-
PDF
- Split View
-
Views
-
Cite
Cite
Claudio Borghi, Alessio Bragagni, There are those who would like zero LDL cholesterol, European Heart Journal Supplements, Volume 26, Issue Supplement_1, April 2024, Pages i19–i22, https://doi.org/10.1093/eurheartjsupp/suae012
Close - Share Icon Share
Abstract
The overwhelming evidence that the reduction of LDL cholesterol (LDLc) levels is associated with a parallel reduction in cardiovascular (CV) risk has led the scientific community to progressively and constantly reduce the optimal therapeutic targets of LDLc, both in patients with known CV disease and in patients undergoing primary prevention. The recent introduction of proprotein convertase subtilisin/kexin type 9 inhibitors has allowed clinicians to observe reductions in LDLc levels that go well beyond the limits set by the main international guidelines; following the ‘the lower the better’ paradigm, it is natural to ask how low LDLc can be reduced, whether this intervention is associated with a further reduction in CV risk and, above all, whether there are no issues related to safety in the use of polypharmacotherapies that determine an extreme reduction in LDLc levels. The purpose of this article is to summarize the main scientific evidence on the topic, trying to provide an answer to all clinicians who ‘would like their LDLc to be—almost—zero’.
Introduction
Since the publication of the latest ESC guidelines for the treatment of dyslipidaemia, the global scientific community has had to further review the recommended optimal levels of LDL cholesterol (LDLc) compared with the past, particularly in subjects at high and very high cardiovascular risk; in the latter, a reduction in LDLc levels of ≥50% compared to baseline levels is recommended with a target of LDLc < 55 mg/dL, which can reach up to <40 mg/dL in the event of a further cardiovascular event (CVE) occurring within the next 2 years.1 Such reductions in LDLc were unthinkable until a few years ago and difficult to obtain only with the aid of a therapy based solely on statins in combination with ezetimibe. The recent introduction of new powerful lipid-lowering drugs, such as proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), has made it possible to obtain reductions of up to 85% in basal LDLc levels; in fact, it is not uncommon for clinicians to follow patients in secondary prevention with LDLc levels well below the targets recommended by the latest ESC guidelines. If the trend in recent years has been to progressively reduce desirable LDLc levels in patients at high and very high cardiovascular risk, some questions arise spontaneously: ‘Having a vast and extremely effective pharmacological therapeutic armamentarium at our disposal, to what extent can we reduce the levels of LDLc safely and what is the optimal LDLc level that can guarantee a further reduction in cardiovascular risk in these patients?’; but above all, can we ask ourselves: ‘So what is LDLc for and why not reduce it—almost—to zero?’.
Notes on the pathophysiology of LDL cholesterol
Cholesterol represents an essential component of cell membranes, as well as a precursor of steroid hormones produced by the adrenal glands and gonads.2 In particular, LDL particles, made up of 80% lipids, are mainly responsible for the transport of cholesterol in the blood and extracellular fluids. Each cell can autonomously regulate both the de novo synthesis of cholesterol starting from acetate and the extracellular uptake through the LDL–LDL receptor (LDLR) interaction.3 The latter mechanism represents an effective way to rapidly satisfy the metabolic requirements of the cell; LDLRs are expressed in particular in the liver and in the adrenal glands, and the LDL–LDLR pathway is mainly responsible for the plasma clearance of cholesterol. It is interesting to note that half of the LDLR receptors are already saturated by their ligand at a concentration of ∼2.5 mg/dL, and that most of the cells are surrounded by interstitial fluid, in which the concentration of LDLc is equal to 20% of plasma levels. Hence, plasma LDLc concentration of 12.5 mg/dL would, theoretically, already be sufficient to guarantee adequate cholesterol uptake to peripheral tissues.2
Extremely reduced LDLc levels are found in some pathologies, such as abetalipoproteinaemia and homozygous hypobetalipoproteinaemia; these conditions are associated with fat malabsorption; gastrointestinal, haematological, and neurological symptoms; and increased ectopic fat deposition in the liver.3 However, these manifestations do not appear to be determined by the reduced concentration of LDLc but by a defective formation of lipoproteins, which determines their intracellular accumulation2; in patients with loss-of-function mutations of the PCSK9 gene and with gene variants resulting in increased LDLR activity, particularly reduced LDLc levels (<15 mg/dL) can be observed without any evidence of pathology.3 Investigations conducted on populations exposed since childhood to particularly low LDLc values or, as in the case of homozygous familial hypercholesterolaemia, to extremely high values remind us that it is not the single value but rather the cumulative exposure over time that determines the burden of cardiovascular disease (CVD): a subject suffering from homozygous familial hypercholesterolaemia will begin to develop signs of ischaemic coronary disease as early as 12.5 years, while a patient with heterozygous familial hypercholesterolaemia will reach the burden of exposure to LDLc necessary to develop ischaemic coronary disease, on average, at 35 years.4 It follows that the early introduction of a lipid-lowering therapy that accelerates the catabolic clearance mechanism mediated by the LDL–LDLR interaction without interfering with the formation of lipoproteins, as in the case of statins and PCSK9i, is not only safe from a mechanistic and theoretical point of view even when reaching extremely low LDLc values but it will also be effective in reducing cumulative exposure over time and consequently cardiovascular risk.3
