-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Marini, Sara Belleggia, Leonardo Brugiatelli, Matteo Francioni, Ilaria Battistoni, Matilda Shkoza, Giulia Pongetti, Luca Angelini, Leonardo Belfioretti, Maria Vittoria Matassini, The golden hour in shock management: do a lot, do it quickly, European Heart Journal Supplements, Volume 26, Issue Supplement_1, April 2024, Pages i78–i83, https://doi.org/10.1093/eurheartjsupp/suae023
Close - Share Icon Share
Abstract
Cardiogenic shock can be defined as a state of inadequate organ perfusion linked primarily to cardiac pump dysfunction. The two predominant causes of this condition are acute myocardial infarction and acutely decompensated heart failure (ADHF). In recent years, a significant increase in cases of cardiogenic shock from ADHF has been described. Recent evidence has defined that the factors with the greatest impact on the prognosis in this context are the early clinical assessment, the definition of the aetiology, the timely application of pharmacological therapies, or individualized mechanical supports for the circulation. Haemodynamic monitoring can help in the phenotyping of cardiogenic shock and therefore guide therapeutic choices, especially if implemented with the aid of advanced monitoring tools such as the Swan–Ganz catheter. Finally, the presence of a dedicated shock team in the ‘hub’ centres is fundamental, which facilitates the choice of the best therapeutic strategy on a case-by-case basis.
Introduction
Cardiogenic shock is defined as a state of inadequate organ perfusion due primarily to cardiac pump dysfunction.
Recent epidemiological data show a reduction in the incidence of cardiogenic shock caused by coronary syndrome in favour of cases linked to other causes, and in particular to acutely decompensated heart failure (ADHF).1
Despite numerous advances in reperfusion and circulation support therapy, mortality remains high, with a range from 25% to 70% depending on the case series.2
The definitions of cardiogenic shock used in clinical trials or guidelines are various, classically based on the presence of hypotension (systolic blood pressure less than 90 mmHg or mean arterial pressure less than 65 mmHg) in the presence of signs of organ hypo-perfusion such as oligo-anuria, increase in lactate, and alteration of mental status. Haemodynamic parameters such as cardiac index less than 2.2 mL/min/m2 and pulmonary wedge pressure greater than 15 mmHg have sometimes been used in the definition of cardiogenic shock together with other indices such as cardiac power output (CPO). (Table 1).
| . | SHOCK trial . | IABP-SHOCK II . | European Society of Cardiology guidelines (2021) . |
|---|---|---|---|
| Definition of Shock | —Clinical criteria: Myocardial infarction complicated by left ventricular dysfunction Systolic pressure < 90 mmHg for >30 min or support to maintain systolic pressure > 90 mmHg and end-organ hypo-perfusion (urine output < 30 mL/h or cold extremities) —Haemodynamic criteria: Cardiac index < 2.2 L/min/m2 and pulmonary capillary pressure > 15 mmHg | −Clinical criteria: Acute myocardial infarction Systolic blood pressure < 90 mmHg for >30 min or catecholamines to maintain systolic blood pressure > 90 mmHg and clinical pulmonary congestion and impaired end-organ perfusion (altered mental status, cold/clammy skin on limbs, urine output < 30 mL/h, or lactate > 2.0 mmol/L) | −Clinical criteria: Systolic blood pressure < 90 mmHg with adequate volume and clinical or laboratory signs of hypo-perfusion. —Clinical hypo-perfusion: Cold extremities, oliguria, mental confusion, dizziness, reduced pulse pressure —Laboratory hypo-perfusion: Metabolic acidosis, elevated serum lactate, elevated serum creatinine |
| . | SHOCK trial . | IABP-SHOCK II . | European Society of Cardiology guidelines (2021) . |
|---|---|---|---|
