthy years of life after a heart attack. This highlights the importance of healthy lifestyle and medication adherence after a heart attack.
Key finding:• adherence to lifestyle advice and medications could add over 7 healthy years of life after a heart attack
Introduction
Patients with established atherosclerotic cardiovascular disease (ASCVD) are at particularly high risk of recurrent cardiovascular events, with 18% of patients experiencing a recurrent event as early as the first year after myocardial infarction.1 This risk is largely modifiable by lifestyle and drug interventions targeted at the major risk factors—smoking, overweight, physical inactivity, hypertension, dyslipidaemia, and diabetes mellitus (DM)—that collectively account for 80–90% of the population-level burden of ASCVD.2,3 Alarmingly, both in trial settings and in practice, the majority of secondary prevention patients maintain unhealthy lifestyles and adherence to preventive medication declines over time.4–10 This poor adherence to standard preventive measures and decreasing attention for risk optimization of healthcare professionals after the first year post-event are central contributing factors to why guideline-mandated treatment targets are not met, with consequently high rates of recurrent events.11
Some patients do achieve risk factor targets with initial lifestyle and drug treatment, yet remain at high risk for recurrent events, so-called residual risk. For these patients, international guidelines mandate increasingly strict treatment targets, with rapidly increasing numbers of pharmaceutical interventions to reach these targets, such as anti-thrombotic,12,13 anti-inflammatory,14–16 glucose-lowering,17,18 and lipid-modifying19–23 options.24 A central challenge in ASCVD risk management is therefore to identify which patients require which treatment intensification and at which level: lifestyle, adherence to conventional medication, or selective use of novel medication. With the increasing number of pharmaceutical options, on top of current suboptimal levels of conventional prevention, the complexity of ASCVD therapeutic regimens is increasing, with challenges in decision-making for both clinicians and patients.25–27
Preventive decision-making can be assisted by quantifying long-term individual risk of recurrent events, both with current treatment and with maximal treatment of all modifiable risk factors, as recommended by the 2021 ESC cardiovascular prevention guidelines.24 This may support optimal use of standard preventive treatment and selective use of novel drugs. We therefore aimed to quantify ASCVD risk under current risk factor management and the potential of maximal prevention strategies in a large database of patients with recent acute coronary syndrome (ACS) or coronary revascularization, using the SMART-REACH lifetime risk prediction model.28
Methods
Design and patient selection
We performed a multi-study analysis in patients with a first or recurrent ACS (acute myocardial infarction or unstable angina pectoris) or coronary revascularization (percutaneous or coronary artery bypass graft surgery) (i.e. index event). We aimed to include a heterogeneous, multi-ethnic sample, representative of the target population, to ensure external validity. Therefore, patients from six large contemporary studies, both trial and non-trial patients, were assessed for eligibility: (i) Randomized Evaluation of Secondary Prevention by Outpatient Nurse SpEcialists (RESPONSE) 1,4 (ii) RESPONSE 2,5 (iii) OPTImal CArdiac Rehabilitation (OPTICARE)29, the Dutch participants of (iv) European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE), IV30 (v) EUROASPIRE V,6 and (vi) HEalthy Life in an Urban Setting (HELIUS) multi-ethnic cohort study.31 Individual study details can be found in Supplementary material online, Tables S1 (study designs) and S2 (inclusion and exclusion criteria per study). We included all patients (i) with risk factor data available at 6 months or later after index event, considering this as a period of lifestyle and medical optimization, and (ii) aged ≥45 years at time of follow-up.
Data extraction
Data collection for each study has been described in detail.4–6,29–32 For the current analysis, we extracted individual-level data on age, sex, index event, educational level, ethnicity, and cardiovascular history. Data on smoking status, body mass index (BMI), physical activity level, systolic blood pressure (SBP), lipids, glucose, glycated haemoglobin (HbA1c), and medication use were extracted at one time point ≥6 months after index event. Methodology of measurements of SMART-REACH prediction variables are specified in Supplementary material online, Table S3.
Outcome measures and definitions
Our primary outcome was 10-year and lifetime (i.e. until 90 years of age) risk of recurrent myocardial infarction, stroke, or vascular death with current treatment, estimated using the SMART-REACH lifetime model.28 Secondary outcomes included the following:
Current risk factor management
Percentage of patients not meeting targets for lifestyle- and drug-modifiable risk factors according to the ESC guidelines for cardiovascular disease prevention applicable at time of the original study is as follows: (ii) smoking, (ii) BMI ≥25 kg/m2 (overweight) and BMI ≥30 kg/m2 (obese); (iii) self-reported physical activity <150 min/week, (iv) SBP ≥140 mmHg, (v) low-density lipoprotein cholesterol (LDL-c) ≥1.8 mmol/L (≥70 mg/dL), and (vi) fasting glucose ≥7.0 mmol/L, non-fasting ≥11.1 mmol/L, or HbA1c ≥48 mmol/mol (patients without DM) or HbA1c ≥53 mmol/mol (patients with DM). Risk factor targets from the applicable ESC guidelines (2006, 2011, and 2016) were uniform, with an exception for the LDL-c target of <2.5 mmol/L (<100 mg/dL) amongst RESPONSE 1 participants, as this study applied the 2006 ESC guidelines.33–35 We used waist circumference thresholds stratified by sex and BMI category (<25, 25–29.9, 30–34.9, ≥35), which better indicate an increased 10-year risk of coronary events and all-cause mortality compared with the single waist circumference thresholds (women >88 cm; men >102 cm), as recommended by most guidelines.36,37 Data on risk factors were assessed at one time point ≥6 months after index event.
Current prevention medication use
The proportion of patients using conventional ASCVD preventive medication is classified by effect: blood pressure-lowering, anti-thrombotic, lipid-lowering, and diabetic medication.
