-
PDF
- Split View
-
Views
-
Cite
Cite
Xing-chen Meng, Xin-xin Guo, Zhen-yan Peng, Chun Wang, Ran Liu, Acute effects of electronic cigarettes on vascular endothelial function: a systematic review and meta-analysis of randomized controlled trials, European Journal of Preventive Cardiology, Volume 30, Issue 5, April 2023, Pages 425–435, https://doi.org/10.1093/eurjpc/zwac248
Close - Share Icon Share
Abstract
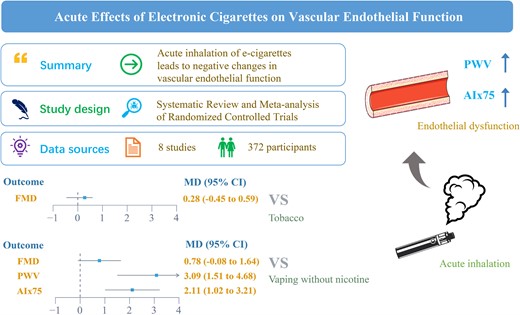
The effects of e-cigarettes on endothelial function remained controversial. The study aimed to investigate the effects of e-cigarettes on vascular endothelial function.
PubMed, Web of Science, Embase, and Cochrane Library were searched up to December 2021. We only included the studies in which the control group included vaping without nicotine and tobacco. Pairwise and network meta-analyses were conducted for flow-mediated dilation (FMD), pulse wave velocity (PWV), and heart rate corrected augmentation index (AIx75). Eight studies involving 372 participants were eligible for this review. Compared with vaping without nicotine, e-cigarettes significantly increase in PWV (mean difference = 3.09; 95% confidential interval: 1.51–4.68, P < 0.001) and AIx75 (mean difference = 2.11; 95% confidential interval: 1.02–3.21, P < 0.001) indicators, but not affect FMD (mean difference = 0.78; 95% confidential interval: −0.08 to 1.64, P = 0.075). But compared with traditional tobacco, e-cigarettes did not affect FMD (mean difference = 0.28, 95% confidential interval: −0.45 to 0.59, P = 0.084). According to surface under the cumulative ranking curve (SUCRA), the e-cigarette ranked first for FMD (SUCRA = 97%), tobacco ranked first for PWV (SUCRA = 75%), and AIx75 (SUCRA = 99%).
In summary, evidence from our pooled analyses indicated that acute inhalation of e-cigarettes leads to negative changes in vascular endothelial function. E-cigarettes cannot be used as an alternative to public health strategies for tobacco control and should not be considered cardiovascular safety products. More future research should be conducted to verify our findings.
Introduction
The electronic cigarette, namely portable battery-powered equipment that delivers nicotine by heating a solution, is generally called e-liquid.1 Solvents contained in e-liquids, usually include propylene glycol and or vegetable glycerine, in addition to concentrated flavours, nicotine, and additives.2 Recently, the number of people using electronic cigarettes (e-cigarettes) has grown substantially in the USA, particularly among the youth.3 Similarly, the use of e-cigarettes among teenagers has also increased rapidly in developed countries such as Finland4 and the UK.5 It is estimated that ∼48.5 million Europeans have used electronic cigarettes at least once, while vaping is prevalent in nearly 7.5 million Europeans currently.6 In addition, the rate of e-cigarette use among women has gradually increased in recent years, with gender differences in tobacco use among adolescents substantially lower than in adults.7
Evidence emerging during the last decades demonstrated a causal link between tobacco smoking and cardiovascular disease, including coronary disease, myocardial infarction, stroke, and heart failure.8 Vascular endothelium plays a significant role in regulating coagulation, vascular tone, and smooth muscle growth.9 Endothelial dysfunction has been related to cardiovascular disease, including atherosclerosis, coronary artery diseases, and hypertension.10 Many studies reported that tobacco smoking causes endothelial dysfunction and blood vessel damage.11 Nevertheless, the effects of e-cigarette smoking on the cardiovascular system remains unknown.12 Studies investigating the relationships between e-cigarette use and markers of endothelial dysfunction risk, such as heart rate corrected augmentation index (AIx75),13,14 flow-mediated dilation (FMD),15,16 and pulse wave velocity (PWV),13,17 yielded inconsistent findings. PWV, AIx75, and FMD are well-established indicators of endothelial dysfunction and arterial stiffness.18 Additionally, e-cigarettes have emerged as an alternative to tobacco cigarettes; however, science is inconclusive as e-cigarettes have not been thoroughly investigated, including their short- and long-term risks and benefits.19 The increased use of e-cigarettes has led to active debate in the public health sphere regarding their use and regulation.20 However, it is unclear whether e-cigarettes can be used as a safe alternative to traditional cigarettes and as an alternative to public health strategies for tobacco control.
