-
PDF
- Split View
-
Views
-
Cite
Cite
Giovanna Manzi, Tommaso Recchioni, Alexandra Mihai, Paolo Severino, Lucia Ilaria Birtolo, Domenico Filomena, Andrea D’Amato, Massimo Mancone, Silvia Papa, Roberto Badagliacca, Carmine Dario Vizza, The right ventricle: the co-star in the complex history of heart failure, European Heart Journal Supplements, Volume 27, Issue Supplement_1, February 2025, Pages i109–i114, https://doi.org/10.1093/eurheartjsupp/suae092
Close - Share Icon Share
Abstract
The natural history of patients with heart failure (HF), mainly affecting the left ventricle in the initial stages, is marked by the progressive involvement of the right ventricle (RV), which in the advanced stages of the disease becomes dilated and dysfunctional. The geometrical, functional, and pathological interdependence binding the two ventricles underlies this progressive path. Researchers’ and clinicians’ efforts must be aimed at interrupting the inevitable trajectory of HF, by preventing the development of pulmonary hypertension (PH) and RV dysfunction or the transition from isolated post-capillary PH to combined pre- and post-capillary PH. The search for drugs targeting the pulmonary circulation is another goal of PH researchers. Studies conducted so far on drugs currently approved for patients with pulmonary arterial hypertension have yielded contradictory results; an in-depth analysis of these trials could help researchers profile the patients with HF who might benefit from these treatments.
Introduction
Pulmonary hypertension (PH) and right ventricular (RV) dysfunction can complicate the clinical course of patients with heart failure (HF) with reduced and preserved ejection fraction (HFrEF and HFpEF), increasing the risk of mortality. For this reason, Guazzi1 defined the relationship between PH and HF as a dangerous liaison. The prevalence of PH in HF is variable, depending on the stage of HF, on the definition of PH, and on the method used for pulmonary artery pressure (PAP) measurement in the different studies. Using right heart catheterization (RHC), the prevalence of PH was reported to be between 40 and 75% in HFrEF, whereas utilizing either echocardiography or RHC the prevalence of PH in HFpEF ranged between 36 and 83%.
The switch in HF evolution from a left ventricular (LV) phenotype, characterized by an abnormal LV with an otherwise normal RV, to RV phenotype, dominated by a dilated and functionally impaired RV, negatively influences patients’ exercise tolerance and outcome increasing mortality. Thus, a complete evaluation of patients with HF must include the careful study of the right heart: understanding where the patient is on the HF trajectory is essential for clinicians to best direct therapeutic intervention.
Mechanisms underlying the right ventricular dysfunction in patients with heart failure: ventricular interdependence
Initially considered as a merely passive conduit for systemic venous return, the idea about RV has changed dramatically over the years.
Three forms of interdependence underlie the intimate connection between RV and LV, thus justifying the development of RV dysfunction in patients with HF:
Geometrical interdependence: RV and LV share the interventricular septum (IVS) and coronary blood flow, have intertwined muscle bundles, and are enclosed by the same pericardium that is highly resistant to acute distension. Of course, volume and pressure of one ventricle influence the compliance of the other one. A clear example of this close anatomical connection comes from pulmonary arterial hypertension (PAH): due to the constraints of the relatively fixed pericardial space and the consequent change in the transseptal pressure gradient during diastole, the hypertensive dilated RV displaces the IVS leftward and alters LV geometry, reducing LV filling and LV output.