Potential benefit with additional treatment
Estimated individual benefit from maximal prevention treatment is expressed as follows: (i) the reduction of absolute 10-year and lifetime risk, (ii) the proportion of patients with 10-year risk <10%, and (iii) the number of cardiovascular event-free life years gained. We modelled a theoretical scenario, in which all participants received ‘maximal’ ASCVD treatment, defined as non-smoking, meeting strict risk factor targets regardless of whether this is achieved with lifestyle changes or medication (SBP ≤120 mmHg, LDL-c <1.8 mmol/L, and HbA1c ≤53 mmol/mol) and additional use of novel drug strategies: dual anti-platelet therapy, colchicine, and, if diagnosed with DM, use of glucagon-like peptide-1 agonists (GLP-1a) and sodium–glucose cotransporter 2 inhibitors (SGLT2i).
Statistical analysis
We report patients’ demographics, risk factor prevalence, and medication using means and standard deviations (SD), medians and the interquartile range (IQR), and numbers and valid percentages (percentage of total number of non-missing cases), as appropriate. The SMART-REACH is a Fine and Gray competing risk model, used in secondary prevention to estimate the 10-year and lifetime (i.e. until age 90 years) risk of recurrent myocardial infarction, stroke, or vascular death, based on the following cardiovascular risk factors: age, sex, smoking, DM, SBP, total cholesterol, creatinine, number of cardiovascular disease manifestations (coronary artery disease, cerebrovascular disease, and/or peripheral artery disease), atrial fibrillation, heart failure, and geographical region (Netherlands).28 Specification of definitions and measurements of the SMART-REACH predictors per study can be found in Supplementary material online, Table S3. We used linear regression to visualize the relationship between age and estimated 10-year and lifetime risk generalized additive model method with a smoothing function, R-package mgcv and ggplot). The SMART-REACH model was also used to estimate the effect of maximal risk factor therapy, by applying relative risk reductions based on hazard ratios from meta-analyses to the estimated individual risk (Supplementary material online, Table S4). The difference in median life expectancy free of myocardial infarction or stroke with current vs. maximal treatment was shown with survival curves (R-package survminer). A two-sided P-value < 0.05 was considered statistically significant. All statistical analyses were performed using R statistical software (version 3.6.1).
Missing data
Missing data patterns were visually explored (R-package VIM) and assumed to be ‘missing completely at random’ and ‘missing at random’ (i.e. not depending on the missing data), allowing us to impute the missing values based on participants with complete data. In case of missing risk factors needed for the SMART-REACH risk estimation, we used single imputation by predictive mean matching (R-package MICE), deriving the missing values from observed values in patients with matching risk profiles. Single imputation has been shown to be a robust method for dealing with missing values in cardiovascular prediction models.38 Number of missing predictors and mean/median values before and after imputation can be found in Supplementary material online, Table S5.
Sensitivity analysis
We performed several sensitivity analyses to explore the robustness of our findings. First, we estimated SMART-REACH risks using two different methods for dealing with missing data, i.e. completed cases and mean/median imputation (see Supplementary material online, Table S5). Second, we performed a stratified analysis by type of study, as we expected differences in risk estimates in trial vs. non-trial patients. Finally, we performed analysis excluding those who reported an ASCVD event at very young age (<35 years), since these events may be driven by different (genetic) risk factors such as elevated lipoprotein(a) and familial hyperlipidaemia, which are not included in our SMART-REACH model.
Results
Participants in RESPONSE 1, RESPONSE 2, EUROASPIRE IV, EUROASPIRE V, OPTICARE, and HELIUS with ACS or revascularization were assessed for eligibility (n = 3934). A total of 3230 participants (24% women) met the inclusion criteria for our pooled cohort, with mean age 61 (SD 8) years and at median 1.1 (IQR 1.0–1.8) years after the index event (Figure 1). Manifestations of vascular disease in addition to the index event were prior myocardial infarction (18%), stroke (7%), and peripheral artery disease (4%) (Table 1). Twenty-six percent of patients were of non-European origin. Patient characteristics stratified per study can be found in Supplementary material online, Table S6.
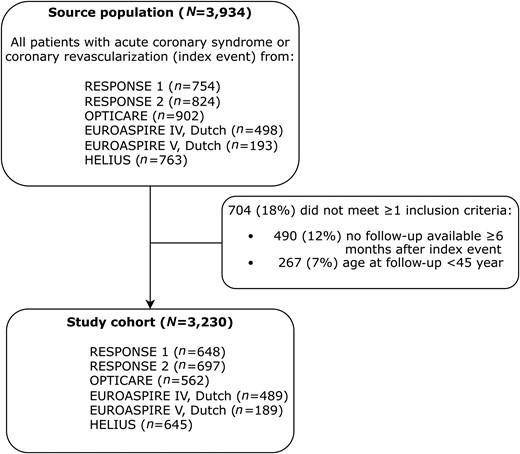
Inclusion flowchart. Patients can lack ≥1 inclusion criteria; therefore, the number of non-included patients per criterion adds up to >100%. Abbreviations: RESPONSE, Randomized Evaluation of Secondary Prevention by Outpatient Nurse SpEcialists; OPTICARE, OPTImal CArdiac Rehabilitation; EUROASPIRE, European Action on Secondary and Primary Prevention by Intervention to Reduce Events (only Dutch participants); HELIUS, HEalthy Life in an Urban Setting.
