-
PDF
- Split View
-
Views
-
Cite
Cite
Giovanni Battista Bonfioli, Matteo Pagnesi, Leonardo Calò, Marco Metra, Towards a phenotype profiling of the patients with heart failure and preserved ejection fraction, European Heart Journal Supplements, Volume 27, Issue Supplement_1, February 2025, Pages i115–i121, https://doi.org/10.1093/eurheartjsupp/suae095
Close - Share Icon Share
Abstract
The prevalence of heart failure with preserved ejection fraction (HFpEF) is increasing and prognosis remains poor, with a high risk of mortality or hospitalizations for worsening heart failure events. Apart from sodium-glucose cotransporter-2 inhibitors and diuretics, the management of HFpEF is nowadays based on the different aetiologies and cardiovascular or non-cardiovascular comorbidities. A great heterogeneity of clinical profiles has been described in HFpEF, with several recent studies focused on the identification of different HFpEF phenotypes. In this review, we summarize available evidence on phenotype profiling in HFpEF, describing the different phenotypes with the relative therapeutic implications, and reporting other specific clinical conditions relevant for HFpEF differential diagnosis.
Introduction
Patients with heart failure (HF) have been traditionally subdivided into different categories based on their left ventricular (LV) ejection fraction (LVEF). Based on the latest guidelines,1 HF with preserved EF (HFpEF) is defined by an LVEF ≥50%. Its prevalence, compared with patients with reduced or mildly reduced LVEF (i.e. ≤40 and 41–49%, respectively), is progressively rising, and HFpEF is nowadays the most common form of HF.2,3
Patients with HFpEF are commonly older, female, and with a higher burden of comorbidities. The reasons for the increase in its prevalence are multiple, including: (i) ageing of the general population; (ii) a higher burden of comorbidities, not only directly associated with ageing, but also due to a better outcome of the patients with coronary artery disease (CAD) and an increased incidence of obesity, metabolic syndrome, diabetes, and hypertension, conditions that may contribute to a systemic pro-inflammatory state leading to cardiac remodelling through coronary microvascular endothelium inflammation;4 and (iii) improved diagnostic techniques. In the last few years, some diagnostic scores have been implemented, such as the H2FPEF score and HFA-PEFF score, the latter including exercise stress echocardiography and invasive haemodynamic measurements in selected cases.5,6
While the prognosis of HF with reduced EF (HFrEF) has improved, thanks to the introduction of new evidence-based medical treatments, the prognosis of HFpEF has remained substantially unchanged in the last years with no medications shown as beneficial in randomized controlled trials in these patients. Only sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been demonstrated to reduce the risk of cardiovascular death or hospitalization for HF in patients with HFpEF and are recommended for its treatment in the latest guidelines update.7–9 Recently, finerenone, a non-steroidal mineralocorticoid receptor antagonist (MRA), already recommended in patients with diabetic nephropathy to reduce the risk of HF hospitalizations,7 has shown a relative reduction of 16% in the primary outcome of worsening HF events and cardiovascular death and a relative reduction of 18% of worsening HF events vs. placebo among patients with HF and LVEF ≥40% enrolled in the FINEARTS-HF trial.10
Nowadays, apart from SGLT2i and diuretic therapy when needed, the management of HFpEF is based on the different aetiologies and comorbidities (both cardiovascular and non-cardiovascular). Therefore, further assessment of patients’ phenotypes and specific clinical conditions associated with HFpEF is important to refine its management. This review focuses on current evidence relative to phenotype profiling in patients with HFpEF, describing the characteristics of the different phenotypes and the consequent therapeutic implications.