Functional interdependence: due to the in-series connection between the RV and LV, the chronic increase in left-sided filling pressure is passively backward transmitted to the pulmonary circulation, up to the pulmonary arteries. The subsequent remodelling of pulmonary vasculature, reversible in the short-term, becomes irreversible in the long-term because of dysregulation of smooth muscle tone and structural alterations. The resulting increase in pulmonary vascular resistance (PVR) induces pathological changes of the RV, including hypertrophy, fibrosis, and dilatation. In the early stages, the RV faces the increased afterload through different mechanisms representing a pathophysiological continuum whose final purpose is to maintain an adequate right ventricular–pulmonary arterial (RV–PA) coupling.2 When these compensatory mechanisms are exhausted in the advanced stages of the disease, RV–PA uncoupling occurs, ultimately leading to right HF. The pattern of RV adaptation to the increased afterload (concentric hypertrophy vs. eccentric hypertrophy) influences the prognosis of patients. In this regard, great lessons come again from the experiences conducted in patients with PAH. Enrolling idiopathic pulmonary arterial hypertension patients who underwent cardiac magnetic resonance, Badagliacca et al. firstly introduced the RV mass/volume (M/V) ratio as a parameter allowing the distinction between RV eccentric (low M/V ratio) and concentric hypertrophy (high M/V ratio) and demonstrated its prognostic role. Patients with a low RV M/V ratio and low cardiac index (CI) showed a 28.8 increase in the hazard ratio (HR) of clinical worsening compared with patients with high RV M/V ratio and high CI patients (P = 0.0001).3 The same authors, evaluating 60 consecutive patients with PAH, found that RV M/V ratio < 0.46 was associated with greater RV filling pressures [right atrial pressure (RAP)] and pericardial effusion, worse CI, more advanced World Health Organization (WHO) functional class, and exercise tolerance impairment compared with patients with RV M/V ratio > 0.46, despite similar PVR, mean pulmonary arterial pressure (mPAP), and pulmonary arterial compliance.4
Pathological interdependence: many primary or secondary myocardial diseases (such as idiopathic dilated cardiomyopathy, myocarditis, ischaemic, or infiltrative diseases) can affect both LV and RV simultaneously, although there is usually predominant involvement of one chamber over the other.
The haemodynamic criteria
The haemodynamic PH pattern complicating the course of patients with left heart disease (LHD) and HF is post-capillary. In line with the recommendations of the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines and with the 7th World Symposium on PH, post-capillary PH is defined by the elevation of mPAP above 20 mmHg combined with a pulmonary arterial wedge pressure (PAWP) > 15 mmHg. The further distinction between isolated post-capillary PH (IpcPH) and combined pre- and post-capillary PH (CpcPH) is based on the measurement of PVR, ≤2 and >2 Wood units (WU), respectively. Indeed, PVR resulted in strongly predicting survival and outperforming diastolic pressure gradient (DPG) and transpulmonary pressure gradient (TPG), two haemodynamic measures previously used to distinguish the two phenotypes of post-capillary PH.
However, when interpreting haemodynamic data, physicians must always consider the clinical context of individual patients, keeping in mind acute conditions and general treatment measures (e.g. diuretics) that can strongly influence haemodynamics. Thus, for optimal decision-making, patients presenting with PAWP between 12 and 18 mmHg should be carefully evaluated, combining clinical history, cardiovascular risk factors, echocardiographic findings, and response to provocative tests, such as fluid challenge. PAWP values >18 mmHg after the rapid infusion of 500 mL saline or 7 mL/kg over 5–10 min in patients with lower baseline PAWP may be suggestive of LHD. Instead, the role of exercise provocation tests to differentiate Group 1 vs. Group 2 PH is limited.
The prognostic role of right ventricular systolic function in heart failure
RV dysfunction, mainly when combined with PH, is associated with dismal prognosis, as resulted from the following experiences. Ghio et al.5 enrolled 377 consecutive patients with HF and a LV ejection fraction (EF) < 35%, who were classified into four subgroups based on PAP and thermodilution-derived right ventricle ejection fraction (RVEF). Through Cox survival analysis, the authors demonstrated that the 215 patients with high PAP and low RVEF had the worst prognosis, with higher mortality rates compared with the other subgroups. Of note, prognosis of patients with high PAP and preserved RVEF was very similar to patients with normal PAP, highlighting the strong prognostic role of RV performance in patients with HF. Some years later, Lam et al.6 demonstrated the relevant association of PH with poor outcome in 244 patients affected by HFpEF, followed up over 3 years. PH, defined by systolic PAP above 35 mmHg evaluated by echocardiography, was found in 83% of patients with HFpEF and reported to be associated with mortality. In this study, the high values of pulmonary capillary wedge pressure (PCWP) were estimated through the ratio of early transmitral flow velocity (E) to early mitral annular diastolic velocity (e′). Due to the lack of haemodynamic data, the distinction between IpcPH and combined post-capillary PH was not possible.