The cardiovascular safety of e-cigarettes is an important issue, and endothelial dysfunction is an important factor in cardiovascular disease. Hence, we conducted a systematic literature review and meta-analysis to investigate the effects of e-cigarettes on endothelial function compared with, two control groups, traditional tobacco, and vaping without nicotine.
Methods
The study protocol was registered in PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/, identifier CRD 42022310565). This systematic review was performed in compliance with the PRISMA guidelines.
Literature search strategy
PubMed, Embase, Web of Science, and Cochrane Library databases were used to search electronic literature up to December 2021. Medical Subject Heading (MeSH) phrases used were ‘E-Cigarette Vapor’, ‘Electronic Cigarette Vapor’, ‘Electronic Nicotine Delivery System’, ‘Electronic Cigarettes’, ‘E-Cig’, ‘E-Cigarette’, ‘Electronic Cigarette’, ‘Vaping’, ‘Vape’ in conjunction with ‘brachial artery’, ‘vasodilation’, ‘endothelium’, ‘vascular’, ‘endothelial function’, ‘flow-mediated dilation, ‘Vascular Stiffnesses’, ‘arterial stiffness’, ‘arterial compliance’, ‘arterial distensibility’, ‘arterial stiffen’, and ‘PWV’. If the references included in the literature met the requisite criteria, further screening was conducted to identify potentially related studies.
Inclusion and exclusion criteria
The meta-analysis included studies limited to randomized controlled trials which compared e-cigarette vapour with nicotine to vaping without nicotine or tobacco control group. The primary outcome was vascular function based on FMD, PWV, or AIx75. The populations in the included studies were classified into healthy smokers and healthy non-smokers. Studies that did not meet the above inclusion criteria were excluded. Animal experiments, case reports, systematic reviews, meta-analyses, editorials, and studies with insufficient information were also excluded.
Data extraction
All eligible articles and data were extracted independently by two researchers (X.C.M. and X.X.G.). Means and standard deviations (SDs) associated with baseline and post-intervention outcomes were included in the meta-analysis. The mean difference (MD) was the effect size. MDs and SDs were determined using the following formulae: MD = Mfinal − Mbaseline, , and . We assumed R = 0.5, according to Higgins et al.21 In the absence of ready access to exhaustive statistical data, the information was obtained from images using the software Engauge Digitizer (version 10.8; http://markummitchell.github.io/engauge-digitizer/). Where we were unable to extract data from trial publications, we planned to contact the author(s), seeking clarification or data as required. In the event of no answer, the study was excluded from the meta-analysis. The extracted information included study design, baseline characteristics of study populations, type of control and intervention, and outcome measures.
Data analysis
The MD with a 95% confidence interval (CI) was used for each outcome. Heterogeneity was assessed with I2 and χ2 statistics; either I2 > 50% or P-value of χ2 test <0.10 was considered as statistically significant heterogeneity. A random-effects model was used to acquire the pooled MD and associated 95% CI. We performed subgroup analyses using pre-specified covariates, including control type and population type. Finally, the restricted maximum likelihood method was used for meta-regression to analyse the relationship between e-cigarette inhalation and endothelial function. Results were considered statistically significant with two-sided P-values ≤0.05. Assessment of the risk of bias for each study was determined using the Cochrane Risk of Bias tool. Sensitivity analyses were used to assess the robustness of the findings of this meta-analysis and identify potential outlier studies. A funnel plot was presented visually to assess publication bias. Because of the limited number of included studies (n < 10), the interpretations of funnel plot symmetry are limited and thus Egger’s test was further used to assess publication bias. In addition, for each index (FMD, PWV, and AIx75), random-effects network meta-analysis was conducted to determine the pooled effect of each intervention relative to every other intervention. Network meta-analysis is an extension of pairwise meta-analysis, which enables simultaneous comparison of multiple interventions while maintaining the internal randomization of each trial to facilitate the estimation of all possible pairwise effects and obtain a meaningful ranking. Summary MD and their corresponding 95% CI were presented in a league table for each index (FMD, PWV, and AIx75). The relative ranking of different interventions was estimated by the distribution of the ranking probabilities and the surface under the cumulative ranking curve (SUCRA). Additionally, the SUCRA values of the three indices (FMD, PWV, and AIx75) were averaged to obtain a summary SUCRA score for each intervention and rank the different interventions according to their overall effect on endothelial function. Further, a common network-specific heterogeneity parameter was assumed. Calculations were fitted in a frequentist framework.22 All statistical analyses were performed using Stata version 15.1 (Stata Corporation, TX, USA).
