-
PDF
- Split View
-
Views
-
Cite
Cite
Iryna Dykun, Raluca Mincu, Stefanie Hendricks, Bastian Balcer, Matthias Totzeck, Tienush Rassaf, Amir A Mahabadi, Efficacy of lipid-lowering therapy beyond statins to prevent cardiovascular events: a meta-analysis, European Journal of Preventive Cardiology, Volume 27, Issue 15, 1 October 2020, Pages 1675–1678, https://doi.org/10.1177/2047487319866992
Close - Share Icon Share
Lipid-lowering therapy is a key cornerstone in secondary prevention in patients with coronary artery disease.1 Current guidelines suggest a low-density lipoprotein (LDL) cholesterol level of 70 mg/dl or a reduction of at least 50% of baseline LDL-cholesterol for patients with cardiovascular disease manifestations.2 With statin therapy alone, a majority of patients miss these thresholds.3,–5 Ezetimibe and pro-protein convertase subtilisin kexin 9 (PCSK9) inhibitors are suggested as an additional treatment option. Large-scale outcome studies have been performed on different agents.6,,,,,–12 We performed a meta-analysis of existing trials, evaluating how lipid-lowering therapy beyond statins impacts cardiovascular outcomes.
This study was performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, in accordance with the meta-analysis of observational studies in epidemiology (MOOSE) recommendations and the Cochrane handbook for systematic reviews of interventions. The study was registered with PROSPERO (CRD 42018099610).
We performed a systematic search using the Pubmed, Cochrane, SCOPUS and Web of Science databases for studies. We also reviewed scientific meetings and clinical trial registers (www.clinicaltrials.gov). Data published until 1 November 2018 were included. The search was specific and sensitive using medical subject headings terms and free text and considered studies published in the English language. Search terms used were ‘ezetimibe’ or ‘evolocumab’ or ‘alirocumab’ or ‘bococizumab’ and ‘statin’ and ‘cardiovascular events’. Only randomised controlled trials comparing ezetimibe or PCSK-9 inhibitors in addition to statin therapy versus statin therapy alone were included. At least one clinical outcome of interest within a follow-up of more than 6 months had to be reported. The primary endpoint of this meta-analysis was defined as a combination of cardiovascular death, non-fatal myocardial infarction, non-fatal ischaemic stroke and urgent coronary revascularisation. Data are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity was assessed using the I2 statistic. All statistical analyses were performed using Revman 5.3 (The Cochrane Collaboration).
This initial search resulted in 450 citations. Of those, 198 articles were evaluated in full-text screening; 189 records were removed based on study design (non-randomised studies or systematic review/meta-analyses), undesired outcomes and short follow-up duration (<6 months). Finally, nine trials enrolling 100,566 patients were included in this meta-analysis. The study characteristics are summarised in Table 1. The primary endpoint of this meta-analysis was reduced by 18% with a treatment with ezetimibe or a PCSK-9 inhibitor in addition to statins (OR 0.82, 95% CI 0.75–0.89, P < 0.0001, see Supplementary Figure 1). Statistical heterogeneity was substantial (I2 = 63%). In a sensitivity analysis, we stratified for trials with acute coronary syndrome as the inclusion criteria and for trials also including patients with stable angina pectoris. For trials including only acute coronary syndrome patients, the primary endpoint was reduced by 14% (OR 0.86, 95% CI 0.82–0.91, P < 0.0001). For trials also including patients for other indications, the primary endpoint was reduced by 25% (OR 0.75, 95% CI 0.63–0.91, P = 0.03).