| Definition of Shock | —Clinical criteria: Myocardial infarction complicated by left ventricular dysfunction Systolic pressure < 90 mmHg for >30 min or support to maintain systolic pressure > 90 mmHg and end-organ hypo-perfusion (urine output < 30 mL/h or cold extremities) —Haemodynamic criteria: Cardiac index < 2.2 L/min/m2 and pulmonary capillary pressure > 15 mmHg | −Clinical criteria: Acute myocardial infarction Systolic blood pressure < 90 mmHg for >30 min or catecholamines to maintain systolic blood pressure > 90 mmHg and clinical pulmonary congestion and impaired end-organ perfusion (altered mental status, cold/clammy skin on limbs, urine output < 30 mL/h, or lactate > 2.0 mmol/L) | −Clinical criteria: Systolic blood pressure < 90 mmHg with adequate volume and clinical or laboratory signs of hypo-perfusion. —Clinical hypo-perfusion: Cold extremities, oliguria, mental confusion, dizziness, reduced pulse pressure —Laboratory hypo-perfusion: Metabolic acidosis, elevated serum lactate, elevated serum creatinine |
| . | SHOCK trial . | IABP-SHOCK II . | European Society of Cardiology guidelines (2021) . |
|---|---|---|---|
| Definition of Shock | —Clinical criteria: Myocardial infarction complicated by left ventricular dysfunction Systolic pressure < 90 mmHg for >30 min or support to maintain systolic pressure > 90 mmHg and end-organ hypo-perfusion (urine output < 30 mL/h or cold extremities) —Haemodynamic criteria: Cardiac index < 2.2 L/min/m2 and pulmonary capillary pressure > 15 mmHg | −Clinical criteria: Acute myocardial infarction Systolic blood pressure < 90 mmHg for >30 min or catecholamines to maintain systolic blood pressure > 90 mmHg and clinical pulmonary congestion and impaired end-organ perfusion (altered mental status, cold/clammy skin on limbs, urine output < 30 mL/h, or lactate > 2.0 mmol/L) | −Clinical criteria: Systolic blood pressure < 90 mmHg with adequate volume and clinical or laboratory signs of hypo-perfusion. —Clinical hypo-perfusion: Cold extremities, oliguria, mental confusion, dizziness, reduced pulse pressure —Laboratory hypo-perfusion: Metabolic acidosis, elevated serum lactate, elevated serum creatinine |
| . | SHOCK trial . | IABP-SHOCK II . | European Society of Cardiology guidelines (2021) . |
|---|---|---|---|
| Definition of Shock | —Clinical criteria: Myocardial infarction complicated by left ventricular dysfunction Systolic pressure < 90 mmHg for >30 min or support to maintain systolic pressure > 90 mmHg and end-organ hypo-perfusion (urine output < 30 mL/h or cold extremities) —Haemodynamic criteria: Cardiac index < 2.2 L/min/m2 and pulmonary capillary pressure > 15 mmHg | −Clinical criteria: Acute myocardial infarction Systolic blood pressure < 90 mmHg for >30 min or catecholamines to maintain systolic blood pressure > 90 mmHg and clinical pulmonary congestion and impaired end-organ perfusion (altered mental status, cold/clammy skin on limbs, urine output < 30 mL/h, or lactate > 2.0 mmol/L) | −Clinical criteria: Systolic blood pressure < 90 mmHg with adequate volume and clinical or laboratory signs of hypo-perfusion. —Clinical hypo-perfusion: Cold extremities, oliguria, mental confusion, dizziness, reduced pulse pressure —Laboratory hypo-perfusion: Metabolic acidosis, elevated serum lactate, elevated serum creatinine |
It is important to underline that cardiogenic shock can present with hypo-perfusion even in the absence of hypotension (due to a compensatory mechanism of increase in peripheral resistance), outlining a phenotype with a greater risk of mortality compared with a picture of hypotension alone without signs of hypoperfusion.3
In 2019, the Society for Cardiovascular Angiography and Interventions (SCAI) proposed a classification of severity of cardiogenic shock that well summarizes the dynamic and progressive nature of this syndrome, categorizing patients into a spectrum of five stages (Table 2).