Results
Description of selected trials
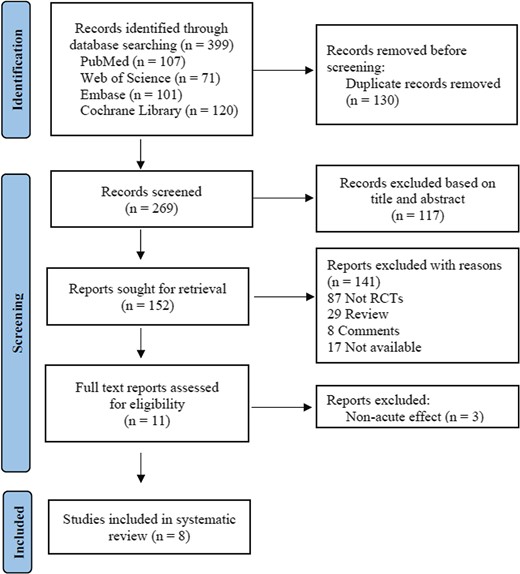
The flow chart of the sifting process is shown in Figure 1. A total of 399 papers were identified from four electronic databases, but 247 were excluded based on the title and abstract, including 130 duplications. A total of 152 full-text papers were retrieved for further review, and 141 studies were excluded for the following reasons: 87 were not RCTs; 29 were review articles; 8 were commentaries, and 17 were not available as full-text articles. Finally, three studies were excluded for non-acute effect, and eight studies with adequate data were selected in this meta-analysis.
Study characteristics
The main characteristics of the included studies are summarized in Table 1. The studies were published between 2016 and 2020. Two studies involved a parallel design, while six had a cross-over design. The sample sizes ranged from 30 to 80 for a total of 372. The average age of participants ranged between 22.9 and 48 years. The proportion of men varied from 33.3 to 72%. Nicotine concentrations ranged from 3 to 24 mg/mL. The included studies were assessed for <1 h.
| First author . | Year . | Control group . | Population . | n (control/intervention) . | Age . | Male (%) . | Nicotine (mg/mL) . | Timepoint of assessment . | Design . | Wash period . |
|---|---|---|---|---|---|---|---|---|---|---|
| Antoniewicz et al. | 2019 | Vaping without nicotine | Healthy smokers | (15/15) | 26.0 ± 3.0 | 40 | 19 | 10 min | RCD | 1 week |
| Franzen et al. | 2018 | NO + | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h |
| TC | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h | ||
| Chaumont et al. | 2018 | NO + | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week |
| Sham-vaping | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week | ||
| Haptonstall et al. | 2020 | Vaping without nicotine | Healthy non-smokers (HNS) + | (39/41) | 26.3 ± 5.2 | 46.8 | NA | 5 min | RCD | 4 weeks |
| Vaping without nicotine | HS | (23/22) | 27.4 ± 5.45 | 73.5 | NA | 5 min | RCD | 4 weeks | ||
| Biondi-Zoccai et al. | 2019 | Heat-not-burn cigarette + | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week |
| TC | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week | ||
| Cossio et al. | 2020 | Vaping without nicotine | Healthy smokers | (16/16) | 24 ± 3 | 56.3 | 5.4 | Immediately | RCT | NA |
| Carnevale et al. | 2016 | Tobacco | HS + | (20/20) | 28.7 ± 5.8 | 45 | 16 | 30 min | RCD | 1 week |
| Tobacco | Healthy non-smokers | (20/20) | 27.3 ± 4.7 | 50 | 16 | 30 min | RCD | 1 week | ||
| Ikonomidis et al. | 2018 | Vaping without nicotine | Healthy smokers | (35/35) | 48 ± 5 | 44 | 12 | 7 min | RCT | NA |
| First author . | Year . | Control group . | Population . | n (control/intervention) . | Age . | Male (%) . | Nicotine (mg/mL) . | Timepoint of assessment . | Design . | Wash period . |
|---|---|---|---|---|---|---|---|---|---|---|
| Antoniewicz et al. | 2019 | Vaping without nicotine | Healthy smokers | (15/15) | 26.0 ± 3.0 | 40 | 19 | 10 min | RCD | 1 week |
| Franzen et al. | 2018 | NO + | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h |
| TC | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h | ||
| Chaumont et al. | 2018 | NO + | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week |
| Sham-vaping | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week | ||
| Haptonstall et al. | 2020 | Vaping without nicotine | Healthy non-smokers (HNS) + | (39/41) | 26.3 ± 5.2 | 46.8 | NA | 5 min | RCD | 4 weeks |
| Vaping without nicotine | HS | (23/22) | 27.4 ± 5.45 | 73.5 | NA | 5 min | RCD | 4 weeks | ||
| Biondi-Zoccai et al. | 2019 | Heat-not-burn cigarette + | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week |
| TC | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week | ||
| Cossio et al. | 2020 | Vaping without nicotine | Healthy smokers | (16/16) | 24 ± 3 | 56.3 | 5.4 | Immediately | RCT | NA |
| Carnevale et al. | 2016 | Tobacco | HS + | (20/20) | 28.7 ± 5.8 | 45 | 16 | 30 min | RCD | 1 week |
| Tobacco | Healthy non-smokers | (20/20) | 27.3 ± 4.7 | 50 | 16 | 30 min | RCD | 1 week | ||
| Ikonomidis et al. | 2018 | Vaping without nicotine | Healthy smokers | (35/35) | 48 ± 5 | 44 | 12 | 7 min | RCT | NA |
HS, healthy EC vapers; NO, vaping without nicotine; RCD, randomized cross-over design; RCT, randomized controlled trial; TC, tobacco.