| N = 3230 . | % . | n/N . |
|---|---|---|
| Socio-demographics | ||
| ȃAge in years, mean (SD) | 61 | 8.4 |
| ȃMen | 76 | 2467 |
| ȃEuropean | 74 | 2077/2795 |
| ȃHigher education (universities of applied sciences and research universities) | 23 | 704/3108 |
| ȃYears between index event and risk factor measurements, median (IQR) | 1.1 | 1.0–1.8 |
| Index event | ||
| ȃAcute coronary syndrome (myocardial infarction or unstable angina pectoris) | 69 | 2243 |
| ȃElective coronary revascularization (PCI or CABG) | 31 | 987 |
| Prior cardiovascular disease | ||
| ȃMyocardial infarction | 18 | 454/2585 |
| ȃStroke | 7 | 199/3042 |
| ȃPeripheral artery disease | 4 | 106/2666 |
| Cardiovascular risk factors/comorbidity | ||
| ȃDiabetes mellitus | 25 | 812/3222 |
| ȃHistory of hypertension | 50 | 1612/3228 |
| ȃHistory of dyslipidemia | 50 | 1417/2852 |
| ȃFamily history of premature atherosclerosis | 54 | 1333/2481 |
| N = 3230 . | % . | n/N . |
|---|---|---|
| Socio-demographics | ||
| ȃAge in years, mean (SD) | 61 | 8.4 |
| ȃMen | 76 | 2467 |
| ȃEuropean | 74 | 2077/2795 |
| ȃHigher education (universities of applied sciences and research universities) | 23 | 704/3108 |
| ȃYears between index event and risk factor measurements, median (IQR) | 1.1 | 1.0–1.8 |
| Index event | ||
| ȃAcute coronary syndrome (myocardial infarction or unstable angina pectoris) | 69 | 2243 |
| ȃElective coronary revascularization (PCI or CABG) | 31 | 987 |
| Prior cardiovascular disease | ||
| ȃMyocardial infarction | 18 | 454/2585 |
| ȃStroke | 7 | 199/3042 |
| ȃPeripheral artery disease | 4 | 106/2666 |
| Cardiovascular risk factors/comorbidity | ||
| ȃDiabetes mellitus | 25 | 812/3222 |
| ȃHistory of hypertension | 50 | 1612/3228 |
| ȃHistory of dyslipidemia | 50 | 1417/2852 |
| ȃFamily history of premature atherosclerosis | 54 | 1333/2481 |
In case of any missing values, the valid percentage of the total number of non-missing cases (N) are presented.
Abbreviations: SD, standard deviation; IQR, interquartile range; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery.
| N = 3230 . | % . | n/N . |
|---|---|---|
| Socio-demographics | ||
| ȃAge in years, mean (SD) | 61 | 8.4 |
| ȃMen | 76 | 2467 |
| ȃEuropean | 74 | 2077/2795 |
| ȃHigher education (universities of applied sciences and research universities) | 23 | 704/3108 |
| ȃYears between index event and risk factor measurements, median (IQR) | 1.1 | 1.0–1.8 |
| Index event | ||
| ȃAcute coronary syndrome (myocardial infarction or unstable angina pectoris) | 69 | 2243 |
| ȃElective coronary revascularization (PCI or CABG) | 31 | 987 |
| Prior cardiovascular disease | ||
| ȃMyocardial infarction | 18 | 454/2585 |
| ȃStroke | 7 | 199/3042 |
| ȃPeripheral artery disease | 4 | 106/2666 |
| Cardiovascular risk factors/comorbidity | ||
| ȃDiabetes mellitus | 25 | 812/3222 |
| ȃHistory of hypertension | 50 | 1612/3228 |
| ȃHistory of dyslipidemia | 50 | 1417/2852 |
| ȃFamily history of premature atherosclerosis | 54 | 1333/2481 |
| N = 3230 . | % . | n/N . |
|---|---|---|
| Socio-demographics | ||
| ȃAge in years, mean (SD) | 61 | 8.4 |
| ȃMen | 76 | 2467 |
| ȃEuropean | 74 | 2077/2795 |
| ȃHigher education (universities of applied sciences and research universities) | 23 | 704/3108 |
| ȃYears between index event and risk factor measurements, median (IQR) | 1.1 | 1.0–1.8 |
| Index event | ||
| ȃAcute coronary syndrome (myocardial infarction or unstable angina pectoris) | 69 | 2243 |
| ȃElective coronary revascularization (PCI or CABG) | 31 | 987 |
| Prior cardiovascular disease | ||
| ȃMyocardial infarction | 18 | 454/2585 |
| ȃStroke | 7 | 199/3042 |
| ȃPeripheral artery disease | 4 | 106/2666 |
| Cardiovascular risk factors/comorbidity | ||
| ȃDiabetes mellitus | 25 | 812/3222 |
| ȃHistory of hypertension | 50 | 1612/3228 |
| ȃHistory of dyslipidemia | 50 | 1417/2852 |
| ȃFamily history of premature atherosclerosis | 54 | 1333/2481 |
In case of any missing values, the valid percentage of the total number of non-missing cases (N) are presented.
Abbreviations: SD, standard deviation; IQR, interquartile range; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery.
Current risk factor management and medication use
In total, 98% of patients had at least one modifiable risk factor; 91% had unhealthy lifestyles including smoking, overweight, and insufficient physical activity; and 82% did not meet at least one drug-modifiable risk factor target (LDL-c, SBP, HbA1c, or glucose levels). The combination of all three standard cardioprotective medications (or four if diabetic, including glucose-lowering medication) was used by 70%. In patients without a diagnosis of DM, we found that 8% met the diagnostic criteria for DM (Table 2 and Supplementary material online, Table S5).
