-
PDF
- Split View
-
Views
-
Cite
Cite
Leonardo Calo', Mario Tatangelo, Germana Panattoni, Cinzia Crescenzi, Marianna Squeglia, Francesca Fanisio, Fabiana Romeo, Federica Toto, Ermenegildo de Ruvo, Marco Rebecchi, Unlocking the enigma: decoding premature ventricular complexes for effective clinical assessment and risk management, European Heart Journal Supplements, Volume 26, Issue Supplement_1, April 2024, Pages i23–i28, https://doi.org/10.1093/eurheartjsupp/suae006
Close - Share Icon Share
Abstract
The identification of ventricular premature complexes during a cardiological evaluation necessitates the implementation of diagnostic processes aimed at discerning the clinical context that may predispose individuals to a high risk of sudden cardiac death. Epidemiological studies reveal that ventricular premature beats occur in approximately 75% of healthy (or seemingly healthy) individuals, as long as there is no evidence of underlying structural heart disease, such as benign idiopathic ventricular extrasystole originating from the right and left ventricular outflow tracts. In the real world, however, ventricular ectopic beats with morphologies very similar to seemingly benign occurrences are not uncommon. They are notable in subjects exhibiting rapid and complex repetitive forms during exercise testing and Holter electrocardiogram. Additionally, these subjects may display more or less extensive scarring signs on cardiac magnetic resonance and may have a family history of cardiomyopathy and/or sudden cardiac death. Therefore, the purpose of this review is to critically analyse the process of evaluating premature ventricular complexes, which is crucial for accurate risk stratification. The latter cannot overlook some inevitable elements, including morphology, origin, complexity, and the associated clinical setting (absence or presence of structural heart disease).
Introduction
Premature ventricular complexes (PVCs) are commonly found in up to 75% of healthy individuals and are generally considered harmless in the absence of structural heart disease (SHD). However, PVCs can occasionally indicate an underlying SHD and may be associated with an increased risk of sudden cardiac death (SCD).1 For this reason, the presence of PVCs often presents an insidious challenge in clinical evaluation and risk stratification.
The most common form of idiopathic and benign PVCs is characterized by a left bundle branch block (LBBB) with inferior axis morphology, indicating a right ventricular outflow tract (RVOT) origin. However, in some cases, these PVCs may be an expression of underlying arrhythmogenic right ventricular cardiomyopathy (ARVC).2 Recent studies have highlighted that PVCs with right bundle branch block (RBBB) morphology, particularly those with an intermediate/superior axis in the frontal plane, are more often associated with evidence of left ventricular (LV) scar at cardiac magnetic resonance (CMR) than other right or left morphologies.3,4
This review focuses on the characteristics of premature ventricular contractions in terms of morphology, distribution, complexity, and response to exercise. It describes the possible underlying myocardial substrates and critically analyses the evaluation process of PVCs necessary for accurate risk stratification. The diagnosis, management, and clinical disorders associated with PVCs will be presented in this review.
Diagnostic workup
As one of the most common types of cardiac arrhythmias, PVCs are typically identified during electrocardiographic examinations. Symptoms may include palpitations, pre-syncope, dyspnoea, and fatigue. Investigating the cause of PVCs is crucial to identify potential impacts on the patient’s overall health and to optimize treatment.
Family history is important to clarify potential hereditary disorders associated with PVCs and the risk of sudden death. Personal and physiological history is also essential to identify potential causes of PVC and address them. Factors such as the use of stimulants, hormonal changes, and the presence of stress, insomnia, and gastric diseases can be easily treatable causes.
The 12-lead electrocardiogram (ECG) is useful for providing initial evidence of PVC frequency and remains the best non-invasive tool for determining the location of PVC origin. An exercise test (ET) is advisable for all patients with PVCs; their behaviour during exercise could be a predictor of SHD, although it may not replicate a specific trigger mechanism for arrhythmia. For this purpose, 24-hour Holter monitoring is useful in evaluating both PVC frequency and its causal relationship with specific situations in the patient’s life (such as the specific sport practiced, situations of emotional stress, and those related to digestion).
In general, a single wearable ECG patch is probably sufficient for evaluating frequency (preferably over 48 or 72 h), while a 12-lead ambulatory monitoring is a fundamental aid when multiple origins of PVCs are suspected or in patients with channelopathies. Ambulatory monitoring is also useful for correlating the patient’s symptoms with (or without) the appearance of PVCs.