The strike association of PH with mortality was also demonstrated in the setting of acute decompensated heart failure (ADHF) by Aronson et al.7 in 242 patients, with a mean left ventricular ejection fraction (LVEF) of 25 ± 13%. Using post-treatment (nesiritide or nitroglycerine) haemodynamic assessment, 58 patients were classified as no PH, 124 with passive PH (mPAP > 25 mmHg, PCWP > 15 mmHg, and PVR ≤ 3 WU) and 60 with reactive PH (mPAP > 25 mmHg, PCWP > 15 mmHg, and PVR > 3 WU). The presence and type of PH have been shown to have an impact on short-term outcome: contrary to the positive haemodynamics response displayed with therapy by patients without PH and with passive PH, patients with reactive PH did not present improvements in mPAP, PVR, or CI in response to the reduction in filling pressures. Furthermore, patients with reactive PH had a worse prognosis, with Kaplan–Meier survival-free estimates at 6 months of 48.3%, significantly lower than 91.4% of patients with normal mPAP, and 76.7% of patients with passive PH. After multivariable adjustments, reactive PH remained an independent predictor of death, with an adjusted HR of 4.8 and 2.8 compared with patients without PH and with passive PH, respectively.
Pulmonary hypertension: a therapeutic target for patients with HFpEF
The primary steps in the management of patients with longstanding HF include the correction of the underlying substrate and the optimization of HF treatment lowering LV filling pressures, with the aim to prevent the development of pulmonary vascular disease with vasoconstriction and remodelling of the pulmonary arterial bed (Figure 1).
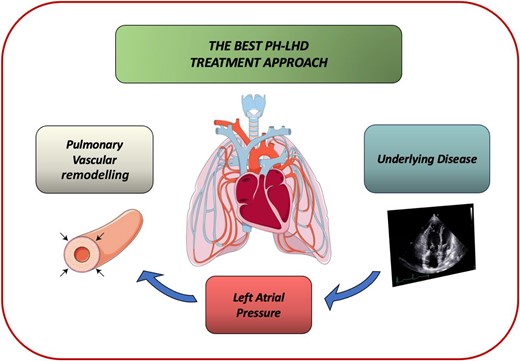
The best pulmonary hypertension-left heart disease therapeutic approach includes the correction of the underlying substrate, the optimization of heart failure treatment to lower left ventricular filling pressures, and the prevention and treatment of pulmonary hypertension.
However, given the development of PH that frequently complicates the clinical course of these patients, ongoing research is focused on investigating currently approved PAH drugs in this population. Nevertheless, studies conducted so far have yielded conflicting results, and no proven therapy has been shown to improve outcomes.
Given the role of deficiency of nitric oxide-soluble guanylate cyclase–cyclic guanosine monophosphate (NO-sGC-cGMP) signalling in the pathogenesis of PH in HFpEF, drugs targeting this pathway may be promising.
At this regard, Guazzi et al.8 performed a double-blind, 1-year study, including 44 patients with HFpEF with pulmonary artery systolic pressure > 40 mmHg, randomly assigned to placebo or to the phophodiesterase-5 inhibitor (PDE5-i) sildenafil 50 mg thrice daily. The unloading effect of the drug with the consequent improvement in RV function was witnessed by the haemodynamics and echocardiographic success reported in the active treatment arm: in patients receiving sildenafil, at 6 and 12 months the authors recorded (i) a significant decrease from baseline in PA, right atrial (RA), and RV end-diastolic pressures and (ii) a significant increase in tricuspid annular plane systolic excursion (TAPSE), CI, E/A ratio, and LV internal dimensions.
Despite the strong pathophysiological rational and Guazzi’s results, the RELAX9 trial failed to demonstrate beneficial effects of sildenafil on exercise capacity and clinical status in HFpEF. Compared with placebo, 24 weeks of treatment with sildenafil did not alter peak oxygen consumption and 6-min walk test nor did it improve clinical status, quality of life, and LV remodelling compared with placebo. Despite the high median values of pulmonary artery systolic pressure (estimated by echocardiography) of the overall enrolled population (41 mmHg), the study was not specifically targeted to patients with HFpEF and PH, and the RHC was not required for enrolment.