| N = 3230 . | % . | n/N . |
|---|---|---|
| ≥1 lifestyle-related or drug-modifiable risk factor | 98 | 3126/3193 |
| Lifestyle-related risk factors: ≥1 not on target | 91 | 2835/3121 |
| ȃPersistent smoking | 23 | 737/3179 |
| ȃBMI, kg/m2, mean (SD) | 29 | 4.6 |
| ȃȃNormal (target) weight (BMI <25 kg/m2) | 21 | 654/3123 |
| ȃȃOverweight (BMI ≥25 <30 kg/m2) | 47 | 1476/3123 |
| ȃȃObesity class I (BMI ≥30 <35 kg/m2) | 23 | 706/3123 |
| ȃȃObesity class II and III (BMI ≥35 kg/m2) | 9 | 286/3123 |
| ȃȃCentral obesity stratified by BMI category | 59 | 1815/3063 |
| ȃPhysical inactivity (<150 min/week) | 32 | 928/2864 |
| Drug-modifiable risk factors: ≥1 not on target | 82 | 2549/3109 |
| ȃSystolic blood pressure | ||
| ȃ≥130 mmHg | 64 | 2046/3211 |
| ȃ≥140 mmHg | 40 | 1278/3211 |
| ȃȃUsing blood pressure lowering medication | 87 | 1104/1278 |
| ȃLDL-cholesterol | ||
| ȃNot on applicable ESC guideline-recommended target | 65 | 1938/3002 |
| ȃȃUsing lipid lowering medication | 79 | 1526/1938 |
| ȃ≥1.4 mmol/L | 92 | 2754/3002 |
| ȃ≥1.8 mmol/L | 76 | 2281/3002 |
| ȃ≥2.5 mmol/L | 38 | 1143/3002 |
| ȃHbA1c | ||
| ȃ≥53 mmol/mol in patients with diagnosis of DM | 43 | 292/686 |
| ȃȃUsing glucose lowering medication | 95 | 276/292 |
| ȃ≥48 mmol/mol, fasting glucose ≥7.0 or non-fasting glucose ≥11.1 in patients without diagnosis of DM | 8 | 138/1782 |
| Standard cardioprotective medication use: use of all three (or four if diabetic) | 70 | 2212/3158 |
| ȃAntiplatelet or anticoagulation | 88 | 2766/3162 |
| ȃLipid-lowering medication | 85 | 2670/3148 |
| ȃBlood pressure lowering medication | 86 | 2726/3154 |
| ȃGlucose lowering medication (if diagnosed with DM) | 76 | 621/812 |
| N = 3230 . | % . | n/N . |
|---|---|---|
| ≥1 lifestyle-related or drug-modifiable risk factor | 98 | 3126/3193 |
| Lifestyle-related risk factors: ≥1 not on target | 91 | 2835/3121 |
| ȃPersistent smoking | 23 | 737/3179 |
| ȃBMI, kg/m2, mean (SD) | 29 | 4.6 |
| ȃȃNormal (target) weight (BMI <25 kg/m2) | 21 | 654/3123 |
| ȃȃOverweight (BMI ≥25 <30 kg/m2) | 47 | 1476/3123 |
| ȃȃObesity class I (BMI ≥30 <35 kg/m2) | 23 | 706/3123 |
| ȃȃObesity class II and III (BMI ≥35 kg/m2) | 9 | 286/3123 |
| ȃȃCentral obesity stratified by BMI category | 59 | 1815/3063 |
| ȃPhysical inactivity (<150 min/week) | 32 | 928/2864 |
| Drug-modifiable risk factors: ≥1 not on target | 82 | 2549/3109 |
| ȃSystolic blood pressure | ||
| ȃ≥130 mmHg | 64 | 2046/3211 |
| ȃ≥140 mmHg | 40 | 1278/3211 |
| ȃȃUsing blood pressure lowering medication | 87 | 1104/1278 |
| ȃLDL-cholesterol | ||
| ȃNot on applicable ESC guideline-recommended target | 65 | 1938/3002 |
| ȃȃUsing lipid lowering medication | 79 | 1526/1938 |
| ȃ≥1.4 mmol/L | 92 | 2754/3002 |
| ȃ≥1.8 mmol/L | 76 | 2281/3002 |
| ȃ≥2.5 mmol/L | 38 | 1143/3002 |
| ȃHbA1c | ||
| ȃ≥53 mmol/mol in patients with diagnosis of DM | 43 | 292/686 |
| ȃȃUsing glucose lowering medication | 95 | 276/292 |
| ȃ≥48 mmol/mol, fasting glucose ≥7.0 or non-fasting glucose ≥11.1 in patients without diagnosis of DM | 8 | 138/1782 |
| Standard cardioprotective medication use: use of all three (or four if diabetic) | 70 | 2212/3158 |
| ȃAntiplatelet or anticoagulation | 88 | 2766/3162 |
| ȃLipid-lowering medication | 85 | 2670/3148 |
| ȃBlood pressure lowering medication | 86 | 2726/3154 |
| ȃGlucose lowering medication (if diagnosed with DM) | 76 | 621/812 |
In case of any missing values, the valid percentage of the total number of non-missing cases (N) are presented.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; LDL-cholesterol, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; DM, diabetes mellitus.