Society for Cardiovascular Angiography and Interventions classification revised 2022
| A- At risk . | Beginning . | Classic . | Deteriorating . | E-Extremis . |
|---|---|---|---|---|
| Haemodynamically stable | Hypotension or hypo-perfusion, without therapy | Hypotension and hypo-perfusion or with therapy | Failure of the initial stabilization attempt | Refractory shock |
| —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg OR —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices | —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices OR 1 drug or 1 device without hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 5–10 mmol/L —ALT > 500 U/L OR —2–5 drugs/device OR 1 drug or 1 device with hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: >10 mmol/L —PH < 7.2 OR More than 3 medications/devices OR Out-of-hospital cardiac arrest |
| A- At risk . | Beginning . | Classic . | Deteriorating . | E-Extremis . |
|---|---|---|---|---|
| Haemodynamically stable | Hypotension or hypo-perfusion, without therapy | Hypotension and hypo-perfusion or with therapy | Failure of the initial stabilization attempt | Refractory shock |
| —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg OR —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices | —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices OR 1 drug or 1 device without hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 5–10 mmol/L —ALT > 500 U/L OR —2–5 drugs/device OR 1 drug or 1 device with hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: >10 mmol/L —PH < 7.2 OR More than 3 medications/devices OR Out-of-hospital cardiac arrest |
Society for Cardiovascular Angiography and Interventions classification revised 2022
| A- At risk . | Beginning . | Classic . | Deteriorating . | E-Extremis . |
|---|---|---|---|---|
| Haemodynamically stable | Hypotension or hypo-perfusion, without therapy | Hypotension and hypo-perfusion or with therapy | Failure of the initial stabilization attempt | Refractory shock |
| —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg OR —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices | —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices OR 1 drug or 1 device without hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 5–10 mmol/L —ALT > 500 U/L OR —2–5 drugs/device OR 1 drug or 1 device with hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: >10 mmol/L —PH < 7.2 OR More than 3 medications/devices OR Out-of-hospital cardiac arrest |
| A- At risk . | Beginning . | Classic . | Deteriorating . | E-Extremis . |
|---|---|---|---|---|
| Haemodynamically stable | Hypotension or hypo-perfusion, without therapy | Hypotension and hypo-perfusion or with therapy | Failure of the initial stabilization attempt | Refractory shock |
| —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg OR —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices | —Systolic blood pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 2–5 mmol/L —ALT 200–500 U/L AND —No drugs —No devices OR 1 drug or 1 device without hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: 5–10 mmol/L —ALT > 500 U/L OR —2–5 drugs/device OR 1 drug or 1 device with hypotension or hypo-perfusion | Systolic pressure < 90 mmHg/mean arterial pressure < 65 mmHg AND —Lactates: >10 mmol/L —PH < 7.2 OR More than 3 medications/devices OR Out-of-hospital cardiac arrest |
Numerous subsequent studies have demonstrated the correlation between SCAI staging and in-hospital mortality, also highlighting a worse prognosis for the same stage in patients with myocardial infarction compared with exacerbation of heart failure.4
At a pathophysiological level, cardiogenic shock begins as a haemodynamic alteration characterized by a reduced cardiac output (CO) with consequent multi-organ systemic hypo-perfusion, which in turn transforms the shock into a haemometabolic condition, triggering a negative spiral that proves fatal in a high percentage of cases.
Evaluation and treatment protocols for cardiogenic shock should require an approach similar to that used for ST-segment elevation myocardial infarction where early diagnosis and immediate activation of a rescue network with rapid revascularization times have significantly reduced mortality.
Cardiogenic shock, therefore, represents a time-dependent critical condition that requires a targeted therapeutic planning strategy from the first clinical contact, defining a short period of time as the ‘golden hour’ for classification and initial management.5
In fact, like the much better known ‘door to balloon’, timely approaches of mechanical support to the circle (‘door to support’) are also gaining evidence in the literature.
Aetiology
Identification of the cause underlying the clinical presentation is of crucial importance to guide the therapeutic choice with obvious prognostic implications.
Numerous studies have highlighted notable differences in terms of haemodynamic profile, therapeutic response, and prognosis among cardiogenic shock secondary to myocardial infarction compared with that due to exacerbation of chronic heart failure rather than from acute de novo decompensation.
As suggested by European guidelines, it may be useful to follow the algorithm defined by the acronym CHAMPIT (acute Coronary syndrome, Hypertensive emergency, Arrhythmia, Mechanical causes, Pulmonary embolism, Infections, cardiac Tamponade) to guide the initial etiological diagnosis.6
Initial diagnosis
First-line diagnostic tests to be performed upon patient arrival include 12-lead electrocardiogram, ultrasound, blood gas analysis, and blood chemistry tests.
The electrocardiogram allows the identification of tachycardia, bradycardia, and myocardial infarction with or without ST-segment elevation, allowing the immediate activation of the catheterization laboratory.
In an emergency, a point-of-care) ultrasound study is recommended, aimed at evaluating biventricular cardiac contractility, verifying the presence of congestion (pulmonary B lines, collapsibility of the inferior vena cava, E/e′ ratio, and velocity time integral aortic variability) during passive leg raising, venous excess ultrasound score, and identifying the causes of cardiogenic shock (Takotsubo, valvular disease, decompensated heart failure, heart attack, etc.).
Thorough monitoring of the blood pressure curve should be quickly initiated in order to obtain continuous control of the variability of the blood pressure pulse (possible indirect index of response to fluids) as well as facilitating frequent blood chemistry and blood gas analysis.