The first evidence: reduction of cardiovascular risk with statins and ezetimibe
It is now well established that the reduction of LDLc determines a parallel reduction in CVEs both in patients at risk of CVD and in patients with established CVD5; in particular, as highlighted by the Cholesterol Treatment Trialists’ (CTT) Collaboration, every 39 mg/dL reduction in LDLc corresponds to a 22% drop in CVEs. Over the last two decades, several clinical studies have further demonstrated how an increasingly greater decrease in LDLc levels is associated with a progressive reduction in the risk of CVD: in the PROVE IT-TIMI 226 (The Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) study, in a population with recent acute coronary syndrome (ACS), a high-intensity statin therapy (atorvastatin 80 mg), which allowed to obtain average LDLc levels equal to 62 mg/dL, compared to a standard statin therapy (pravastatin 40 mg), which allowed to reach average LDLc levels of 95 mg/dL, proved to be superior in reducing the composite endpoint of death, myocardial infarction (MI), stroke, and unstable angina; in particular, the decrease in CVE was higher in groups of patients with LDLc levels ≤40 mg/dL {hazard ratio [HR] 0.61 (95% confidence interval [CI], 0.40–0.91)}. No adverse events related to myopathy or elevation of liver enzymes were related to the LDLc levels achieved with the therapy.6 Subsequently, in the JUPITER7 (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) study, a population of 17 802 apparently healthy patients but with elevated C-reactive protein values and LDLc values < 130 mg/dL were randomized to rosuvastatin 20 mg or placebo; after 1.9 years, a significant reduction in the incidence of major CVEs was observed in patients receiving rosuvastatin. In the rosuvastatin therapy group, LDLc levels were, on average, 54 mg/dL.
Finally, in the IMPROVE-IT8 (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) study, the association between simvastatin 40 mg and ezetimibe 10 mg compared with monotherapy with simvastatin 40 mg was tested in a population of patients with recent ACS. The primary endpoint, a composite of cardiovascular death, non-fatal MI, hospitalization for unstable angina, and non-fatal stroke, was 32.7% in the simvastatin–ezetimibe group vs. 34.7% in the simvastatin monotherapy group (2.0 percentage point reduction in absolute risk, HR 0.936; 95% CI, 0.89–0.99; P = 0.016); it should be noted that in the combination therapy group, LDLc levels were, on average, 54 mg/dL.
The new era: beyond the limits of statins with PCSK9 inhibitors
The introduction of monoclonal antibodies capable of inhibiting the functionality of PCSK9 has allowed clinicians to push the reduction of LDLc even further (Figure 1): the FOURIER9 (Further cardiovascular OUtcomes Research with PCSK9 Inhibition subjects with Elevated Risk) study has demonstrated how, in patients with atherosclerotic CVD, the addition of evolocumab to a statin allowed to reduce, on average, LDLc levels by 59%, reducing the risk of death from CVD, MI, stroke, and hospitalization for unstable angina by 15% compared to placebo, in an average of 2.2 years of follow-up. Patients on evolocumab therapy, starting from an average LDLc of 92 mg/dL, reached an average LDLc value of 30 mg/dL, while 10% of patients on evolocumab therapy (n = 2669) had even reached values lower than 19 mg/dL, without increases in adverse events. In the GLAGOV2 (GLobal Assessment of Plaque reGression With a PCSK9 antibOdy as Measured by intraVascular Ultrasound) study, the use of evolocumab compared to placebo was shown to reduce the volume of atheromas assessed by intravascular ultrasound; the average LDLc achieved in patients receiving PCSK9i therapy was 36.6 mg/dL, with minimum values reached up to 20 mg/dL. Furthermore, in a secondary analysis conducted by Giugliano et al.,10 it was observed that in patients treated with evolocumab, there was a reduction in the risk for major CVEs, which remained constant as LDLc levels decreased to lower concentrations at 8 mg/dL measured at the fourth week of treatment; in this last subgroup, the lowest risk of CVE in the absence of significant differences in the incidence of adverse events has been reported, when compared with the groups of patients with higher LDLc values.9
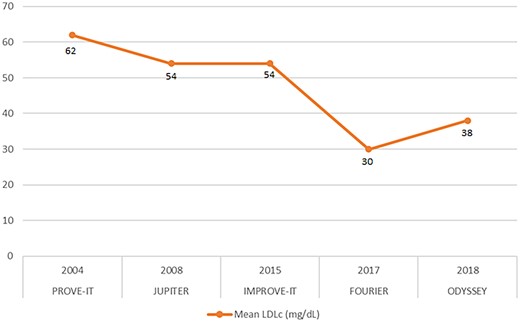
The graph shows the average LDL cholesterol values obtained (in mg/dL) in the groups of patients receiving therapy in the respective trials.