Interestingly, sildenafil has also been investigated in a randomized controlled trial enrolling patients undergoing left-sided valvular surgery with PH, defined by mPAP > 25 mmHg and PAPs ≥ 45 mmHg.10 Indeed, after correction of the valvular lesion, PH may persist in up to 75% of patients with moderate or severe preoperative PH or sometimes even develop late in patients without preoperative PH. In this small study by Jiang et al., a single oral dose of the drug relieved both the right and the left heart (e.g. reduction in mPAP, PVR index, LV work, and LV stroke work) and significantly reduced the time on mechanical ventilation and the duration of hospitalization. Given the positive effects of sildenafil in the immediate post-surgery, Bermejo et al.11 conducted the SIOVAC trial to investigate the long-term efficacy of the drug in patients with persistent PH after successful correction of the underlying valvular heart disease (mPAP ≥ 30 mmHg by RHC). Fifty-seven per cent of patients included in this trial showed a PVR > 3 WU, consistent with CpcPH. Nevertheless, sildenafil was associated with worse clinical outcomes than placebo.
Two studies explored the effects of riociguat, a sGC stimulator currently approved for PAH and chronic thromboembolic PH, in patients with symptomatic HFpEF and PH despite optimized standard HF therapy, defined by LVEF > 50%, mPAP > 25 mm Hg, and PAWP > 15 mm Hg at rest. The DILATE-1 trial12 evaluated riociguat acute haemodynamic and echocardiographic effects: no significant change was reported in the primary efficacy variable, represented by the peak decrease in mPAP from baseline up to 6 h, in the riociguat 2 mg group compared with placebo. However, the drug significantly increased stroke volume and CI and reduced the size of the RV (decrease in RV end-diastolic area); no changes were observed in PAWP or PVR. The small number of patients with a DPG ≥ 7 mmHg, previously used to differentiate isolated and combined post-capillary PH, precluded a valid statistical evaluation of this population.
In line with these results, the next haemoDYNAMIC trial13 demonstrated that chronic treatment with riociguat in PH-HFpEF increased cardiac output, with a placebo-corrected change from baseline of 0.54 L/min, without affecting PAWP and systemic vascular resistance (SVR). The decrease in TPG and PVR observed in this trial after 26 weeks of treatment in contrast to the unchanged values of the same parameters reported in the DILATE-1 may be explained by an additional effect on the pulmonary vasculature beyond vasodilation obtained with chronic administration compared with acute administration. Note the higher number of dropouts reported in the riociguat-treated group compared with placebo and the lack of drug effects on clinical variables, such as quality-of-life scores or exercise capacity.
The list of drugs acting on the NO-sGC-cGMP pathway studied in patients with HFpEF also includes tadalafil, another PDE5 inhibitor. Its efficacy and safety have been recently investigated in the PASSION, a Phase 3 study enrolling patients with HFpEF with CpcPH (PVR > 3 WU).14 Although the trial was interrupted due to discontinuation of study drug supply, the analysis of patients included demonstrated that the primary endpoint, time to the first composite event of adjudicated HF hospitalization or all-cause death, occurred more frequently in patients randomized to tadalafil than in those assigned to placebo.
Patients with HF and PH with a pre-capillary component may theoretically benefit from treatment with endothelin receptor antagonists class. The MELODY-1,15 a Phase II exploratory study designed to evaluate safety and tolerability of macitentan 10 mg in patients with LVEF > 30% and CpcPH confirmed by RHC, included a high rate of patients with HFpEF (76.4%). Compared with placebo, more patients in the macitentan group experienced fluid retention or worsening in New York Heart Association (NYHA) functional class from baseline and serious adverse events. As concerns the haemodynamic effects of macitentan, the decrease in PVR was similar in both groups, whereas no change was reported in PAWP values. Finally, the SERENADE trial, which was stopped early due to slow enrolment, did not show positive results in favour of macitentan over placebo, neither in the primary endpoint of change in NT-proBNP from baseline nor in the time to worsening HF.