| N = 3230 . | % . | n/N . |
|---|---|---|
| ≥1 lifestyle-related or drug-modifiable risk factor | 98 | 3126/3193 |
| Lifestyle-related risk factors: ≥1 not on target | 91 | 2835/3121 |
| ȃPersistent smoking | 23 | 737/3179 |
| ȃBMI, kg/m2, mean (SD) | 29 | 4.6 |
| ȃȃNormal (target) weight (BMI <25 kg/m2) | 21 | 654/3123 |
| ȃȃOverweight (BMI ≥25 <30 kg/m2) | 47 | 1476/3123 |
| ȃȃObesity class I (BMI ≥30 <35 kg/m2) | 23 | 706/3123 |
| ȃȃObesity class II and III (BMI ≥35 kg/m2) | 9 | 286/3123 |
| ȃȃCentral obesity stratified by BMI category | 59 | 1815/3063 |
| ȃPhysical inactivity (<150 min/week) | 32 | 928/2864 |
| Drug-modifiable risk factors: ≥1 not on target | 82 | 2549/3109 |
| ȃSystolic blood pressure | ||
| ȃ≥130 mmHg | 64 | 2046/3211 |
| ȃ≥140 mmHg | 40 | 1278/3211 |
| ȃȃUsing blood pressure lowering medication | 87 | 1104/1278 |
| ȃLDL-cholesterol | ||
| ȃNot on applicable ESC guideline-recommended target | 65 | 1938/3002 |
| ȃȃUsing lipid lowering medication | 79 | 1526/1938 |
| ȃ≥1.4 mmol/L | 92 | 2754/3002 |
| ȃ≥1.8 mmol/L | 76 | 2281/3002 |
| ȃ≥2.5 mmol/L | 38 | 1143/3002 |
| ȃHbA1c | ||
| ȃ≥53 mmol/mol in patients with diagnosis of DM | 43 | 292/686 |
| ȃȃUsing glucose lowering medication | 95 | 276/292 |
| ȃ≥48 mmol/mol, fasting glucose ≥7.0 or non-fasting glucose ≥11.1 in patients without diagnosis of DM | 8 | 138/1782 |
| Standard cardioprotective medication use: use of all three (or four if diabetic) | 70 | 2212/3158 |
| ȃAntiplatelet or anticoagulation | 88 | 2766/3162 |
| ȃLipid-lowering medication | 85 | 2670/3148 |
| ȃBlood pressure lowering medication | 86 | 2726/3154 |
| ȃGlucose lowering medication (if diagnosed with DM) | 76 | 621/812 |
| N = 3230 . | % . | n/N . |
|---|---|---|
| ≥1 lifestyle-related or drug-modifiable risk factor | 98 | 3126/3193 |
| Lifestyle-related risk factors: ≥1 not on target | 91 | 2835/3121 |
| ȃPersistent smoking | 23 | 737/3179 |
| ȃBMI, kg/m2, mean (SD) | 29 | 4.6 |
| ȃȃNormal (target) weight (BMI <25 kg/m2) | 21 | 654/3123 |
| ȃȃOverweight (BMI ≥25 <30 kg/m2) | 47 | 1476/3123 |
| ȃȃObesity class I (BMI ≥30 <35 kg/m2) | 23 | 706/3123 |
| ȃȃObesity class II and III (BMI ≥35 kg/m2) | 9 | 286/3123 |
| ȃȃCentral obesity stratified by BMI category | 59 | 1815/3063 |
| ȃPhysical inactivity (<150 min/week) | 32 | 928/2864 |
| Drug-modifiable risk factors: ≥1 not on target | 82 | 2549/3109 |
| ȃSystolic blood pressure | ||
| ȃ≥130 mmHg | 64 | 2046/3211 |
| ȃ≥140 mmHg | 40 | 1278/3211 |
| ȃȃUsing blood pressure lowering medication | 87 | 1104/1278 |
| ȃLDL-cholesterol | ||
| ȃNot on applicable ESC guideline-recommended target | 65 | 1938/3002 |
| ȃȃUsing lipid lowering medication | 79 | 1526/1938 |
| ȃ≥1.4 mmol/L | 92 | 2754/3002 |
| ȃ≥1.8 mmol/L | 76 | 2281/3002 |
| ȃ≥2.5 mmol/L | 38 | 1143/3002 |
| ȃHbA1c | ||
| ȃ≥53 mmol/mol in patients with diagnosis of DM | 43 | 292/686 |
| ȃȃUsing glucose lowering medication | 95 | 276/292 |
| ȃ≥48 mmol/mol, fasting glucose ≥7.0 or non-fasting glucose ≥11.1 in patients without diagnosis of DM | 8 | 138/1782 |
| Standard cardioprotective medication use: use of all three (or four if diabetic) | 70 | 2212/3158 |
| ȃAntiplatelet or anticoagulation | 88 | 2766/3162 |
| ȃLipid-lowering medication | 85 | 2670/3148 |
| ȃBlood pressure lowering medication | 86 | 2726/3154 |
| ȃGlucose lowering medication (if diagnosed with DM) | 76 | 621/812 |
In case of any missing values, the valid percentage of the total number of non-missing cases (N) are presented.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; LDL-cholesterol, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; DM, diabetes mellitus.
Ten-year and lifetime risk of recurrent events under current treatment
At median 1.1 years after the index event, the median (IQR) 10-year risk of recurrent events with current treatments was 20% (15–27) and lifetime risk 54% (47–63). Especially in younger and middle-aged patients, 10-year risk is relatively low but increases when estimated for lifetime exposure (Figure 2). In contrast, ratios between lifetime and 10-year risk decreased with increasing age, with these ratios reversing in patients aged >80 years.
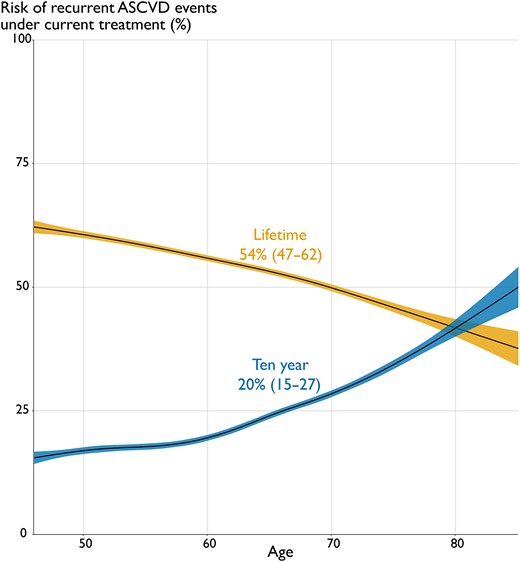
Relation between estimated 10-year and lifetime ASCVD risk with age. Linear regression relations with 95% confidence intervals between age (x-axis) and the SMART-REACH e#stimated 10-year risk (blue) and lifetime risk (yellow) of myocardial infarction, stroke, or cardiovascular mortality under current treatment (y-axis). Abbreviation: ASCVD, atherosclerotic cardiovascular disease.