The dosage of lactate as a marker of hypo-perfusion and tissue hypoxia is strictly recommended with particular attention to lactate clearance, which correlates with the prognosis more than the single initial value (normality cut-off 2 mmol/L, while the clearance value is prognostically favourable if the second blood lactate level measured 8 h after the first is less than 3.1 mmol/L).7
Blood chemistry tests including troponin, renal, and hepatic function are useful in further discriminating the genesis of shock and for the SCAI severity classification.
Also desirable and necessary is the positioning of a central venous catheter useful for continuous monitoring of the CVP (central venous pressure, approximate index of volume status, and pre-load) and for the evaluation of SvO2 (central venous saturation, target > 65%) index of occult hypo-perfusion in the absence of lactate elevation. Furthermore, the evaluation of delta CO2 (difference between arterial and venous CO2 from central venous access) is also an expression of early and sensitive tissue hypo-perfusion even in conditions in which SvCO2 is less sensitive (e.g. in septic or mixed shock).
Haemodynamic parameters
Despite the reduction in its use in light of the lack of robust scientific evidence, as the SCAI severity class increases, the availability of additional parameters such as CO, CPO, pulmonary artery pulsatility (PAPi) index, and vascular resistance, derived from the insertion of a Swan–Ganz catheter, is important in order to identify a latent right dysfunction and/or characterize the possible evolution towards an inflammatory/septic state. This strategy allows the orientation and ‘customization’ of the vasoactive therapeutic choice, both pharmacological and mechanical.8
The CPO and cardiac power index (CPI) represent the hydraulic energy indices of the heart pump based on the physical principle according to which power = flow × pressure. In the shock trial, values of CPO < 0.53 and CPI < 0.33 were shown to strongly correlate with in-hospital mortality in patients in cardiogenic shock and this index is the main ‘haemodynamic’ prognostic factor in patients with cardiogenic shock.9
Therapy
The therapy of cardiogenic shock is based on two fundamental cornerstones: the treatment of the underlying cause (e.g. myocardial revascularization in case of acute infarction) and the supportive therapy aimed at improving perfusion and oxygenation through the use of vasoactive drugs and mechanical support devices for the circulation.
Fluid administration with boluses of saline or Ringer's lactate (250 mL over 15–10 min) should be considered in non-congestive patients with a pre-load-dependent haemodynamic profile.
The type of ventilatory support (non-invasive vs. invasive) for the patient should be assessed upon arrival based on the clinical presentation and blood gas analysis data, also taking into consideration the haemodynamic benefit given by the reduction of the afterload of positive pressure ventilation.
As is well known, the use of vasoactive drugs (inotropes, vasopressors, and inodilators) is associated with an increase in in-hospital morbidity and mortality through mechanisms of increased cardiac work, oxygen consumption, and arrhythmic inducibility. Therefore, their administration should be individualized based on the patient's haemodynamic needs, for the shortest possible time, at the minimum sufficient dosage and taking into account the prevailing haemodynamic profile.
The European Society of Cardiology guidelines recommend the use of Class IIb/B norepinephrine as a first-line vasopressor.6
In the OPTIMA CC (Study Comparing the Efficacy and Tolerability of Epinephrine and Norepinephrine in Cardiogenic Shock) comparison study, epinephrine showed a higher incidence of lactic acidosis, higher heart rate values (greater oxygen consumption), and mortality, even though used at very high doses.
Inotropes may be considered adjunctively to improve CO and organ perfusion (Class IIb/C).6
There are no significant differences between levosimendan, milrinone, and dobutamine, with preference for the latter in conditions of impaired renal function and generally for levosimendan, as also highlighted in a recent Cochrane meta-analysis.10
Vasopressin can have a role in case of hypotension refractory to therapy or in case of right-sided decompensation due to its selectivity of action on the systemic circulation, not impacting on pulmonary vascular resistance. Likewise, in case of prevalent right ventricular dysfunction, dobutamine, levosimendan, or milrinone/enoximone is preferable, especially in patients on beta-blocker therapy.