Research on sotatercept, a first-in-class fusion protein that acts as a ligand trap for selected TGF-β superfamily members, in patients with PH-LHD is ongoing: a Phase 2 study, CADENCE (NCT04945460),16 is evaluating its efficacy and safety vs. placebo in adults with CpcPH due to HFpEF, defined by a baseline LVEF ≥ 50%, a mPAP of >20 mmHg, a PCWP between 15 and 30 mmHg, and a minimum PVR of ≥4 WU.
Pulmonary hypertension: a therapeutic target for patients with HFrEF
Below are the main studies that have tested PAH-specific drugs in patients with HFrEF.
The SilHF17 trial was designed to assess safety, efficacy, and tolerability of phosphodiesterase-5 inhibitors in outpatients with chronic HF with LVEF < 40%, NYHA functional Classes II–III, and a systolic pulmonary pressure >40 mmHg assessed by echocardiography on optimal medical therapy, including diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and aldosterone antagonists. Sixty-nine patients were randomly allocated to either sildenafil 40 mg three times daily (45) or placebo (24). Despite previous findings supporting the acute positive effects of sildenafil on pulmonary circulation, in the SilHF trial sildenafil did not improve symptoms, quality of life, or walk test distance.
Nevertheless, a meta-analysis conducted subsequently by Jiang et al.10 demonstrated the beneficial effects deriving from sildenafil treatment, both at the acute phase and over long-term follow-up, in patients with HFrEF and PH-LHD, assessed by RHC or echocardiography. Sildenafil was superior to placebo (i) in reducing PAP and PVR; (ii) in improving the oxygen consumption at peak exercise (peak VO2), the efficiency of ventilatory response to carbon dioxide output (VE/VCO2 slope), and the ratio of dead space to tidal volume at peak exercise (peak VD/VT) during chronic treatment; and (iii) in improving quality of life assessed using a 16-question chronic HF questionnaire. As suggested by the authors, the afterload reduction with the consequent increase in RV function could probably mediate the improvement in exercise capacity obtained with sildenafil.
The LEPHT trial18 studied the long-term efficacy and safety effects of 16 weeks of riociguat treatment in patients with HF with LVEF ≤ 40% and mPAP ≥ 25 mmHg at rest, symptomatic despite optimized medical therapy. The primary endpoint, placebo-corrected change from baseline to Week 16 in mean PAP, was not met: the reduction in mPAP was significant in both the riociguat 2 mg three times daily group (6.1 ± 1.3 mmHg) and the placebo group (4.0 ± 1.2 mmHg), with a P-value of 0.10 for pairwise comparison of the two arms. Of note, riociguat 2 mg three times a day significantly increased CI and stroke volume index without changing heart rate or systolic blood pressure and decreased both PVR and SVR compared with placebo (unchanged PVR/SVR ratio, suggestive of similar vasodilator effect on the pulmonary and systemic circulation).
In conclusion, evidence on the potential beneficial effects of sodium glucose cotransporter 2 inhibitors (SGLT-2), vericiguat, and angiotensin receptor–neprilysin inhibitor therapies in patients with IpcPH and CpcPH is limited. In this regard, a small single-centre non-randomized trial including 15 patients with HFrEF (88.2%) and 2 patients with HFpEF (11.8%), who had previously implanted a CARDIOMEMS device, investigated the effects of empagliflozin and dapagliflozin on PA pressure: the observed reduction in systolic, diastolic, and mean PA pressures began 3 weeks after the initiation of treatment with SGLT2-i and reached 3 mmHg after 10 weeks.19 These data by Kirshbaum et al.20,21 extended previous smaller observations.
Conclusions
PH is undoubtedly a therapeutic target in patients with HF. Despite the large amount of available evidence, small sample sizes, differences in study design and methods used to diagnose PH, as well as inclusion criteria and chosen primary and secondary endpoints, all these findings have heavily affected the contradictory results obtained in this field. Future studies are welcome to properly phenotype the ideal patient to be treated with PH-specific therapies, preferably including patients with HF with CpcPH. Indeed, the elevation in mPAP in patients with IpPH is necessary to maintain adequate forward blood flow, so in this population drugs specifically targeting the pulmonary vasculature are dangerous, whereas patients with CpPH, who have an increase in mPAP beyond that necessary to maintain cardiac output, may benefit from PAH therapies.
Funding
No funding obtained.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: none declared.