| First author . | Year . | Control group . | Population . | n (control/intervention) . | Age . | Male (%) . | Nicotine (mg/mL) . | Timepoint of assessment . | Design . | Wash period . |
|---|---|---|---|---|---|---|---|---|---|---|
| Antoniewicz et al. | 2019 | Vaping without nicotine | Healthy smokers | (15/15) | 26.0 ± 3.0 | 40 | 19 | 10 min | RCD | 1 week |
| Franzen et al. | 2018 | NO + | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h |
| TC | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h | ||
| Chaumont et al. | 2018 | NO + | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week |
| Sham-vaping | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week | ||
| Haptonstall et al. | 2020 | Vaping without nicotine | Healthy non-smokers (HNS) + | (39/41) | 26.3 ± 5.2 | 46.8 | NA | 5 min | RCD | 4 weeks |
| Vaping without nicotine | HS | (23/22) | 27.4 ± 5.45 | 73.5 | NA | 5 min | RCD | 4 weeks | ||
| Biondi-Zoccai et al. | 2019 | Heat-not-burn cigarette + | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week |
| TC | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week | ||
| Cossio et al. | 2020 | Vaping without nicotine | Healthy smokers | (16/16) | 24 ± 3 | 56.3 | 5.4 | Immediately | RCT | NA |
| Carnevale et al. | 2016 | Tobacco | HS + | (20/20) | 28.7 ± 5.8 | 45 | 16 | 30 min | RCD | 1 week |
| Tobacco | Healthy non-smokers | (20/20) | 27.3 ± 4.7 | 50 | 16 | 30 min | RCD | 1 week | ||
| Ikonomidis et al. | 2018 | Vaping without nicotine | Healthy smokers | (35/35) | 48 ± 5 | 44 | 12 | 7 min | RCT | NA |
| First author . | Year . | Control group . | Population . | n (control/intervention) . | Age . | Male (%) . | Nicotine (mg/mL) . | Timepoint of assessment . | Design . | Wash period . |
|---|---|---|---|---|---|---|---|---|---|---|
| Antoniewicz et al. | 2019 | Vaping without nicotine | Healthy smokers | (15/15) | 26.0 ± 3.0 | 40 | 19 | 10 min | RCD | 1 week |
| Franzen et al. | 2018 | NO + | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h |
| TC | Healthy smokers | (15/15) | 22.9 ± 3.5 | 33.3 | 24 | 15 min | RCD | 24 h | ||
| Chaumont et al. | 2018 | NO + | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week |
| Sham-vaping | Healthy smokers | (25/25) | 23.0 ± 0.4 | 72 | 3 | 10 min | RCD | 1 week | ||
| Haptonstall et al. | 2020 | Vaping without nicotine | Healthy non-smokers (HNS) + | (39/41) | 26.3 ± 5.2 | 46.8 | NA | 5 min | RCD | 4 weeks |
| Vaping without nicotine | HS | (23/22) | 27.4 ± 5.45 | 73.5 | NA | 5 min | RCD | 4 weeks | ||
| Biondi-Zoccai et al. | 2019 | Heat-not-burn cigarette + | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week |
| TC | Healthy smokers | (20/20) | 35 ± 13 | 30 | NA | Immediately | RCD | 1 week | ||
| Cossio et al. | 2020 | Vaping without nicotine | Healthy smokers | (16/16) | 24 ± 3 | 56.3 | 5.4 | Immediately | RCT | NA |
| Carnevale et al. | 2016 | Tobacco | HS + | (20/20) | 28.7 ± 5.8 | 45 | 16 | 30 min | RCD | 1 week |
| Tobacco | Healthy non-smokers | (20/20) | 27.3 ± 4.7 | 50 | 16 | 30 min | RCD | 1 week | ||
| Ikonomidis et al. | 2018 | Vaping without nicotine | Healthy smokers | (35/35) | 48 ± 5 | 44 | 12 | 7 min | RCT | NA |
HS, healthy EC vapers; NO, vaping without nicotine; RCD, randomized cross-over design; RCT, randomized controlled trial; TC, tobacco.