Phenotypes profiling
Considering the great heterogeneity of clinical profiles in patients with HFpEF, several studies have focused on the identification of different HFpEF phenotypes to better understand its pathogenesis and to refine its management. In a subanalysis of the TOPCAT trial, Cohen et al. distinguished three different phenotypes based on latent-class analysis: the first one composed by young patients with normal LV geometry, filling pressures, arterial stiffness, and natriuretic peptides, with mild symptoms and probably with other clinical features, such as lung disease, that may explain the clinical manifestations; the second one composed by elderly patients, typically women, with small LV and atrial fibrillation (AF); the third one composed by obese and diabetic patients, usually with advanced symptoms. The first group had a lower rate of clinical events during follow-up compared with the others.11
Recently, Larson et al.12 categorized 643 patients with HFpEF undergoing invasive haemodynamic cardiopulmonary testing into 4 distinct, but possibly overlapping, clinically defined phenogroups: the cardiometabolic, the left atrial myopathy, the stiff vascular, and the pulmonary vascular disease. Haemodynamic signatures and determinants of exercise intolerance differed by phenogroup. Patients who fulfilled the criteria to enter more than one phenogroup were found to have more compromised functional capacity and worse outcomes.12 Machine learning has also been recently used to identify distinct artificial intelligence–derived phenotypes in HFpEF.13–15
The 2023 scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension underline the complexity of HFpEF, an entity that cannot be easily restricted into a few phenogroups but is represented by a wide variety of clinical features. Nonetheless, in this vast range of possibilities, phenotype profiling is essential to identify the best treatment16 (Figure 1).
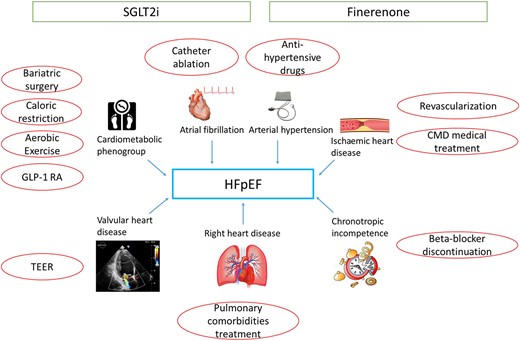
Representation of different phenotypes and conditions associated with heart failure with preserved ejection fraction with the relative treatments. Sodium glucose cotransporter 2 inhibitors and finerenone may have a beneficial effect in all heart failure with preserved ejection fraction patients, while other treatments should be targeted on the patient’s phenotype. CMD, coronary microvascular dysfunction; FMR, functional mitral regurgitation; GLP-1 RA, glucagon-like peptide-1 receptor agonist; SGLT2i, sodium glucose cotransporter 2 inhibitors; STR, secondary tricuspid regurgitation; TEER, transcatheter edge-to-edge repair.
Different phenotypes and relative treatments
Cardiometabolic phenogroup
Type 2 diabetes mellitus (T2DM) and obesity are closely interconnected with each other and their prevalence is rising, representing a major concern for healthcare worldwide. Nowadays, in the western countries, >25% of the population is obese.17 Both T2DM and obesity seem to contribute to a chronic pro-inflammatory state that concurs in the LV remodelling leading to concentric hypertrophy and diastolic dysfunction. Obese patients have an increased epicardial adipose tissue, which may cause a disbalance in the production of adipokines towards a pro-inflammatory state; furthermore, they need a greater volume of circulating blood, demanding an increase in stroke volume and consequently in myocardial wall stress, LV hypertrophy, and remodelling.18,19 The vicious pathogenetic cycle that leads to HFpEF in obese patients is summarized in Figure 2.