Echocardiography is indicated for almost all individuals presenting with PVCs to exclude SHD, particularly reduced LV systolic function (LVEF), and other underlying pathologies that may contribute to the genesis of PVCs or make them more symptomatic. Cardiac magnetic resonance should be considered when the PVC does not originate from a common site, when polymorphic morphologies and the presence of couplets and runs of non-sustained or sustained ventricular tachycardia (NSVT) are present, or when reduced LVEF is observed (Figure 1).
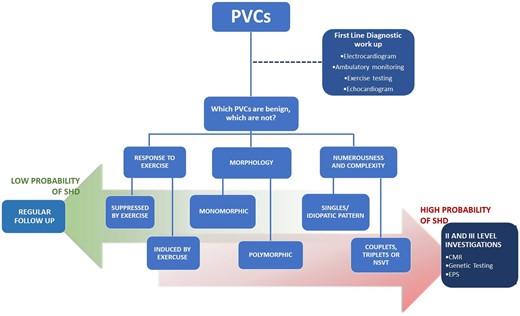
Clinical management of premature ventricular complexes. Risk stratification flow chart for structural heart disease in patients with premature ventricular complexes, clinical evaluation, and subsequent follow-up. CMR, cardiac magnetic resonance; EPS, electrophysiological study; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; SHD, structural heart disease.
Premature ventricular complex evaluation
Left bundle branch block morphology
The most common type of ‘benign PVCs’ originates from the RVOT and is characterized by a LBBB with inferior axis morphology (infundibular pattern). In this context, the LBBB pattern is defined by a negative QRS complex in lead V1, a negative QRS complex in lead aVL, and a positive QRS in the inferior leads (II, III, and aVF), describing an inferior axis. The embryologic common origin and the anatomic relation between RVOT and the LV outflow tract (LVOT) are essential to explain why outflow tract PVCs can share a similar morphology on surface ECG.
Evaluation of precordial transition at V3 could help differentiate RVOT from LVOT origin: late R/S transition beyond V3 is typical of RVOT, while an earlier transition denotes an LVOT origin.5 In patients with outflow tract PVCs and a V3 transition, the variability in coupling intervals has been described as a significant predictor of the origin of PVCs. Specifically, a coupling interval variability of 30 or more is indicative of an origin from LVOT, with a sensitivity of 83% and specificity of 89%.6 This independent predictor underscores the importance of considering coupling interval variability in determining the source of PVCs.
In most cases, the presence of LBBB and an inferior axis configuration indicates a positive outlook and the absence of any underlying structural heart conditions (Figure 2). Nevertheless, in a small number of instances, PVCs exhibiting LBBB/inferior axis should serve as an indicator for ARVC. Novak et al.2 and Hoffmayer et al.7 independently describe electrocardiographic characteristics related to the risk of ARVC in patients with LBBB inferior axis PVCs; these characteristics of PVCs are summarized in Table 1.
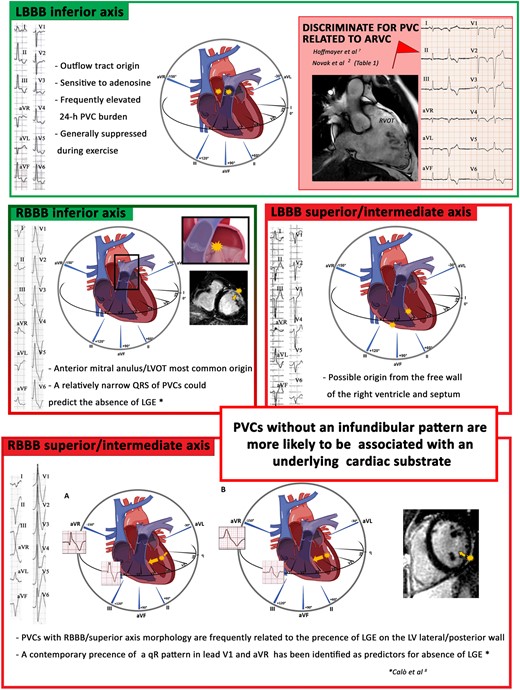
Representative monomorphic premature ventricular complexes, except for the fascicular pattern. The figure shows the main morphologies of premature ventricular complexes and their sites of origin. Green boxes indicate morphologies associated with a lower risk of structural heart disease. The left bundle branch block inferior axis morphology generally indicates an origin from the outflow tract of the right/left ventricle but rarely can be the expression of a predominantly arrhythmogenic right ventricular cardiomyopathy; the electrocardiographic characteristics that can help discriminate a structural cardiomyopathy (arrhythmogenic right ventricular cardiomyopathy) are indicated in Table 1. The right bundle branch block inferior axis morphology instead indicates an origin from the outflow tract of the left ventricle or from the mitral annulus; the duration of the QRS in this case can discriminate the presence/absence of late gadolinium enhancement at cardiac magnetic resonance. The red boxes are highly indicative of structural heart disease, therefore worthy of further diagnostic investigations. A left bundle branch block superior axis morphology could be indicative of an origin from the free wall of the right ventricle or from the mid-ventricular septum. A superior axis right bundle branch block morphology, suggesting a left ventricular origin, are more often associated with scar at CMR. Panel A shows how the depolarization forces can give rise to an initial Q wave in avR and V1 in the absence of scar; Panel B, instead, shows how the presence of scar gives rise to a depolarization gradient mainly directed towards leads V1 and aVR; no Q waves showed at 12-lead electrocardiogram.
