-
PDF
- Split View
-
Views
-
Cite
Cite
Giovanni Amedeo Tavecchia, Elena Gualini, Alice Sacco, Fabrizio Oliva, The role of sodium–glucose co-transporter 2 inhibitors in myocardial infarction: available evidence and future perspectives, European Heart Journal Supplements, Volume 26, Issue Supplement_1, April 2024, Pages i84–i87, https://doi.org/10.1093/eurheartjsupp/suae008
Close - Share Icon Share
Abstract
There is an unmet need for new treatment options for patients with acute myocardial infarction (AMI) as progress in patients’ outcomes has plateaued over the past 15 years. Sodium–glucose co-transporter 2 (SGLT2) inhibitors have demonstrated cardio-renal benefits in various disease states, encompassing diabetes mellitus, chronic kidney disease, and heart failure. Experimental studies further support their use in AMI, demonstrating beneficial effects in animal models by reducing infarct size and mitigating adverse cardiac remodelling. Recently, two clinical trials have been published thus paving the way for a new field to explore. This paper briefly outlines the available evidence and future perspectives regarding the use of SGLT2 inhibitors in this clinical scenario.
Introduction
The introduction of early invasive strategies and the availability of evidence-based secondary prevention therapies have dramatically changed the prognosis of patients with acute myocardial infarction (AMI), resulting in longer survival and a lower risk of recurrent ischaemic events. However, over the past 15 years, progress in patients’ outcomes has slowed and the availability of new treatment options has been limited.1 As a result, there is a significant unmet need for new and effective therapies.
Although traditionally recognized for their efficacy in the management of type 2 diabetes, sodium–glucose co-transporter 2 (SGLT2) inhibitors have emerged as agents associated with improved cardio-renal outcomes, even in individuals without diabetes mellitus. In particular, important benefits have been observed in patients with heart failure (HF), encompassing both reduced ejection fraction and preserved ejection fraction, thus becoming a new treatment paradigm for this condition.2–5 Given the pleiotropic effects of this pharmacological class and the need for new treatment options for patients with AMI, it has been hypothesized that SGLT2 inhibitors may be effective in this patient population.
This paper aims to briefly review the pathophysiological mechanisms that may support the use of SGLT2 inhibitors in AMI and the evidence available to date.
Pathophysiology
Experimental studies have demonstrated multiple benefits from SGLT2 inhibition. In a non-diabetic mouse model of myocardial infarction, empagliflozin was shown to significantly reduce infarct size and myocardial fibrosis, resulting in improved cardiac function.6 Similarly, in a non-diabetic porcine model subjected to left anterior descending artery balloon occlusion, the use of empagliflozin was associated with improved cardiac remodelling, improved left ventricular systolic function, and reduced neurohormonal activation.7 In addition, long-term administration of canagliflozin has been associated with a significant reduction in myocardial infarct size, in both diabetic and non-diabetic rats, regardless of glucose concentration at the time of ischaemia/reperfusion injury.8
These beneficial effects of SGLT2 inhibitors in myocardial infarction can be explained by several mechanisms, as shown in Figure 1. First, it has been postulated that the use of gliflozins may optimize myocardial energetics by switching metabolism away from glucose in favour of ketone bodies; ketones, often considered a ‘super-fuel’, are associated with improved cardiac function and reduced adverse cardiac remodelling.7 Notably, the heart lacks specific SGLT2 receptors under normal conditions; however, SGLT2 receptors are transiently expressed in ischaemic myocardial tissue, allowing SGLT inhibitors to directly affect cardiomyocyte metabolism and attenuate infarct size.9 In addition, SGLT2 inhibitors have been shown to directly inhibit the cardiomyocyte Na+/H+ exchanger 1 (NHE1), whose activity is responsible for autophagic cell death. Inhibition of NHE1 has been shown to be cardioprotective by decreasing intracellular calcium and sodium levels while concurrently increasing mitochondrial calcium levels.10 In terms of cardiac remodelling, SGLT2 inhibitors exhibit remarkable anti-fibrotic properties by increasing the activation of M2 macrophages and suppressing collagen synthesis.11 Finally, anti-inflammatory properties and improved endothelial function induced by SGLT2 inhibitors may mitigate the development and progression of coronary artery disease.
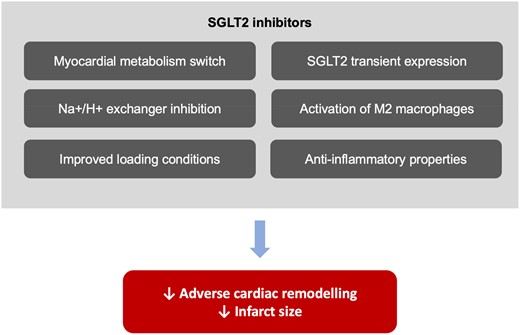
Proposed cardio-protective mechanism of sodium–glucose co-transporter 2 inhibitors in patients with acute myocardial infarction. SGLT2, sodium–glucose co-transporter 2.