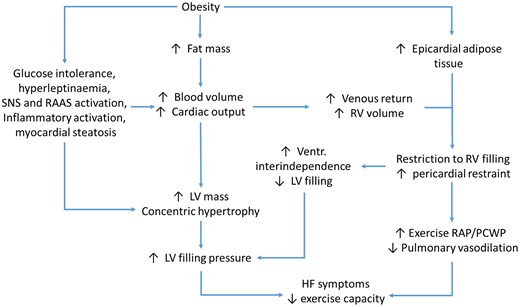
Schematic representation of the vicious circle leading to heart failure with preserved ejection fraction in obese patients. HF, heart failure; LV, left ventricle; PCWP, pulmonary capillary wedge pressure; RAAS, renin angiotensin aldosterone system; RAP, right atrial pressure; RV, right ventricle; SNS, sympathetic nervous system.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are a glucose-lowering class of drugs that act by stimulating insulin secretion by the pancreatic beta cells, with the concomitant inhibition of glucagon secretion by the alpha cells. Apart from the action on insulin production, GLP-1 RAs have wide pleotropic effects that include weight loss through the slowing of gastric emptying and the reduction of hunger mediated by the central nervous system. Beyond their benefits in reducing cardiovascular ischaemic events among patients with or without HF,20 GLP-1 RAs have recently been specifically tested in patients with HFpEF. In the recent STEP-HFpEF-DM and STEP-HFpEF randomized controlled trials (RCTs), semaglutide (2.4 mg subcutaneously, once weekly) showed an improvement in quality of life (QoL) and functional capacity when administered to patients affected by HFpEF and obesity [body mass index (BMI) ≥30 kg/m2], with and without T2DM, respectively.21,22 A pooled analysis of these two trials demonstrated an improvement of QoL and functional capacity, as well as of the secondary hierarchical composite endpoint of all-cause death, HF events, and differences in changes in KCCQ-CSS and 6 min walk distance, with a win ratio of 1.65 (1.42–1.91).23 The combined GLP-1/glucose-dependent insulinotropic polypeptide agonist tirzepatide demonstrated excellent weight-loss efficacy in patients with obesity.24 The SUMMIT trial (NCT04847557) will provide insights on the effects of tirzepatide compared with placebo in obese patients with HFpEF.
Bariatric surgery may be an alternative to medical treatment in patients with BMI ≥40 kg/m2 or BMI ≥35 kg/m2 in the presence of obesity-related complications, such as T2DM, cardiovascular disease, and sleep apnoea. Surgery for BMI ≥30 kg/m2 is only indicated for patients with T2DM refractory to conventional medical treatment.25 Bariatric surgery has demonstrated a reduction in blood pressure and cholesterol levels, but data regarding improved cardiovascular outcomes derive from observational studies and RCTs are needed to confirm these results.18
Apart from medical and interventional measures, caloric restriction and aerobic exercise training in obese patients with HFpEF are related to an improvement in functional capacity and iQoL.26,27 Lifestyle management remains a cornerstone in the treatment of the HFpEF obese phenotype.
Atrial fibrillation
Atrial fibrillation and HFpEF are closely related. Atrial fibrillation may contribute to the pathophysiology of HFpEF due to atrial dilatation and increased filling pressures. Furthermore, AF may negatively impact the clinical course of HFpEF, as many HF hospitalizations may be secondary to AF episodes. The EAST-AFNET 4 trial showed that, in patients with AF, early rhythm control (<1 year) reduced the composite outcome of cardiovascular death, stroke, and hospitalizations for HF or for acute coronary syndrome.28 In a post hoc analysis of the ATHENA trial, dronedarone, compared with placebo, tended to reduce the composite outcome of death or cardiovascular hospitalizations [hazard ratio (HR) 0.79, 95% confidence interval (CI) 0.61–1.03] in patients with LVEF >40% and paroxysmal or persistent AF.29 Catheter ablation represents an effective option for the treatment of the patients with AF and HFpEF. In the CABANA trial, catheter ablation significantly reduced all-cause mortality when compared with medical treatment alone in the subgroup of patients affected by HF, independently by LVEF.30 In the Swedish Heart Failure Registry, catheter ablation was associated with a lower risk of all-cause mortality or first HF hospitalization compared with medical therapy in all patients with HF. Furthermore, in the subgroup of patients with HFpEF, it was associated with a relative reduction of 57% of first HF hospitalization (HR 0.43, 95% CI 0.22–0.85) and 83% of recurrent HF hospitalizations (HR 0.17, 95% CI 0.07–0.42).31 However, a recent meta-analysis has shown a reduction in HF events with AF ablation in the patients with HFrEF but not in those with HFpEF.32
Arterial hypertension
Arterial hypertension is the most prevalent comorbidity in HFpEF with rates of ∼90% in RCTs.8,26,27 There are many links between arterial hypertension and HFpEF: primarily, elevated blood pressure causes LV hypertrophy and fibrosis secondary to microvascular dysfunction and increased arterial stiffness. The treatment of hypertension reduces arterial stiffness and consequently LV adverse remodelling. In a meta-analysis including 613 815 participants from 123 different trials, every 10 mmHg reduction in systolic blood pressure was associated with a significant 20% reduction in major cardiovascular disease events [relative risk (RR) 0.80, 95% CI 0.77–0.83], including a reduction in the risk of HF (RR 0.72, 95% CI 0.67–0.78).33 In the HYVET trial, the use of indapamide in combination with perindopril among patients who did not achieve target blood pressure values with monotherapy, reduced the rate of death from cardiovascular disease by 23% and the rate of HF by 64%.34
Ischaemic heart disease
Epicardial CAD is common in patients with HFpEF and is related to a worse prognosis. In some observational studies, revascularization of diseased vessels was associated with better outcomes.35,36 However, RCTs are needed to determine the efficacy of revascularization in patients with HFpEF.