| ECG characteristic . | P-value . |
|---|---|
| QRS axis > 90° | 0.048 |
| Intrinsicoid deflection > 80 ms | 0.037 |
| QS morphology in lead V1 | 0.003 |
| Lead I QRS duration ≥ 120 ms | 0.005 |
| QRS notching (multiple leads) | 0.014 |
| V5 transition or later | 0.002 |
| ECG characteristic . | P-value . |
|---|---|
| QRS axis > 90° | 0.048 |
| Intrinsicoid deflection > 80 ms | 0.037 |
| QS morphology in lead V1 | 0.003 |
| Lead I QRS duration ≥ 120 ms | 0.005 |
| QRS notching (multiple leads) | 0.014 |
| V5 transition or later | 0.002 |
Bold values depict characteristics with significant P-values.
Summary of the main characteristics of PVCs with LBBB morphology/inferior axis according to the authors: Novak et al.2 showed that three QRS features were significantly more common in patients with early ARVC than idiopathic RVOT-VA; Hoffmayer et al.7 scoring system can be utilized to differentiate between idiopathic and ARVC-related LBBB inferior axis morphology PVCs.
| ECG characteristic . | P-value . |
|---|---|
| QRS axis > 90° | 0.048 |
| Intrinsicoid deflection > 80 ms | 0.037 |
| QS morphology in lead V1 | 0.003 |
| Lead I QRS duration ≥ 120 ms | 0.005 |
| QRS notching (multiple leads) | 0.014 |
| V5 transition or later | 0.002 |
| ECG characteristic . | P-value . |
|---|---|
| QRS axis > 90° | 0.048 |
| Intrinsicoid deflection > 80 ms | 0.037 |
| QS morphology in lead V1 | 0.003 |
| Lead I QRS duration ≥ 120 ms | 0.005 |
| QRS notching (multiple leads) | 0.014 |
| V5 transition or later | 0.002 |
Bold values depict characteristics with significant P-values.
Summary of the main characteristics of PVCs with LBBB morphology/inferior axis according to the authors: Novak et al.2 showed that three QRS features were significantly more common in patients with early ARVC than idiopathic RVOT-VA; Hoffmayer et al.7 scoring system can be utilized to differentiate between idiopathic and ARVC-related LBBB inferior axis morphology PVCs.
Left bundle branch block superior axis morphology supported a diagnosis of PVCs originating from the inferior wall of the right ventricle (Figure 2).
Right bundle branch block morphology
Idiopathic PVCs characterized by a typical RBBB/left or right axis deviation and a duration of ≤130 ms are defined as ‘fascicular’. They are common in children and often associated with a structurally normal heart. Premature ventricular complexes with RBBB pattern are more often associated with underlying pathological structural diseases. In a study published by Muser et al.,3 RBBB pattern was the clinically dominant PVC morphology associated with late gadolinium enhancement (LGE) at CMR compared with an LBBB pattern (51% vs. 5%; P < 0.01). Among patients with RBBB PVCs, a superior axis pattern is most frequently related to a higher evidence of scar at CMR compared with RBBB inferior axis morphology.4 In patients with RBBB PVCs, CMR plays a key role but cannot be proposed for systematic evaluation. A recent study8 identified predictors of the absence of LV scar, such as the contemporary presence of a QR pattern in lead aVR and V1 in the subgroup of patients with RBBB/superior axis and a narrow QRS in the subgroup with RBBB/inferior axis (Figure 2).