Potential benefit with additional therapy
If ASCVD therapy changed from current levels to theoretical, maximal levels, median (IQR) 10-year risk decreased from 20% (15–27) to 6% (4–8) and lifetime risk from 54% (47–63) to 20% (15–27). Figure 3 shows density plots for 10-year (a) and lifetime risk (b), demonstrating the potential of maximal treatment by the shift from peak density to lower risk, where the current lowest risk estimates equal the highest risk estimates. The proportion of patients with 10-year risk of recurrent events <10% increased from 2% to 85%. This risk reduction resulted in a lifetime benefit of an added median 7.3 (IQR 5.4–10.4) event-free years that can be read in Figure 4 as higher 50% probability of event-free survival. The number of added event-free years showed a wide distribution, ranging from 0 to 26 years, with greater estimated overall treatment benefit in younger patients and 25% of patients gaining over 10 years free of myocardial infarction or stroke.
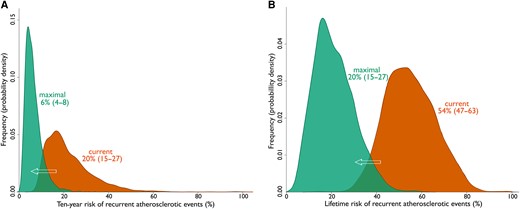
Potential benefit of maximal vs. current ASCVD treatment. Density plots showing the shift in distribution of SMART-REACH estimates for 10-year risk (A) and lifetime risk (B) of recurrent events, when estimated with ‘current’ (orange) and ‘maximal’ (green) treatment scenarios, at median (IQR) 1.1 (1.0–1.8) years after index event. The y-axis is labelled density instead of frequency, with an area under each curve equal to 1. Abbreviation: ASCVD, atherosclerotic cardiovascular disease; IQR, interquartile range.
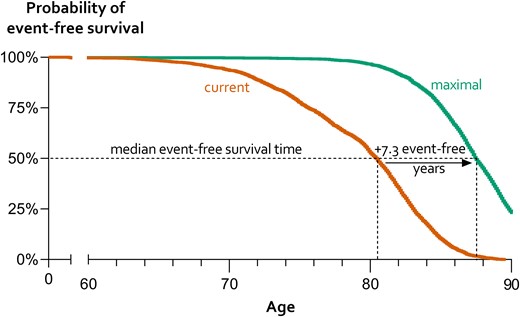
Estimates of the gain in survival time free of myocardial infarction or stroke with maximal vs. current ASCVD treatment. Median event-free survival time can be read at a 50% probability of event-free survival. The green curve presenting a theoretical scenario of maximal treatment shows higher (7.3 years) estimated event-free life expectancy (x-axis) compared with current treatment strategy (orange). Abbreviation: ASCVD, atherosclerotic cardiovascular disease.
Sensitivity analysis
We confirmed the robustness of our results with different methods for dealing with missing data. Ten-year and lifetime risk estimates were, respectively, 26% (18–35) and 55% (47–63) in complete cases [n = 416, mean (SD) age 65 (9) years and 80% men] and 19% (15–25) and 53% (47–60) with imputation of mean/median (see Supplementary material online, Table S6). Stratified analysis for trial (RESPONSE 1, RESPONSE 2, and OPTICARE, n = 1907) vs. non-trial patients (EUROASPIRE and HELIUS, n = 1323) showed lower median (IQR) 10-year risk [18% (14–23) vs. 24% (18–32), P < 0.001], lower lifetime risk [52% (45–58) vs. 60% (50–68), P < 0.001], and less event-free years to be gained from treatment in trial patients [6.7 (5.0–9.6) years vs. 8.3 (6.1–11.4) years, P < 0.001]. Finally, excluding n = 57 (1.8% of sample) patients who reported an ASCVD event at very young age (<35 years) did not affect our overall results significantly [median (IQR) 10-year risk, lifetime risk, and potential event-free years of, respectively, 22% (15–27), 53% (45–62), and 7.0 (5.0–10.5) years].
Discussion
Amongst 3230 patients with established ASCVD, current suboptimal risk factor management results in 10-year risk of 20% (median) of recurrent cardiovascular events. If estimated as lifetime risk, this risk is on average two to three times higher. We found that standard guideline-based preventive therapy is poorly implemented, even in our trial populations, with unhealthy lifestyles in 91% and 82% having ≥1 drug-modifiable risk factor not on target. Finally, using the SMART-REACH model, we found that lifetime risk can potentially be reduced by >50%, which can be communicated to patients in an easy-to-understand way as gaining a median (IQR) of 7.3 (5.4–10.4) years without recurrent ASCVD events. In summary, a strict guideline-based therapy is highly effective not only to improve life expectancy but also to significantly increase the number of total event-free life years. These results highlight the unexploited potential of current (conventional) treatment options, both for lifestyle and pharmacological therapies, in patients with established ASCVD.