Coronary microvascular dysfunction (CMD) is also frequently associated with HFpEF. Coronary microvascular dysfunction is associated with reduced coronary flow reserve and endothelial dysfunction that may lead to the development of cardiac remodelling.37,38 Coronary microvascular dysfunction is associated with worse prognosis and worse diastolic function with more dilated left atrium and higher filling pressures.39 It is not yet known if classical treatments for CMD, such as beta blockers, calcium channel blockers and ranolazine, have an impact on the outcomes of patients with concomitant HFpEF.
Chronotropic incompetence
Patients with HF have a significantly impaired exercise tolerance to which chronotropic incompetence significantly contributes. Beta blockers blunt chronotropic response, and in selected patients, their discontinuation leads to a better functional capacity.40 The use of beta blockers in HFpEF should be considered based on patients’ characteristics and comorbidities. However, in the RAPID-AF trial, the implantation of a pacemaker with rate-adaptive atrial pacing in patients with symptomatic HFpEF and chronotropic incompetence was not associated with an improvement in exercise capacity.41
Right heart dysfunction
Right ventricular dysfunction (RVD) is present in 20–50% of patients with HFpEF and is a predictor of adverse events. The most important mechanism that leads to RVD is post-capillary pulmonary hypertension secondary to raised LV filling and atrial pressures and the loss of left atrial compliance. Furthermore, pulmonary vascular disease may be aggravated by chronic pulmonary congestion and concomitant factors such as ageing, male sex, and chronic obstructive pulmonary disease.42,43 It must be noted that right heart catheterization is recommended to distinguish between primitive pulmonary arterial hypertension and pulmonary hypertension related to HFpEF; exercise testing or fluid challenge may be useful to enhance HFpEF diagnosis.42
Valvular heart disease
The negative cardiac remodelling of HFpEF may lead to secondary valvular heart disease, such as functional mitral regurgitation (FMR) and secondary tricuspid regurgitation (STR). The FMR of HFpEF is typically due to left atrial remodelling (‘atrial’ FMR), with left atrial dilatation and increase in left atrial pressure. These alterations lead to leaflet malcoaptation secondary to annulus dilatation and flattening.44 Observational studies have recently suggested that transcatheter mitral valve edge-to-edge repair can effectively treat atrial FMR with a consequent improvement in HF-related symptoms.45,46
Secondary tricuspid regurgitation has a relevant prognostic impact in patients with HFpEF.47 Tricuspid transcatheter edge-to-edge repair recently demonstrated to be safe and effective in patients with severe tricuspid regurgitation with an improvement in QoL, but further studies are needed especially in the specific context of HFpEF.
Specific clinical conditions (heart failure with preserved ejection fraction differential diagnosis)
In some patients, specific conditions cause HFpEF.48 It is important to distinguish these conditions since they have distinct aetiologies and treatments. These treatable conditions include hypertrophic cardiomyopathy, cardiac amyloidosis, pulmonary hypertension not due to left-sided HF (Groups 1, 3, 4, and 5), specific causes of high-output HF, Fabry disease, cardiac sarcoidosis, and constrictive pericarditis (Table 1).