In addition to these molecular effects, SGLT2 inhibitors have been shown to improve several cardiometabolic parameters. These include reducing hyperglycaemia, promoting weight loss, and lowering blood pressure.12 Together, these favourable effects may contribute to an improved cardiovascular profile in patients with AMI. In addition, gliflozins may improve ventricular loading conditions due to their diuretic and natriuretic effects. Interestingly, SGLT2 inhibitors show a selective reduction in interstitial fluid, thereby limiting the neurohumoral stimulation that may occur as a consequence of loop diuretic use and intravascular volume contraction.13
Available evidence
Despite these intriguing pathophysiological mechanisms observed in animal models, only a limited number of studies have investigated the effects of SGLT inhibitors in patients with AMI. Recently, new evidence has been published regarding two molecules currently used in HF, empagliflozin and dapagliflozin (Table 1).
Available evidence and ongoing trials on sodium–glucose co-transporter 2 inhibitors in acute myocardial infarction
| . | EMMY (2022)14 . | DAPA-MI (2023)15 . | EMPACT-MI16 . |
|---|---|---|---|
| Design | RCT | Registry-based RCT | RCT |
| Intervention | Empagliflozin 10 mg | Dapagliflozin 10 mg | Empagliflozin 10 mg |
| Number of patients | 476 | 4017 | 6522 |
| Study population | AMI | AMI | AMI |
| Additional features | CK > 800 IU/L and troponin > 10×ULN | New Q waves on ECG New LV dysfunction | New LVEF < 45% and/or signs/symptoms of congestion |
| Primary endpoint | Changes in NT-pro-BNP at 6 months | Death, hospitalization for HF, nonfatal AMI, atrial fibrillation/flutter, type 2 diabetes mellitus, NYHA class, body weight decrease | All-cause mortality and time to first HF hospitalization |
| . | EMMY (2022)14 . | DAPA-MI (2023)15 . | EMPACT-MI16 . |
|---|---|---|---|
| Design | RCT | Registry-based RCT | RCT |
| Intervention | Empagliflozin 10 mg | Dapagliflozin 10 mg | Empagliflozin 10 mg |
| Number of patients | 476 | 4017 | 6522 |
| Study population | AMI | AMI | AMI |
| Additional features | CK > 800 IU/L and troponin > 10×ULN | New Q waves on ECG New LV dysfunction | New LVEF < 45% and/or signs/symptoms of congestion |
| Primary endpoint | Changes in NT-pro-BNP at 6 months | Death, hospitalization for HF, nonfatal AMI, atrial fibrillation/flutter, type 2 diabetes mellitus, NYHA class, body weight decrease | All-cause mortality and time to first HF hospitalization |
AMI, acute myocardial infarction; CK, creatine kinase; ECG, electrocardiogram; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro-BNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RCT, randomized controlled trial.
Available evidence and ongoing trials on sodium–glucose co-transporter 2 inhibitors in acute myocardial infarction
| . | EMMY (2022)14 . | DAPA-MI (2023)15 . | EMPACT-MI16 . |
|---|---|---|---|
| Design | RCT | Registry-based RCT | RCT |
| Intervention | Empagliflozin 10 mg | Dapagliflozin 10 mg | Empagliflozin 10 mg |
| Number of patients | 476 | 4017 | 6522 |
| Study population | AMI | AMI | AMI |
| Additional features | CK > 800 IU/L and troponin > 10×ULN | New Q waves on ECG New LV dysfunction | New LVEF < 45% and/or signs/symptoms of congestion |
| Primary endpoint | Changes in NT-pro-BNP at 6 months | Death, hospitalization for HF, nonfatal AMI, atrial fibrillation/flutter, type 2 diabetes mellitus, NYHA class, body weight decrease | All-cause mortality and time to first HF hospitalization |
| . | EMMY (2022)14 . | DAPA-MI (2023)15 . | EMPACT-MI16 . |
|---|---|---|---|
| Design | RCT | Registry-based RCT | RCT |
| Intervention | Empagliflozin 10 mg | Dapagliflozin 10 mg | Empagliflozin 10 mg |
| Number of patients | 476 | 4017 | 6522 |
| Study population | AMI | AMI | AMI |
| Additional features | CK > 800 IU/L and troponin > 10×ULN | New Q waves on ECG New LV dysfunction | New LVEF < 45% and/or signs/symptoms of congestion |
| Primary endpoint | Changes in NT-pro-BNP at 6 months | Death, hospitalization for HF, nonfatal AMI, atrial fibrillation/flutter, type 2 diabetes mellitus, NYHA class, body weight decrease | All-cause mortality and time to first HF hospitalization |
AMI, acute myocardial infarction; CK, creatine kinase; ECG, electrocardiogram; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro-BNP, N terminal pro brain natriuretic peptide; NYHA, New York Heart Association; RCT, randomized controlled trial.