Similarly, from the ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) study,11 it emerged that in a population of patients with recent ACS and already on maximal therapy with a statin and LDLc levels ≥ 70 mg/dL, the addition of alirocumab reduced LDLc levels by 63% compared to placebo and reduced the risk of the primary composite endpoint of death from CVD, non-fatal MI, non-fatal stroke, or hospitalization for unstable angina by 15% at a mean follow-up of 2.8 years. In the first results of the ODYSSEY LONG TERM study, in which alirocumab was administered in addition to a statin at the maximum tolerated dose, patients were observed with LDLc levels consistently lower than 15 mg/dL over time, without increases in adverse events.12
Safety implications: what evidence we have
Since subjects affected by mutations in the genes for PCSK9 and hydroxymethyl-glutaryl-CoA reductase are more at risk of developing diabetes, combination therapies to lower LDLc levels with these two classes of drugs had initially raised this concern: in the FOURIER study, the addition of evolocumab on top of statin therapy did not increase the incidence of type 2 diabetes mellitus both in patients with normal glycaemic profiles and in patients with impaired fasting glycaemia; furthermore, it did not worsen glycaemic control in patients already suffering from diabetes.13
In the ODYSSEY LONG TERM and OSLER I and II studies, an increased incidence of neurocognitive events was initially observed in patients receiving PCSK9i.2,14 The release of the EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects) study allowed us to dispel any doubts, demonstrating that there were no significant neurocognitive differences after 19 months between patients treated with evolocumab and patients in the placebo group, despite a 59% LDLc reduction in the evolocumab group12; similarly, in a review conducted on 14 studies using alirocumab and in which patients with LDLc values lower than 25 mg/dL (n = 839) and 15 mg/dL (n = 314) were analysed, it was demonstrated that, despite the extremely low LDLc values obtained, there was no significant difference in neurocognitive events.15 Similarly, also in the GLAGOV study, no significant differences were highlighted in the incidence of myalgia, diabetes mellitus, or neurocognitive disorders compared to placebo.
In the SPARCL (Stroke Prevention With Aggressive Reductions in Cholesterol Levels) study, the use of atorvastatin 80 mg, despite reducing the incidence of ischaemic stroke in patients with a history of cerebrovascular disease, was associated with an increased incidence of haemorrhagic strokes3; these data, to be confirmed with further studies, do not however appear to be linked to the magnitude of the reduction in LDLc itself.
Finally, in the IMPROVE-IT study, >5000 patients achieved LDLc values < 50 mg/dL and ∼1000 patients < 30 mg/dL9; during the 7 years of follow-up, in neither subgroup was observed an increased incidence of diabetes, haemorrhagic stroke, or neurocognitive disorders.
Conclusions
An ever-increasing amount of scientific evidence is leading clinicians to start lipid-lowering therapies earlier and with more ambitious therapeutic targets; LDLc concentrations at extremely low levels achievable through the use of innovative molecules such as PCSK9i have not only been shown to have no deleterious side effects for health, but indeed, the benefits in terms of protection from CVEs for LDLc concentrations < 20 mg/dL are even more marked9 and should not scare clinicians nor patients, certainly up to concentrations not exactly equal to zero but at least equal to or higher than 12.5 mg/dL.2
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: none declared.