Numerousness and complexity
Holter monitoring examination is essential for assessing the arrhythmic burden in terms of frequency, morphology, relation to exercise, and complexity. The number of PVCs in 24 h is a controversial topic. Traditionally, the presence of more than 500 PVCs per 24 h on Holter monitoring is attributed to a potential risk of SCD in patients with cardiomyopathy.2 On the other hand, frequent PVCs, with a predominant infundibular or fascicular pattern, occurred in athletes without underlying structural diseases with a positive prognosis and should not be considered a risk factor for an associated cardiac disease.4 According to these findings, PVC burden was not significantly different in patients with idiopathic PVCs and in those with SHD,9 respectively. The complexity of the PVCs in terms of polymorphic morphologies and the presence of couplets, triplets, and runs of NSVT, in contrast, was significantly higher in the group with SHD. Regardless of the burden, PVC characteristics may help identify potentially malignant arrhythmias that are more likely to be related to underlying heart disease. Premature ventricular complex couplets with short RR interval or PVCs that are superimposed on the preceding T-wave (‘R on T’ phenomenon) can be caused by myocardial electrical instability and may predispose to complex ventricular arrhythmias5 (Figure 1).
Response to exercise
The ET is an important non-invasive method for exposing arrhythmias. By producing several important physiological changes, particularly the activation of the sympathetic nervous system and an increase in circulating catecholamines, the ET provides a more complete assessment. However, the prognostic information provided by PVCs associated with ET can often be unclear. An increase in the arrhythmic rate at the onset of exercise, disappearance at the peak of exercise, and reappearance during recovery usually suggest benign behaviour for PVCs (Figure 1). On the other hand, the onset or worsening of ventricular arrhythmia with increasing workload may indicate an underlying cardiomyopathy or ion channel disease and may predict the risk of malignant arrhythmias during sports activity.4,5 Although current guidelines do not provide recommendations on exercise stimulation modality, including cycle ergometer, treadmill, and total body training equipment, ET should be tailored to the specific type of exercise responsible for the arrhythmic events/symptoms since a conventional ET may not replicate the specific clinical situation and the arrhythmogenic mechanism triggered by a certain sporting modality. There is no evidence to favour a specific ET protocol, but testing should be maximal, targeting patient exhaustion rather than a specific maximum heart rate (HR). However, it has been shown that a protocol characterized by an abrupt start at high intensity and an equally abrupt stop can be more sensitive in unmasking exercise-induced arrhythmias, especially in particular conditions such as catecholaminergic polymorphic ventricular tachycardia (CPVT).10
Substrate and cardiac magnetic resonance
The presence of PVCs can be associated with several clinical conditions, some of which pose a potential risk of fatal arrhythmias and SCD. In the proper diagnostic framework for patients with PVCs, it is crucial to identify the presence of an underlying arrhythmogenic substrate.
In adult subjects (age > 35 years), the most important arrhythmic substrate is ischaemic heart disease. Sometimes the presence of PVCs may be the only sign of underlying coronary artery disease (CAD). Additionally, patients with CAD, ventricular dysfunction (LVEF ≤ 40%), and NSVT have an indication for programmed electrical stimulation to identify those who benefit from an implantable cardioverter–defibrillator.11
Beyond ischaemic heart disease, various structural cardiomyopathies can be associated with PVCs, both genetic and acquired. Many of these conditions can be diagnosed through standard first and second-level diagnostic evaluations, but some arrhythmic substrates are detectable only by CMR. The term non-ischaemic LV scar (NILVS) refers to the presence of LGE in the sub-epicardial/mid-myocardial layers of the LV, indicating replacement-type myocardial fibrosis, in the absence of significant CAD.