| Trial . | Drug . | Sample size, n . | Year of publication . | Mean age (years) . | Number of men (%) . | LDL-cholesterol inclusion criteria, mg/dl . | LDL-cholesterol reduction, % . | Median follow-up, months . |
|---|---|---|---|---|---|---|---|---|
| IMPROVE-IT | Ezetimibe | 18,144 | 2015 | 64 | 76 | ≥50 mg/dl | 24 | 72 |
| HIJ-PROPER | Ezetimibe | 1734 | 2017 | 67 | 75 | ≥100 mg/dl | 52 | 36 |
| ODYSSEY Outcomes | Alirocumab | 18,924 | 2018 | 58 | 75 | ≥70 m/dl | 61 | 34 |
| ODYSSEY LONG TERM | Alirocumab | 2341 | 2015 | 60 | 62 | ≥70 m/dl | 58 | 18 |
| FOURIER | Evolocumab | 27,564 | 2017 | 63 | 75 | ≥70 mg/dl | 58 | 25 |
| OSLER-1, OSLER-2 | Evolocumab | 4465 | 2015 | 58 | 51 | ≥75 mg/dl | 61 | 11 |
| SPIRE I | Bococizumab | 16,817 | 2017 | 63 | 26 | ≥70 m/dl | 45 | 7a |
| SPIRE II | Bococizumab | 10,621 | 2017 | 63 | 30 | ≥100 mg/dl | 41 | 12a |
| Trial . | Drug . | Sample size, n . | Year of publication . | Mean age (years) . | Number of men (%) . | LDL-cholesterol inclusion criteria, mg/dl . | LDL-cholesterol reduction, % . | Median follow-up, months . |
|---|---|---|---|---|---|---|---|---|
| IMPROVE-IT | Ezetimibe | 18,144 | 2015 | 64 | 76 | ≥50 mg/dl | 24 | 72 |
| HIJ-PROPER | Ezetimibe | 1734 | 2017 | 67 | 75 | ≥100 mg/dl | 52 | 36 |
| ODYSSEY Outcomes | Alirocumab | 18,924 | 2018 | 58 | 75 | ≥70 m/dl | 61 | 34 |
| ODYSSEY LONG TERM | Alirocumab | 2341 | 2015 | 60 | 62 | ≥70 m/dl | 58 | 18 |
| FOURIER | Evolocumab | 27,564 | 2017 | 63 | 75 | ≥70 mg/dl | 58 | 25 |
| OSLER-1, OSLER-2 | Evolocumab | 4465 | 2015 | 58 | 51 | ≥75 mg/dl | 61 | 11 |
| SPIRE I | Bococizumab | 16,817 | 2017 | 63 | 26 | ≥70 m/dl | 45 | 7a |
| SPIRE II | Bococizumab | 10,621 | 2017 | 63 | 30 | ≥100 mg/dl | 41 | 12a |
Early discontinuation by sponsor due to data from other studies in the programme.
| Trial . | Drug . | Sample size, n . | Year of publication . | Mean age (years) . | Number of men (%) . | LDL-cholesterol inclusion criteria, mg/dl . | LDL-cholesterol reduction, % . | Median follow-up, months . |
|---|---|---|---|---|---|---|---|---|
| IMPROVE-IT | Ezetimibe | 18,144 | 2015 | 64 | 76 | ≥50 mg/dl | 24 | 72 |
| HIJ-PROPER | Ezetimibe | 1734 | 2017 | 67 | 75 | ≥100 mg/dl | 52 | 36 |
| ODYSSEY Outcomes | Alirocumab | 18,924 | 2018 | 58 | 75 | ≥70 m/dl | 61 | 34 |
| ODYSSEY LONG TERM | Alirocumab | 2341 | 2015 | 60 | 62 | ≥70 m/dl | 58 | 18 |
| FOURIER | Evolocumab | 27,564 | 2017 | 63 | 75 | ≥70 mg/dl | 58 | 25 |
| OSLER-1, OSLER-2 | Evolocumab | 4465 | 2015 | 58 | 51 | ≥75 mg/dl | 61 | 11 |
| SPIRE I | Bococizumab | 16,817 | 2017 | 63 | 26 | ≥70 m/dl | 45 | 7a |
| SPIRE II | Bococizumab | 10,621 | 2017 | 63 | 30 | ≥100 mg/dl | 41 | 12a |
| Trial . | Drug . | Sample size, n . | Year of publication . | Mean age (years) . | Number of men (%) . | LDL-cholesterol inclusion criteria, mg/dl . | LDL-cholesterol reduction, % . | Median follow-up, months . |