Risk of recurrent cardiovascular events and death is conventionally calculated for the short term, typically 10 years. These short-term risk estimates are widely recommended and used in clinical practice for both primary (SCORE2) and secondary prevention (SMART and SMART-REACH). Currently, lifetime risk estimation is facilitated through the availability of long-term follow-up and advanced statistic methodology, which may provide a more complete estimate of future ASCVD disease burden.28,39 We therefore included lifetime risk in addition to 10-year risk, which is also recommended by the latest ESC guidelines on secondary prevention.24 We showed that especially in younger and middle-aged patients, a 10-year risk is usually relatively low but increases when estimated for lifetime exposure. In contrast, ratios between lifetime and 10-year risk may be reversed in the elderly, when accounting for remaining life expectancy and competing events. The use of lifetime risk rather than 10-year risk for individual decision-making is potentially advantageous when specifically aiming not only to select younger patients for treatment intensification but also to avoid overtreatment in older adults.
We found that more than half of the lifetime risk of recurrent cardiovascular events was modifiable with maximal therapy, i.e. both lifestyle and medical optimization. This potential may be even greater with stricter risk factor targets as recommended by the latest ESC prevention guideline, for example, LDL-c <1.4 mmol/L, which was only met by 8% in our study.24 The remaining risk is explained by ‘non-modifiable’ factors that may not be targetable with current approaches, which includes age, gender, and (poly-)genetic predisposition.40 The modifiable risk was driven by the high prevalence of both unhealthy lifestyles (91%) and insufficient use of standard preventive medication (30%), which clearly outlines the unused potential of basic preventive measures. Of note, these poor results were found in a population mainly extracted from trials in secondary prevention and cardiac rehabilitation in the Netherlands, which is classified as a low-risk country. In these trials, the intervention arm received intensified preventive efforts, including medication titration, and communication strategies such as motivational interviewing and health education. Considering that tailored multidisciplinary secondary prevention and rehabilitation programmes have been shown to be effective, in a context where poor preventive outcomes have been consistent for decades, strategies aimed at improving and implementing such programmes warrant priority.41 Furthermore, as results from the current study are dependent on the absolute level of ASCVD incidence, the conclusions from the current study mostly apply to Europe’s low-risk region. The potential of further preventive efforts may be even greater in higher-risk regions.
For high-risk patients not achieving treatment targets with maximum conventional preventive efforts, or who remain at high residual risk despite achieving mandated treatment targets, tailored use of novel drug therapies to reach stricter targets should be considered, in line with current guidelines.24 Traditionally, the intensity of treatment is recommended to increase with increasing ASCVD risk, but the potential benefit and side effects should be taken into account. Importantly, in our analysis, we calculated risk of recurrent event under ‘ideal’ circumstances, i.e. all risk factors at currently mandated target levels. As our findings show, current levels of risk factor control are far from optimal, and the use of the SMART-REACH model does not address all underlying causes. Our findings therefore serve to highlight the maximum risk reduction that can theoretically be achieved with current ASCVD therapy, even in an above-average healthy post-ACS population.
New risk models estimate and employ individual ‘treatment benefit’, which may assist healthcare professionals in selecting patients for treatment intensification based on the ability to prolong event-free life instead of absolute risk levels.42 A benefit-based treatment strategy to reduce ASCVD risk in high-risk patients has been shown to be more cost-effective than a risk-based strategy.43,44 Additionally, limited yet promising findings indicate that communicating personalized estimates of risk and treatment benefit may increase patients’ knowledge, risk perception, and willingness for therapy and lead to improved decisional certainty in medication use.45,46 This is an important message not only for all individual patients being affected with ASCVD but also for the communities managing the socioeconomic consequences of these conditions. Although younger patients have a longer life expectancy in which they can benefit from treatment, the threshold for intensifying treatment is lower in younger adults. Furthermore, our findings indicate that estimated treatment benefit decreases with increasing age, especially in the elderly. Therefore, a ‘definitive’ threshold for total ASCVD risk should not automatically lead to treatment intensification. However, for patients across the full range of ASCVD risk, the decision to initiate or intensify treatments remains a matter of shared decision-making and may be assisted by communicating treatment benefits.46 Prior to the decision to intensify treatment, the generally poor results of current secondary prevention efforts should be evaluated.
Few studies have reported on distributions and modifiable components of risk in patients with a history of coronary artery disease and only for short-term (maximal 10-year) risk and without calculating the potential benefits of recently introduced treatments.47–49 The median (IQR) 10-year risk in our cohort [20% (15–27)] is consistent with earlier studies in patients with a history of coronary artery disease [14% (10–20) and 16% (10–27)].48,49 However, we found greater potential treatment benefit expressed as the proportion of patients with a residual risk of <10% compared with other studies (85% in our study vs. 52% and 50%).48,49 This could potentially be explained by the underuse of standard medication in the HELIUS population, leaving more room for improvement (see Supplementary material online, Table S5) and the intensive treatment combination applied in our estimates, including the beneficial effects of novel medications, which represents the theoretical maximum ‘best-case’ modifiable risk reduction for our study population.
There are several strengths to our study. First, we ensured external validity by analysing a large, well-documented diverse sample of contemporary patients, representing trial and non-trial patients of multi-ethnic origins, with individual-level data on cardiovascular risk factors. In most cardiovascular studies, racial and ethnic minorities are underrepresented, possibly due to practical challenges in including these groups. The inclusion of the HELIUS multi-ethnic cohort therefore resulted in improved representation of patients with non-European origin (24%) and middle and lower educational levels (77%) in our study. The proportion of women (24%) is comparable with average ASCVD trial patient populations. Second, the definitions and methods of measurements were comparable across all studies, making them suitable for pooling. Third, the SMART-REACH risk score used to estimate risk or recurrent ASCVD events has been developed and externally validated in large European and North American cardiovascular cohorts, and its use is recommended as a first choice option for secondary prevention by the latest ESC prevention guidelines.24,28 Previous studies have shown the validity of lifetime predictions for up to 17 years.50 Finally, our main findings were confirmed by the minimal impact of imputation methods on risk estimates in our extensive sensitivity analysis.