The EMpagliflozin in acute MYocardial infarction (EMMY) study is a randomized, double-blind, controlled trial published in August 2022.14 The study enrolled 476 patients with AMI who were randomized to receive empagliflozin 10 mg daily or placebo within 72 h of percutaneous coronary intervention. The primary endpoint was a change of N-terminal pro-hormone of brain natriuretic peptide (NT-pro-BNP) over 26 weeks of follow-up, while the secondary endpoints were echocardiographic changes in left ventricular ejection fraction (LVEF), left ventricular end-systolic and end-diastolic volumes, and parameters of diastolic dysfunction. The results showed that early initiation of empagliflozin on top of guideline-recommended post-AMI therapy led to a greater reduction in NT-pro-BNP levels compared with placebo; moreover, the empagliflozin group showed significant improvements in functional and structural echocardiographic parameters, with a significant increase of LVEF observed as early as 6 weeks of follow-up. Of note, only a small number of patients in the EMMY population had type 2 diabetes (30 patients in the empagliflozin group and 33 patients in the placebo group). Although the EMMY trial was not powered for hard clinical endpoints, these findings suggest a potential benefit in terms of both biochemical and echocardiographic improvements.
Regarding the use of dapagliflozin, the results of the Dapagliflozin Effects on Cardiometabolic Outcomes in Patients With an Acute Heart Attack (DAPA-MI) study were recently published.15 This international, registry-based, randomized, double-blind placebo-control trial enrolled 4017 patients within 7–10 days of an AMI complicated by impaired regional or global left ventricular systolic dysfunction or new Q waves on the electrocardiogram to receive dapagliflozin 10 mg daily or placebo. Patients who were already eligible to receive SGLT2 inhibitors prior to the event due to other indications, such as diabetes mellitus or HF, were excluded to avoid confounding factors. The primary outcome was originally a composite of cardiovascular death and hospitalization for HF. However, due to a small number of events during the follow-up, the primary outcome was modified into a hierarchical composite of death, hospitalization for HF, non-fatal AMI, atrial fibrillation/flutter, new-onset type 2 diabetes mellitus, New York Heart Association functional class at the last visit, and body weight loss of 5% or more at the last visit. Statistical analysis employed a win ratio analysis method. The primary hierarchical composite outcome was 32.9% wins for dapagliflozin and 24.6% wins for placebo, but the benefit was mainly driven by the cardiometabolic components, particularly the new diagnosis of type 2 diabetes; rates of the composite of time to cardiovascular death/hospitalization for HF were similar in both treatment and placebo groups.
Ongoing trials
Despite the results of these trials, the role of SGLT2i in the setting of AMI is not yet fully defined and there is still an unmet need for new evidence in this scientific scenario. The EMPAgliflozin on Hospitalization for Heart Failure and Mortality in Patients With aCuTe Myocardial Infarction (EMPACT-MI) trial may provide further information.16 In this multinational, randomized, double-blind, placebo-controlled trial 6522 patients with AMI were randomized to receive empagliflozin 10 mg or placebo in addition to standard of care within 14 days of hospital admission.17 Patients were eligible for enrolment if AMI was complicated by acute signs or symptoms of congestion and/or left ventricular dysfunction (LVEF < 45%). Data analysis is ongoing, and the primary composite endpoint will be time to the first hospitalization for HF or all-cause mortality.
Conclusions
Sodium–glucose co-transporter 2 inhibitors, traditionally known for the management of type 2 diabetes, have emerged as agents with cardiovascular benefits, particularly in HF patients. Our manuscript highlights the potential of SGLT2 inhibitors as a therapeutic possibility for AMI. Although the available evidence is limited, there are promising results from recent trials. The EMMY trial with empagliflozin showed a reduction in NT-pro-BNP levels and improvements in echocardiographic parameters, suggesting potential benefits in biochemical and structural benefits; the DAPA-MI trial with dapagliflozin showed an advantage in the composite outcome, mainly driven by cardiometabolic components. In conclusion, although the pathophysiological mechanisms and initial trial results are promising, more extensive and definitive evidence is needed to establish the role of SGLT2 inhibitors in the treatment of AMI. Ongoing trials, such as the EMPACT-MI, are critical to filling the knowledge gaps and determining the potential benefits of SGLT2 inhibitors in improving outcomes for patients with AMI.
Funding
No funding provided.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: none declared.