Risk of bias
The evaluation of bias risk is presented in Table 2. All studies were rated as low risk of bias. In addition, the risk of other bias in most of the included studies was unclear because of insufficient information.
| Reference . | year . | Risk of bias assessment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation . | Allocation concealment . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Selective reporting . | Other bias . | ||
| Antoniewicz et al. | 2019 | Low | Low | Low | Low | Low | Low | Unclear |
| Franzen et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Chaumont et al. | 2018 | Low | Low | Low | Low | Low | Low | Unclear |
| Haptonstall et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Biondi-Zoccai et al. | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Cossio et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Carnevale et al. | 2016 | Low | Low | Low | Low | Low | Low | Low |
| Ikonomidis et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Reference . | year . | Risk of bias assessment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation . | Allocation concealment . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Selective reporting . | Other bias . | ||
| Antoniewicz et al. | 2019 | Low | Low | Low | Low | Low | Low | Unclear |
| Franzen et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Chaumont et al. | 2018 | Low | Low | Low | Low | Low | Low | Unclear |
| Haptonstall et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Biondi-Zoccai et al. | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Cossio et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Carnevale et al. | 2016 | Low | Low | Low | Low | Low | Low | Low |
| Ikonomidis et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
High, high risk of bias; Low, low risk of bias; Unclear, unclear risk of bias.
| Reference . | year . | Risk of bias assessment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation . | Allocation concealment . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Selective reporting . | Other bias . | ||
| Antoniewicz et al. | 2019 | Low | Low | Low | Low | Low | Low | Unclear |
| Franzen et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Chaumont et al. | 2018 | Low | Low | Low | Low | Low | Low | Unclear |
| Haptonstall et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Biondi-Zoccai et al. | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Cossio et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Carnevale et al. | 2016 | Low | Low | Low | Low | Low | Low | Low |
| Ikonomidis et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Reference . | year . | Risk of bias assessment . | ||||||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation . | Allocation concealment . | Blinding of participants and personnel . | Blinding of outcome assessment . | Incomplete outcome data . | Selective reporting . | Other bias . | ||
| Antoniewicz et al. | 2019 | Low | Low | Low | Low | Low | Low | Unclear |
| Franzen et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
| Chaumont et al. | 2018 | Low | Low | Low | Low | Low | Low | Unclear |
| Haptonstall et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Biondi-Zoccai et al. | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Cossio et al. | 2020 | Low | Low | Low | Low | Low | Low | Unclear |
| Carnevale et al. | 2016 | Low | Low | Low | Low | Low | Low | Low |
| Ikonomidis et al. | 2018 | Low | Low | Low | Low | Low | Low | Low |
High, high risk of bias; Low, low risk of bias; Unclear, unclear risk of bias.
Pairwise meta-analysis
Comparison of acute effects of e-cigarettes with tobacco on flow-mediated dilation
Four included studies estimated the acute effects of e-cigarettes on FMD. No single study reported that acute intake of e-cigarettes significantly increased FMD. Even, when four studies were analysed in the meta-analysis (n = 160), when compared with tobacco, consuming e-cigarettes did not affect FMD (MD = 0.28, 95% CI: −0.45 to 0.59, P = 0.084; Figure 2). We found no significant heterogeneity between these studies (I2 = 0.0%, P = 0.677).
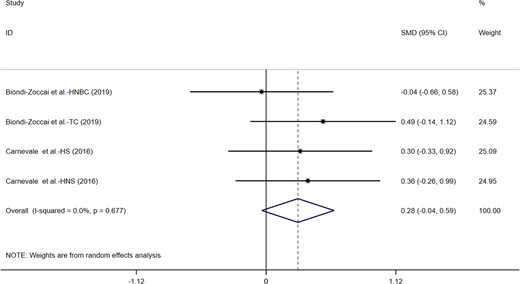
Acute effects of electronic cigarettes on flow-mediated dilation, comparison with traditional tobacco.
Comparison of acute effects of e-cigarettes with vaping without nicotine on flow-mediated dilation
Three studies explored the acute effects of electronic cigarettes on PMD. Only one trial reported that e-cigarettes significantly increased FMD. However, when the data included in the experiment were subjected to meta-analysis (n = 157), e-cigarette consumption did not significantly increase FMD (MD = 0.78; 95% CI: −0.08 to 1.64, P = 0.075; Figure 3). Significant heterogeneity was found between studies (I2 = 84.1%, P = 0.002).
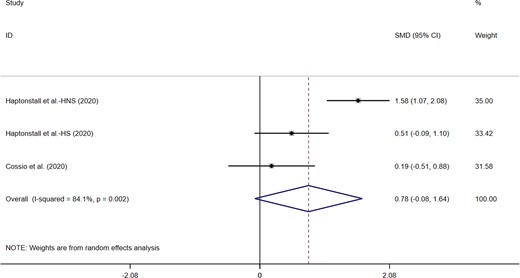
Acute effects of electronic cigarettes on flow-mediated dilation, comparison with vaping without nicotine.
Comparison of acute effects of e-cigarettes with vaping without nicotine on pulse wave velocity
Five studies appraised the effects of electronic cigarettes on PWV. Only one trial found that the e-cigarette use did not affect PWV. Further, pooling of the data from included trials (n = 230) in this study showed that e-cigarettes use significantly increased PWV (MD = 3.09; 95% CI: 1.51–4.68, P < 0.001; Figure 4). We detected significant heterogeneity (I2 = 93.7%, P < 0.001).