Summary of specific heart failure with preserved ejection fraction differential diagnosis and relative treatment
| Condition . | Clinical and instrumental findings . | Therapy . |
|---|---|---|
| Hypertrophic cardiomyopathy (HCM) | Asymmetric or global increase in LV wall thickness | General: avoid dehydration, rhythm control if AF |
| Possible LVOT obstruction | Obstructive HCM: BB, CCB, disopyramide, mavacamten, SRT49 | |
| Non-obstructive HCM: valsartan may slow LV remodelling when administered early50 | ||
| Cardiac amyloidosis | Global increase in LV wall thickness | TTR: tafamidis and other novel treatments51 |
| Restrictive pathophysiology | AL: haematological treatment | |
| Pulmonary hypertension (PH) not due to left-sided HF (Groups 1, 3, 4, and 5) | Type 1: idiopathic/hereditary PH | Aetiology-based treatment |
| Type 3: pulmonary disease–related PH | Specialist referral | |
| Type 4: CTPH | ||
| Type 5: rare disease | ||
| High-output HF | Elevated cardiac output may be due to obesity, arteriovenous shunts, liver disease, lung disease, and myeloproliferative disease | Aetiology-based treatment |
| Fabry disease | X-linked genetic transmission | ERT |
| Systemic symptoms/renal involvement | ||
| Restrictive pathophysiology | ||
| Cardiac sarcoidosis | Usually related to extracardiac sarcoidosis | Immunosoppression |
| Possible symptoms include ventricular arrythmias and heart failure | Specialist referral | |
| Constrictive pericarditis | Related to chest surgery or trauma, history of pericarditis, or connective tissue disease | Anti-inflammatory treatment if active pericarditis |
| Pericardiectomy |
| Condition . | Clinical and instrumental findings . | Therapy . |
|---|---|---|
| Hypertrophic cardiomyopathy (HCM) | Asymmetric or global increase in LV wall thickness | General: avoid dehydration, rhythm control if AF |
| Possible LVOT obstruction | Obstructive HCM: BB, CCB, disopyramide, mavacamten, SRT49 | |
| Non-obstructive HCM: valsartan may slow LV remodelling when administered early50 | ||
| Cardiac amyloidosis | Global increase in LV wall thickness | TTR: tafamidis and other novel treatments51 |
| Restrictive pathophysiology | AL: haematological treatment | |
| Pulmonary hypertension (PH) not due to left-sided HF (Groups 1, 3, 4, and 5) | Type 1: idiopathic/hereditary PH | Aetiology-based treatment |
| Type 3: pulmonary disease–related PH | Specialist referral | |
| Type 4: CTPH | ||
| Type 5: rare disease | ||
| High-output HF | Elevated cardiac output may be due to obesity, arteriovenous shunts, liver disease, lung disease, and myeloproliferative disease | Aetiology-based treatment |
| Fabry disease | X-linked genetic transmission | ERT |
| Systemic symptoms/renal involvement | ||
| Restrictive pathophysiology | ||
| Cardiac sarcoidosis | Usually related to extracardiac sarcoidosis | Immunosoppression |
| Possible symptoms include ventricular arrythmias and heart failure | Specialist referral | |
| Constrictive pericarditis | Related to chest surgery or trauma, history of pericarditis, or connective tissue disease | Anti-inflammatory treatment if active pericarditis |
| Pericardiectomy |
AF, atrial fibrillation; AL, amyloid light chain; BB, beta blockers; CCB, calcium channel blockers; CTPH, chronic thromboembolic pulmonary hypertension; ERT, enzyme replacement therapy; LV, left ventricular; LVOT, left ventricular outflow tract; SRT, septal reduction therapy; TTR, transthyretin.