Non-ischaemic LV scar has emerged in recent years as a predisposing substrate for major ventricular arrhythmias and SCD in young people and athletes.12 In studies of patients with apparent idiopathic ventricular arrhythmias, certain features of PVCs, such as morphology (polymorphic, non-LBBB lower axis morphology), complexity, and exercise-induced patterns, have emerged as predictors of scarring at CMR.3,4,8 Muser et al., in a group of 686 subjects with apparent idiopathic ventricular arrhythmias, identified RBBB/multifocal morphology as one of the elements associated with the presence of a high-risk arrhythmic LGE pattern called ‘ringlike’.13
However, myocardial scarring is a non-specific finding.12 Although an isolated NILVS is traditionally interpreted as a post-myocarditis scar, it can be observed in genetically left-dominant arrhythmogenic cardiomyopathy (desmosomal genes) or other ‘scarring’ phenocopies such as arrhythmogenic cardiomyopathy not related to desmosomal genes (e.g. PLN, FLMN, and LMNA), congenital heart diseases, other isolated cardiomyopathies, or, in the context of neuromuscular diseases, acquired inflammatory diseases (e.g. sarcoidosis).
All these ‘arrhythmogenic cardiomyopathies’ are associated with a distinctly higher risk of SCD, making an etiologic diagnosis crucial. In addition to careful clinical examination, blood tests, imaging, genetic testing, and ECG still remain valuable diagnostic tools. New ECG features are emerging in the diagnosis of arrhythmogenic LV cardiomyopathy to aid the clinician in early diagnosis.14
Genetic testing in premature ventricular complexes
Premature ventricular complexes can occur in any genetic cardiomyopathies during the course of the disease and may be the first presentation, especially in arrhythmogenic cardiomyopathy, where frequent PVCs also represent a diagnostic criterion. The likelihood of underlying cardiomyopathies increases with multifocal PVCs, PVCs with RBBB pattern, and if fibrosis is detected on CMR imaging.15
Genetic testing should be performed only following comprehensive clinical phenotyping when the probability of an inherited cardiac disorder (a cardiomyopathy or an ion channel disease) is reasonably high and if it can provide prognostic information or be useful for family screening. It is also mandatory in the presence of inducible polymorphic PVCs, inducible PVCs in bigeminy, and, in bidirectional couplets at HR > 100 b.p.m., features that suggest possible CPVT.16
Tachycardiomyopathy and treatment
In some patients, frequent PVCs may cause a decrease in LVEF. In the absence of underlying SHD, the diagnosis of tachycardia-induced cardiomyopathy (TIC) is generally considered. When a significant scar is detected by magnetic resonance imaging, the causal link between frequent PVCs and cardiomyopathy may be poor. The molecular mechanism supposed to be the cause of TIC is altered calcium homeostasis. Most patients with TIC are male, asymptomatic (and thus with a longer history of PVCs), with a burden of >10%, with interpolated or epicardial origin of PVCs (wide QRS duration), and a lack of diurnal variation of PVC frequency.17,18
When the elimination of PVCs often leads to improvement of the cardiomyopathy, the diagnosis of idiopathic TIC is certain. Medical management is a reasonable initial strategy. Beta-blockers or non-dihydropyridine calcium channel blockers have limited effectiveness and are indicated in symptomatic patients with idiopathic ventricular tachycardia (VT)/PVCs from an origin other than the RVOT or the left fascicles. Amiodarone and Class IC drugs may reduce PVC burden in >70% of patients, but flecainide should be cautiously used in this setting, and amiodarone is not recommended in patients with idiopathic PVCs due to side effects and the high rate of discontinuation. Thus, catheter ablation of PVCs should always be considered.11
Catheter ablation is recommended as a first-line treatment for symptomatic idiopathic VT/PVCs from the RVOT or the left fascicles. Success rates for monomorphic PVCs originating from the RV outflow are more than 90%. Ablation may fail in the presence of polymorphic PVCs or epicardial or papillary muscle origin. When complete elimination of PVCs is not possible, a reduction of the PVC burden below 10% may be sufficient to determine an improvement of LV dysfunction.11
Conclusion
Premature ventricular complexes and related symptoms are frequently observed in clinical practice. They can occur in patients with or without SHD, so they are best evaluated with a thorough history, physical examination, and 12-lead ECG, usually supplemented with ambulatory monitoring and an echocardiogram. The morphology of PVCs helps in the differential diagnosis between idiopathic ventricular arrhythmias and PVCs related to myocardial abnormalities. Cardiac magnetic resonance and genetic testing are useful in selected patients.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Leonardo Calo, Mario Tatangelo, Germana Panattoni and Cinzia Crescenzi contributed equally to the study and are co-first authors.
Conflict of interest: none declared.