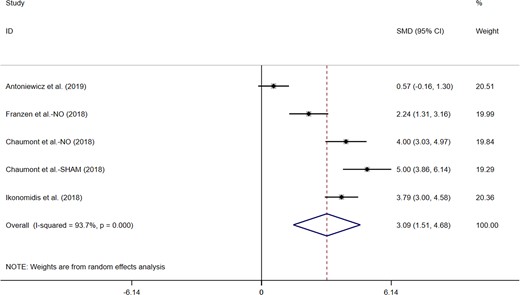
Acute effects of electronic cigarettes on pulse wave velocity, comparison with vaping without nicotine.
Comparison of acute effects of e-cigarettes with vaping without nicotine on heart rate corrected augmentation index
Five studies explored the acute effects of e-cigarettes on AIx75. Four trials reported that e-cigarettes significantly decreased AIx75. The meta-analysis of data in the included trials (n = 230) demonstrated that vaping has significantly enhanced AIx75 (MD = 2.11; 95% CI: 1.02–3.21, P < 0.001; Figure 5). Significant heterogeneity between studies was detected (I2 = 90.5%, P < 0.001).

Acute effects of electronic cigarettes on heart rate corrected augmentation index, comparison with vaping without nicotine.
Network meta-analysis
Figure 6 shows the network diagrams for FMD (Figure 6A), PWV (Figure 6B), and AIx75 (Figure 6C). Based on the contribution matrix, the outcome is attributed to indirect comparison.
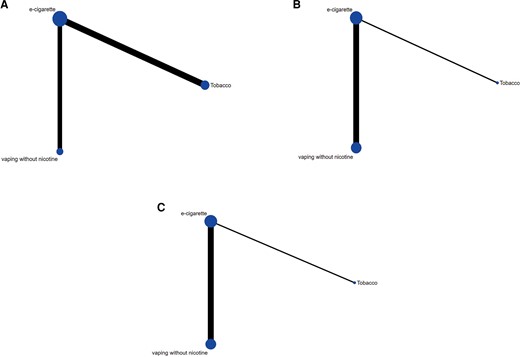
Network diagrams for flow-mediated dilation (A), pulse wave velocity (B), and heart rate corrected augmentation index (C). The size of the nodes is proportional to the total number of participants allocated to each intervention approach and the thickness of the lines is proportional to the number of studies evaluating each direct comparison.
The effect size estimates summarized in Supplementary material online, Appendix S3.1.1 compare the relative effects of each intervention on FMD. The e-cigarette was found to be more effective in increasing FMD than vaping without nicotine (MD = 0.75; 95% CI: 0.22–1.28). However, no significant difference was found between e-cigarettes and tobacco (MD = −0.83; 95% CI: −1.85 to 0.18). The effect size estimates are summarized in Supplementary material online, Appendix S3.2.1, comparing the relative effect of each of the interventions on PWV. The e-cigarette enhanced the PWV compared with vaping without nicotine (MD = 0.51; 95% CI: 0.27–0.75). Likewise, no significant difference was found between e-cigarettes and tobacco (MD = 0.01; 95% CI: −0.48 to 0.51). The effect size estimates are summarized in Supplementary material online, Appendix S3.3.1, comparing the relative effects of every intervention on AIx75. E-cigarette usage also increased AIx75 more effectively compared with vaping without nicotine (MD = 6.45; 95% CI: 5.69–7.21). Further, the e-cigarette reduced the AIx75 compared with tobacco (MD = −1.81; 95% CI: −3.44 to −0.17).
The SUCRA values for each index (FMD, PWV, and AIx75) and the summary SUCRA scores for each intervention are summarized in Supplementary material online, AppendicesS3.1.2, S3.2.2, and S3.3.2. For FMD, the e-cigarette scored the highest SUCRA value (97%), followed by vaping without nicotine (28%) and tobacco use (25%). For PWV, tobacco use yielded the highest SUCRA value (75%), followed by e-cigarette (74%) and vaping without nicotine (2%). For AIx75, tobacco use yielded the highest SUCRA score (99%), followed by e-cigarette use (51%) and vaping without nicotine (0%).
Subgroup analysis and meta-regression
Control groups that were not exposed to nicotine were subjected to subgroup analyses. The effects of e-cigarette intake on FMD, PWV, and AIx75 in subgroups reported by eligible studies are presented in Table 1. For FMD, the results suggested heterogeneity of the trial populations, and a significantly greater increase in FMD was observed in studies involving healthy non-smokers. For PWV and AIx75, sources of significant heterogeneity were not identified in the subgroup analysis, given the relatively few studies associated with specific e-cigarettes. We found that the population may be the source of heterogeneity for PWV (standard error = 0.183, t = −4.13, P = 0.026), following meta-regression to explore potential sources of heterogeneity for FMD, PWV, and AIx75.
Sensitivity analysis
A sensitivity analysis was conducted to assess the robustness of our results. The overall results revealed that the removal of any of the trials from our study did not significantly change the acute effect of vaping on FMD, PWV, and AIx75, which suggested that our conclusions are robust and reliable.