Summary of specific heart failure with preserved ejection fraction differential diagnosis and relative treatment
| Condition . | Clinical and instrumental findings . | Therapy . |
|---|---|---|
| Hypertrophic cardiomyopathy (HCM) | Asymmetric or global increase in LV wall thickness | General: avoid dehydration, rhythm control if AF |
| Possible LVOT obstruction | Obstructive HCM: BB, CCB, disopyramide, mavacamten, SRT49 | |
| Non-obstructive HCM: valsartan may slow LV remodelling when administered early50 | ||
| Cardiac amyloidosis | Global increase in LV wall thickness | TTR: tafamidis and other novel treatments51 |
| Restrictive pathophysiology | AL: haematological treatment | |
| Pulmonary hypertension (PH) not due to left-sided HF (Groups 1, 3, 4, and 5) | Type 1: idiopathic/hereditary PH | Aetiology-based treatment |
| Type 3: pulmonary disease–related PH | Specialist referral | |
| Type 4: CTPH | ||
| Type 5: rare disease | ||
| High-output HF | Elevated cardiac output may be due to obesity, arteriovenous shunts, liver disease, lung disease, and myeloproliferative disease | Aetiology-based treatment |
| Fabry disease | X-linked genetic transmission | ERT |
| Systemic symptoms/renal involvement | ||
| Restrictive pathophysiology | ||
| Cardiac sarcoidosis | Usually related to extracardiac sarcoidosis | Immunosoppression |
| Possible symptoms include ventricular arrythmias and heart failure | Specialist referral | |
| Constrictive pericarditis | Related to chest surgery or trauma, history of pericarditis, or connective tissue disease | Anti-inflammatory treatment if active pericarditis |
| Pericardiectomy |
| Condition . | Clinical and instrumental findings . | Therapy . |
|---|---|---|
| Hypertrophic cardiomyopathy (HCM) | Asymmetric or global increase in LV wall thickness | General: avoid dehydration, rhythm control if AF |
| Possible LVOT obstruction | Obstructive HCM: BB, CCB, disopyramide, mavacamten, SRT49 | |
| Non-obstructive HCM: valsartan may slow LV remodelling when administered early50 | ||
| Cardiac amyloidosis | Global increase in LV wall thickness | TTR: tafamidis and other novel treatments51 |
| Restrictive pathophysiology | AL: haematological treatment | |
| Pulmonary hypertension (PH) not due to left-sided HF (Groups 1, 3, 4, and 5) | Type 1: idiopathic/hereditary PH | Aetiology-based treatment |
| Type 3: pulmonary disease–related PH | Specialist referral | |
| Type 4: CTPH | ||
| Type 5: rare disease | ||
| High-output HF | Elevated cardiac output may be due to obesity, arteriovenous shunts, liver disease, lung disease, and myeloproliferative disease | Aetiology-based treatment |
| Fabry disease | X-linked genetic transmission | ERT |
| Systemic symptoms/renal involvement | ||
| Restrictive pathophysiology | ||
| Cardiac sarcoidosis | Usually related to extracardiac sarcoidosis | Immunosoppression |
| Possible symptoms include ventricular arrythmias and heart failure | Specialist referral | |
| Constrictive pericarditis | Related to chest surgery or trauma, history of pericarditis, or connective tissue disease | Anti-inflammatory treatment if active pericarditis |
| Pericardiectomy |
AF, atrial fibrillation; AL, amyloid light chain; BB, beta blockers; CCB, calcium channel blockers; CTPH, chronic thromboembolic pulmonary hypertension; ERT, enzyme replacement therapy; LV, left ventricular; LVOT, left ventricular outflow tract; SRT, septal reduction therapy; TTR, transthyretin.
Conclusions
Heart failure with preserved ejection fraction is a complex syndrome characterized by a huge heterogeneity in terms of clinical presentation, pathophysiologic pathways, phenogroups, and therapeutic response. Currently, treatment of HFpEF is based on the administration of SGLT2i, the use of diuretics for patients with fluid overload, and the identification and treatment of associated comorbidities. A special interest has raised in the last years about the cardiometabolic phenogroup of HFpEF, mainly due to the increasingly growing number of patients with cardiovascular-kidney-metabolic conditions and the results of recent RCTs demonstrating the benefits of the GLP-1 RA semaglutide in obese HFpEF and of the non-steroidal MRA finerenone in unselected HFpEF. However, a prompt diagnosis and treatment of other comorbidities, as well as further research aimed at identifying clinically relevant HFpEF phenotypes and developing phenogroup-specific therapies, are important to refine the management of HFpEF.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
These authors have equally contributed as first authors.
Conflict of interest: M.P. has received personal fees from Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Novartis, Roche Diagnostics, and Vifor Pharma. M.M. has received consulting honoraria as a member of trial committees or advisory boards for Abbott Vascular, Actelion, Amgen, Bayer, Edwards Therapeutics, Servier, Vifor Pharma, and Windtree Therapeutics. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.