In case of cardiogenic shock due to acute myocardial infarction, emergency revascularization is recommended as supported by the evidence deriving from the 1999 SHOCK trial (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock), which demonstrated a lower 6-month mortality in the group subjected to revascularization earlier compared with the medical therapy group (50% vs. 63%, P = 0.027).11
Patients presenting with extreme cardiogenic shock, deteriorating, or not haemodynamically stabilized with two vasoactive agents may benefit from mechanical circulatory support in an individualized manner (Class IIa/C recommendation).6
Choices for left ventricular support include the aortic balloon pump [intra-aortic balloon pump (IABP)] and microaxial flow pumps [Impella cardiac power (CP), Impella 5–5.5]. Right ventricular assist systems include Impella RP and Tandem-Heart right atrium–pulmonary artery devices and Protek Duo.
Finally, to improve organ perfusion in cases of severe biventricular dysfunction and concomitant Acute Respiratory Distress Syndrome, the veno-arterial extracorporeal membrane oxygenation (ECMO) is available.
The use of IABP has decreased over time due to the lack of survival benefits in the IABP-SHOCK (Intra-Aortic Balloon Pump in Cardiogenic Shock) trial; note that 86% of patients had this device implanted after the procedure of angioplasty.12
It is currently considered for patients in refractory shock not due to myocardial infarction (Class IIb/C) or for patients with cardiogenic shock from myocardial infarction in the presence of mechanical complications, as a bridge to more advanced supports (Class IIa/C).6
Although Impella appears promising, little data are available regarding its beneficial effect on mortality. In the IMPRESS trial (IMPella vs. IABP reduces mortality in ST-elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention in severe cardiogenic SHOCK), 48 patients were randomized to Impella CP or IABP to undergo primary angioplasty for STEMI and cardiogenic shock. The results of this trial indicated that vascular complications were greater in the Impella group, with no significant differences in survival between the two groups.
Similarly, ECMO could improve haemodynamic stability during cardiopulmonary resuscitation and also increase the afterload of the left ventricle, making its use reasonable when associated with devices that allow ‘unloading’ of the left ventricle (e.g. IABP, Impella, septostomy, and hybrid circuit configurations). However, the data provided by the ECLS-SHOCK (Extra Corporeal Life Support in Infarct-Related Cardiogenic shock) and ECMO-CM (Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock) trials presented recently were not satisfactory, albeit with limitations due to the small sample size and the general severity of these patients.
Devices such as Impella and ECMO require the insertion of large calibre cannulae into the main vessels and carry with them a high risk of complications, including access site complications and bleeding.
In cardiogenic shock, the rate of serious bleeding from placement of IMPELLA varies in the literature from 8.5% to 31%; therefore, the use of this device should take place in selected patients (pre-implantation evaluation of vascular access) with the supervision of a team of experts.
The availability of devices that can be implanted in the medium term via the transaxillary route such as IMPELLA 5.0 and 5.5 also allows the clinical picture to be stabilized to the point of allowing active mobilization of the patient (biking/walking), a result of great importance both for weaning from support and during bridge towards definitive therapy with left ventricular assist device or cardiac transplant.
There are little data available in the literature regarding the use of the aforementioned mechanical supports (both univentricular and biventricular types) in the early stages of cardiogenic shock, even before the administration of inotropes or myocardial revascularization.
The ‘Detroit cardiogenic shock initiative’ is a single-arm, multi-centre pilot study that evaluated the feasibility of early application of mechanical supports to the circulation in a small group of patients with cardiogenic shock (n = 41), with good results in terms of mortality compared with the ‘historical’ control cohort (85% vs. 51%, P < 0.001), and with a 67% increase in CPO following the index procedure.13
The Altshock-2 study (early IABP in acute decompensated heart failure complicated by cardiogenic shock) is underway in Italy, a prospective, randomized, multi-centre, open-label trial with blinded outcome evaluation, in which 200 patients with cardiogenic shock due to ADHF will be randomized to early implantation of an IABP or vasoactive treatments.
The possible evidence-based implementation of early mechanical support for the circulation in the management of patients with cardiogenic shock could lead to an increase in survival by supporting the principle of ‘door to support’ interventions, aimed at anticipating the deleterious effects of the negative spiral of cardiogenic shock.
Shock teams and networks
The variety of presentation of cardiogenic shock, the severity and potential causes, the absence of strong evidence for the proposed treatments (e.g. mechanical supports), and the need for personalized therapies make decision-making even more complex and the presence of an essential multi-disciplinary team.
The introduction of the ‘hub-and-spoke’ model has demonstrated positive effects on the outcomes of care in a ‘real-life’ setting.14
The ‘hub’ hospital is equipped with a multi-disciplinary team made up of an interventional cardiologist, emergency specialist, cardiac surgeon, and specialist in advanced heart failure.