Publication bias
Egger’s test was used to evaluate the publication bias. Because of the limited number of included studies of FMD, PWV, and AIx75, Egger’s test was used to assess the publication bias. The Egger’s test of control groups that did not contain nicotine revealed no publication bias in terms of FMD (intercept = −14.97; standard error = 4.84; 95% CI: −76.46 to 46.51; t = −3.09; two-tailed P-value 0.199), PWV (intercept = 16.71; standard error = 9.21; 95% CI: −12.61 to 46.04; t = 1.81; two-tailed P-value 0.167), and AIx75 (intercept = 8.60; standard error = 4.75; 95% CI: −6.52 to 23.73; t = 1.81; two-tailed P-value 0.168). In case of control groups exposed to tobacco, the Egger’s test revealed no publication bias associated with FMD (intercept = 103.44; standard error = 25.85; 95% CI: −7.78 to 214.66; t = 4.00; two-tailed P-value 0.057).
Discussion
Although tobacco, as a traditional cardiovascular risk factor, has been recognized and many interventions have also been carried out, the cardiovascular hazards of e-cigarette use have yet to be reported comprehensively. In addition, e-cigarettes were used as an alternative to traditional tobacco in some studies.23 The trial conducted by Franzen et al.14 showed that e-cigarettes did not significantly increase arterial stiffness compared with conventional cigarettes. This finding indicated that e-cigarettes alleviated the cardiovascular burden of tobacco smoking when they are used as alternatives to tobacco smoking, which warrants further investigation given the high cardiovascular risk associated with e-cigarette usage.24 Skotsimara et al.25 reported that inhalation of e-cigarettes significantly increased the heart rate and blood pressure. Several cross-sectional studies have shown that e-cigarette use is significantly associated with poor cardiovascular health outcomes. A cross-sectional study by Alzahrani et al.24 showed that after adjusting for smoking behaviour, daily e-cigarette users were twice as likely to develop myocardial infarction as those who never used e-cigarettes. Another study by Vindhyal et al.26 also demonstrated that e-cigarette use increased the incidence of myocardial infarction and stroke. This study is consistent with a cross-sectional study conducted by Ndunda and Muutu,27 suggesting that e-cigarette users had a higher incidence of stroke, myocardial infarction, angina, or coronary heart disease than those who did not use e-cigarettes. This further suggests the cardiovascular harm associated with e-cigarettes. As far as we know, this is the first meta-analysis involving a number of randomized controlled trials to investigate the effect of e-cigarette usage on endothelial function based on three indicators, FMD, PWV, and AIx75.
FMD is a non-invasive technique that can be used to quantitatively measure endothelial function28 and is an important physiological stimulus regulating vascular tension and homeostasis.29 FMD is mainly mediated by nitric oxide (NO) and therefore reflects endothelium-dependent vasodilation.30 Previous studies have shown that tobacco is a key factor affecting FMD.31 Changes in FMD are a sign of cerebrovascular disease and it is also a trigger of atherosclerotic heart disease.32 The results of our current meta-analysis, which compared e-cigarettes with vaping without nicotine, revealed that acute inhalation of e-cigarettes does not substantially increase FMD. Further, when e-cigarettes were compared with tobacco, the results suggested that e-cigarettes did not alter FMD significantly. For FMD, subgroup analysis of vaping without nicotine as a control group revealed heterogeneity among the different study populations. Besides, we found a significantly greater decrease in FMD heterogeneity when trials were removed from the study population of healthy non-smokers. This suggests that for healthy smokers, the endothelial function damage caused by e-cigarettes may be obscured by traditional tobacco. When e-cigarette use was compared with vaping without nicotine or tobacco, our results suggested that e-cigarettes did not affect FMD significantly, in our meta-analysis. This shows that FMD may not be a sensitive indicator of the effects of e-cigarettes on endothelial function, especially during short-term exposure. Further, considering the possible effect of e-cigarettes on vascular smooth muscle tension, we analysed two other indicators, PWV and AIx75.
PWV is a widely used and established method for measuring arterial stiffness,33 reflecting the elasticity of arterial endothelium.34 AIx75 is to measure the reflection of arterial pulse wave,35 indicating vascular smooth muscle tension and endothelial function.36 As heart rate may affect arterial stiffness, arterial stiffness is expressed as AIx75 with a correction rate of 75 per minute.37 Elastic fibre loss and increased arterial wall fibrosis are the main physiological characteristics.38 Previous studies have shown that atherosclerosis increases PWV and AIx75, thereby increasing cardiac load, reducing coronary perfusion, and increasing the risk of myocardial ischaemia.39 When comparing e-cigarette use with vaping without nicotine, interestingly our results revealed e-cigarette use leads to an increase in PWV and AIx75 after acute exposure. This suggests that PWV and AIx75 are sensitive indicators of exposure to e-cigarettes, indicating that e-cigarettes increase arterial stiffness, especially in healthy non-smokers.