Some aspects of our study warrant consideration. First, our study population was extracted from studies with different designs—experimental and observational—using different inclusion and exclusion criteria (see Supplementary material online, Tables S1–S3). This heterogeneity improved our external validity, but we acknowledge some inherent limitations. For example, 59% of patients in our sample participated in (randomized) clinical trials and therefore were subject to selection, selective loss to follow-up, and stricter risk factor control than the average post-ACS patient. Furthermore, patients >80 years were underrepresented, and patients with severe heart failure were excluded in all three randomized controlled trials, which influences the SMART-REACH risk estimates. Finally, all data came from the Netherlands, which is classified as a low-risk country, and half of these trial patients were subject to an intervention including behavioural support and strict risk factor control, leaving fewer possibilities for improvement. On the other hand, the EUROASPIRE and HELIUS studies have less exclusion criteria and are more representative of usual care, as expected; this was associated with a higher prevalence of comorbidities and lower drug use (see Supplementary material online, Table S5). Our stratified analysis confirmed the consequently lower risk and smaller potential risk reduction in trial patients vs. non-trial patients.
Second, the index event of HELIUS participants was retrospectively self-reported and on average longer ago [median (IQR) 7 (3–13) years] and therefore potentially subject to recall bias, with the additional risk that we could have included a small number of patients without an actual ASCVD event. However, sensitivity analysis taking this into account by excluding patients with events at very young age did not change our overall findings. The advantage of including the HELIUS population was an improved generalizability of our results for sex, education level, and ethnicity.
Third, atrial fibrillation and heart failure was not assessed in all studies, resulting in large portions of missing data. This was partially addressed by a robust method for imputation; nevertheless, this may have led to an underestimation of standard errors and, thus, overestimation of the level of certainty from our risk estimates.
Finally, we estimated lifetime treatment benefit of a theoretical ‘maximal’ ASCVD treatment combination, which has limitations. For example, risk factors are considered to remain constant the rest of a patient’s life, and treatment benefit is only estimated for a limited set of cardiovascular outcomes. Also, estimates of treatment effect were limited to mean results obtained from large studies with high methodological quality (Supplementary material online, 7), ignoring possible individual variation and the beneficial effects of, e.g. cardiac rehabilitation, behavioural therapy, and weight loss, and increasing physical activity. We addressed this limitation by estimating the effects of meeting risk factor targets, whether achieved with lifestyle changes or medication. For drugs, we assumed prolonged use of dual anti-platelet therapy and use of colchicine by all patients, and a combination of SGLT2i and GLP-1a in diabetics. While these new drugs are effective in reducing ASCVD risk in selected, high-risk patients, they are considered neither appropriate, safe, nor cost-effective in routine practice.24 In addition, it is clinically implausible to assume 100% compliance and toleration of all drugs that might lower ASCVD risk by all patients. As a result, while overall findings meant to highlight the untapped potential of preventive efforts, our present findings may slightly underestimate the potential gain with maximal risk factor control for non-trial patients, or patients in moderate- to high-risk countries, but possibly overestimate the achievable gain in practice.
Conclusions
Amongst 3230 patients with established ASCVD, suboptimal basic preventive therapy results in an avoidable high lifetime risk of recurrent cardiovascular events. Having all risk factors within optimal guideline-recommended target ranges could theoretically result in gaining median (IQR) 7.3 (5.4–10.4) years free from recurrent acute myocardial infarction or stroke. These high levels of modifiable risk highlight the unexploited potential of lifestyle optimization and current drug therapy, prior to the selective use of novel drugs, to prolong cardiovascular event-free life.
Author contributions
Authorship: T.T., M.Sn., W.S.R., R.P., and H.J. contributed to the conception of the work. M.Sn., N.H., M.Su., E.M.C., H.G., J.D., W.S.R., R.P., and H.J. contributed to the acquisition of the data. T.T., M.Sn., H.J., W.S.R., R.P., and S.H. contributed to the analysis and/or interpretation of the data. T.T., M.Sn., and H.J. drafted the manuscript including figures. W.S.R., R.P., E.M.C., F.M., F.V., H.G., J.D., H.J., N.H., and M.Su. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Frank Visseren, (M.D.), Professor, Fabrice (M.A.C. Martens, M.D., PhD), Jaap W. Deckers, (M.D.), Professor, Harald Thune Jørstad, (MD, PhD), Ron J.G. Peters, (M.D.), Professor, Wilma J.M. Scholte op Reime, Professor, Henrike Galenkamp, (PhD), Steven H.J. Hageman, (M.D., PhD), Marjolein Snaterse, (PhD), Tinka van Trier, (M.D.), Eric Moll van Charante, (M.D.), Professor, Madoka Sunamura, (M.D., PhD), and Nienke ter Hoeve, (PhD)
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Acknowledgements
The authors would like to thank all participants and staff who took part in the data collection of the included studies.
Funding
None declared.
Data availability
The data underlying this article were provided by RESPONSE 1, RESPONSE 2, OPTICARE, EUROASPIRE, and HELIUS study groups under licence/by permission. Data will be shared on request to the corresponding author with permission of these parties.
References
Author notes
Conflict of interest: T.T. declares to have no disclosures; M.Sn. declares to have no disclosures; S.H. has no disclosures; N.H. has no disclosures; M.Su. has no disclosures; E.M.C. has no disclosures; H.G. has no disclosures; J.D. has no disclosures; F.M. has no disclosures for this study; F.V. has no disclosures; W.S.R. has no disclosures; R.P. declares has no disclosures; H.J. received unrestricted research grants to institution from Bayer, Amgen, and Sanofi and is a member of the national advisory board Amgen (The Netherlands). These funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.




Comments