‘Spoke’ hospitals include centres equipped with catheterization laboratory without the availability of advanced circulation supports or hospitals not equipped with catheterization laboratory, both referring to the ‘hub’ centre.
The hub centre should be equipped with a shock team that provides adequate information to the spoke centres regarding the need for escalation of treatments, the need for advanced support, the right catheterization for the choice of appropriate support, adequate perioperative management and monitoring, and possible weaning from supports.
The implementation of regional protocols regarding shock both due to myocardial infarction (acute myocardial infarction) and acute-on-chronic decompensation (ADHF) was found to be both feasible and associated with improved survival.
A multi-centre observational study by Papolos A. et al. that compared intensive care units with or without shock teams evaluated how the presence of a shock team was associated with more extensive use of right catheterization and reduced administration of vasoactive agents. Centres with a shock team used fewer mechanical supports but more often advanced supports (e.g. IMPELLA and ECMO).
In this model, the emergency room had a strategic role as it was involved in the early identification of patients with cardiogenic shock, initial stabilization, and triage to the appropriate type of management.
Conclusions
Cardiogenic shock is a complex syndrome burdened by high mortality.
Its management should include a protocol that is as standardized as possible and includes a team of expert multidisciplinary professionals.
Among all the variables to consider, it is clear how crucial a timely clinical framework is in activating the alarm code that can be translated into a management strategy equivalent to the ‘golden hour’ of myocardial infarction (e.g. such as the one proposed in Figure 1).
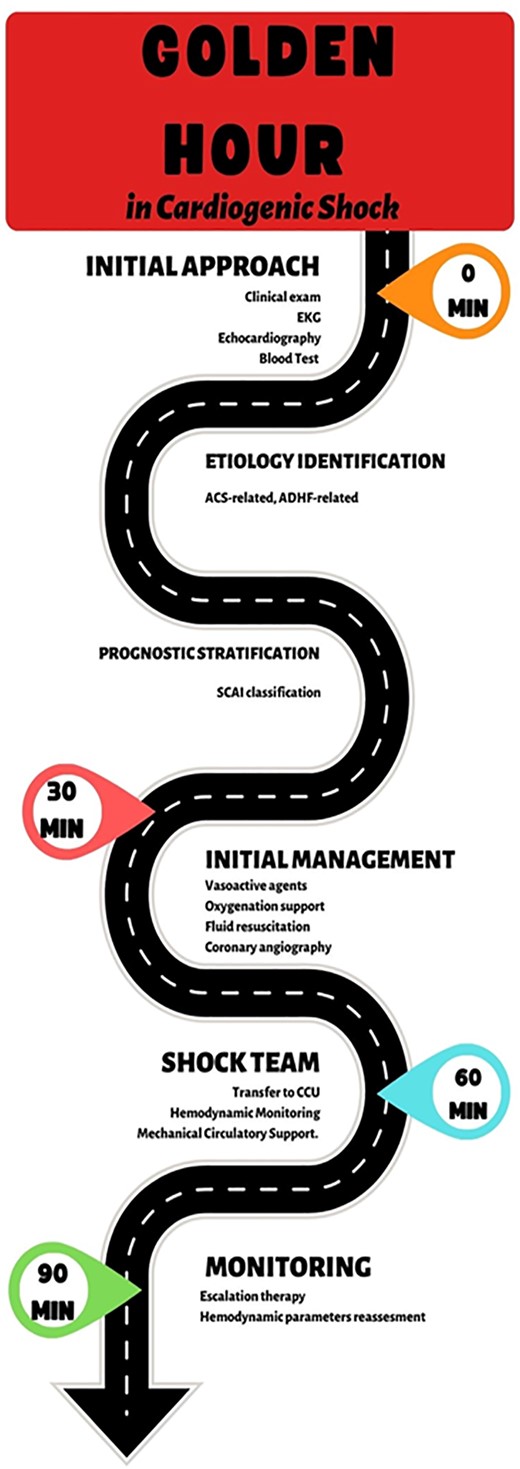
Modified from Polyzogopoulou E., Bezati S., Karamasis G., Boultadakis A., Parissis J. Early Recognition and Risk Stratification in Cardiogenic Shock: Well Begun Is Half Done. J Clin Med. 2023 Apr 1; 12(7):2643.
Further studies will be necessary to support with solid evidence the principle of ‘door to support’ circle of assistance interventions, aimed at anticipating the deleterious effects of the negative spiral of cardiogenic shock.
Funding
No funding provided.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: none declared.