|---|---|---|---|---|---|---|---|---|
| IMPROVE-IT | Ezetimibe | 18,144 | 2015 | 64 | 76 | ≥50 mg/dl | 24 | 72 |
| HIJ-PROPER | Ezetimibe | 1734 | 2017 | 67 | 75 | ≥100 mg/dl | 52 | 36 |
| ODYSSEY Outcomes | Alirocumab | 18,924 | 2018 | 58 | 75 | ≥70 m/dl | 61 | 34 |
| ODYSSEY LONG TERM | Alirocumab | 2341 | 2015 | 60 | 62 | ≥70 m/dl | 58 | 18 |
| FOURIER | Evolocumab | 27,564 | 2017 | 63 | 75 | ≥70 mg/dl | 58 | 25 |
| OSLER-1, OSLER-2 | Evolocumab | 4465 | 2015 | 58 | 51 | ≥75 mg/dl | 61 | 11 |
| SPIRE I | Bococizumab | 16,817 | 2017 | 63 | 26 | ≥70 m/dl | 45 | 7a |
| SPIRE II | Bococizumab | 10,621 | 2017 | 63 | 30 | ≥100 mg/dl | 41 | 12a |
Early discontinuation by sponsor due to data from other studies in the programme.
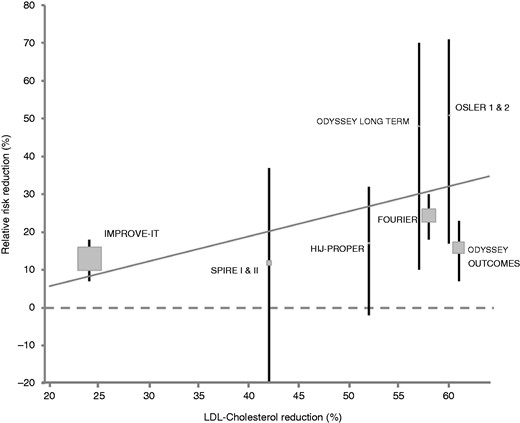
Correlation of mean relative low-density lipoprotein (LDL) reduction by non-statins and corresponding mean risk reduction of the composite primary endpoint.
Overall, our analysis was limited by the number of included trials. However, given the large cohorts of included studies, our analysis includes over 100,000 patients, enabling the description of robust effect sizes. In addition, heterogeneity was of concern in some analyses.
Intensified LDL-lowering therapy with ezetimibe or PCSK-9 inhibitors, in addition to statins, reduces the risk of major cardiovascular events, predominantly by a reduction of myocardial infarction and stroke. There is a linear relationship between LDL reduction and cardiovascular risk reduction, which enables physicians to base treatment decisions for lipid-lowering therapy according to the individual’s patient risk profile. Further studies with longer follow-up are needed to investigate the effect of intensified lipid-lowering therapy on mortality.
Supplemental Material
Supplemental material for Efficacy of lipid-lowering therapy beyond statins to prevent cardiovascular events: a meta-analysis
Supplemental Material for Efficacy of lipid-lowering therapy beyond statins to prevent cardiovascular events: a meta-analysis by Iryna Dykun, Raluca Mincu, Stefanie Hendricks, Bastian Balcer, Matthias Totzeck, Tienush Rassaf and Amir A Mahabadi in European Journal of Preventive Cardiology
Author contribution
Iryna Dykun, Raluca Mincu, Tienush Rassaf and Amir Mahabadi contributed to the conception or design of the work. Iryna Dykun, Raluca Mincu, Stefanie Hendricks, Bastian Balcer, Matthias Totzeck, Tienush Rassaf and Amir Mahabadi contributed to the data acquisition, analysis and interpretation of data for the work. Iryna Dykun and Amir Mahabadi drafted the manuscript. Raluca Mincu, Stefanie Hendricks, Bastian Balcer, Matthias Totzeck and Tienush Rassaf critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.




Comments