In network meta-analysis, we ranked three interventions (e-cigarette, tobacco, and vaping without nicotine) according to their effects on endothelial function (FMD, PWV, and AIx75). Among the three interventions, e-cigarettes showed the strongest effect and the highest SUCRA values for FMD. Tobacco use showed the strongest effect and the highest SUCRA values for PWV and AIx75. Besides, when e-cigarette use was compared with vaping without nicotine, stronger effects were observed. Based on the AIx75 indicator, e-cigarette use showed a protective effect compared with tobacco. However, according to the three indicators, the negative effects of e-cigarette and tobacco on endothelial function were obvious. The difference between e-cigarettes and tobacco was not obvious. Compared with tobacco, we cannot conclude that e-cigarettes were beneficial in terms of endothelial function. This is consistent with the results of our pairwise meta-analysis, which further enhances the reliability of our conclusion.
Given the complex composition of e-cigarettes, their adverse effects on endothelial function may be related to the ingredients contained in e-cigarettes, such as heavy metals, organic solvents, or other unknown substances. Previous studies have shown that solvents in e-cigarettes, such as ethylene glycol and vegetable glycerine, can generate carcinogenic carbonyl compounds such as acetaldehyde, formaldehyde, and acrolein when heated.40 These carbonyl compounds exhibit toxicity via formation of adducts with proteins and DNA, causing oxidative stress and inflammation.41 Excessive oxidative stress uncouples endothelial NO synthase to reduce NO bioavailability,42 and impaired NO bioavailability contributes to impaired FMD.43 Further, a recent experimental study showed that platelets exposed to e-cigarette vapour exhibited increased expression of proinflammatory molecules compared with platelets exposed to traditional tobacco extracts.44 Therefore, these aerosols may cause arterial stiffness and ischaemia/reperfusion injury through inflammation and excessive oxidative stress, resulting in endothelial dysfunction.
Previous studies25,45 have also shown that switching from traditional tobacco to e-cigarettes may reduce cardiovascular damage. The negative cardiovascular effects of tobacco smoking,46 such as tachycardia, arteriosclerosis, and endothelial damage, are well established. Although e-cigarettes are less harmful than traditional tobacco, our study confirmed that short-term exposure to e-cigarettes still generates endothelial stress and injury, indicating that it is not an unequivocal alternative to traditional tobacco. Due to the lack of monitoring regarding the composition and concentration of xenobiotics in e-cigarettes in most countries, its long-term toxicity requires increased attention. In addition, the position paper of the European Association of Preventive Cardiology47 indicated that e-cigarettes increased arterial stiffness and have harmful effects on vascular endothelial function, consistent with our findings. In summary, our study suggests that acute exposure to e-cigarettes affects endothelial function, arterial stiffness, and vascular smooth muscle tension, and may increase the risk of cardiovascular diseases such as acute coronary syndrome and atherosclerosis. Finally, the impact of e-cigarettes on other organs should be considered, and the safety and standardized management of electronic smoke requires comprehensive analysis.
This meta-analysis has several advantages. This work is based on the current guidelines,48,49 including the use of multiple sensitivity analyses of the results, and the use of updated assessment tools to investigate the risk of bias. Additionally, only randomized controlled studies were included for enhanced reliability of the results. However, our meta-analysis has some limitations. First, some of the literature was unavailable during full-text search and therefore was not included in the studies. Additionally, currently available studies are mostly based on qualitative analysis and report preliminary findings regarding e-cigarettes, suggesting the need for quantitative data involving nicotine exposure. In the future, a more comprehensive experimental design is recommended to explore the harms of e-cigarettes and traditional cigarettes.
Conclusion
In summary, our pooled analysis of the evidence suggests that acute inhalation of e-cigarettes can lead to negative changes in endothelial function, while PMV and Alx75 are sensitive indicators of endothelial function during short-term e-cigarette exposure. Compared with tobacco, e-cigarettes did not cause significant changes in endothelial function; however, there is no clear evidence that e-cigarettes are superior to conventional tobacco in terms of modulation of endothelial function. Evidence is inadequate to support the current view that e-cigarettes represent an alternative to traditional tobacco and therefore cannot be used in public health strategies for tobacco control. Further quantitative studies investigating e-cigarette nicotine are needed to elucidate the toxicity and harms of e-cigarettes. Given the limitations associated with the current studies, additional well-designed trials are still needed.
Authors’ contributions
X.C.M. and R.L. contributed to the conception and design of the systematic review and meta-analysis. X.X.G. and Z.Y.P. were involved in the acquisition and analysis of the data. C.W. interpreted the results. X.C.M. drafted this manuscript. All authors provided critical revisions of the protocol and approved the submission of the final manuscript.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Acknowledgement
The authors sincerely thank the support from each participant.
Funding
This study was funded by National Natural Science Foundation of China grants (81872579, 82173479).
Data availability
There is no data linked to this manuscript.
References
Author notes
Conflict of interest: None declared.




Comments