-
PDF
- Split View
-
Views
-
Cite
Cite
Leonardo De Luca, Carmine Riccio, Alessandro Navazio, Serafina Valente, Manlio Cipriani, Marco Corda, Alfredo De Nardo, Giuseppina Maura Francese, Cosimo Napoletano, Emanuele Tizzani, Loris Roncon, Pasquale Caldarola, Michele Massimo Gulizia, Domenico Gabrielli, Fabrizio Oliva, Furio Colivicchi, ANMCO position paper on the management of hypercholesterolaemia in patients with acute coronary syndrome, European Heart Journal Supplements, Volume 25, Issue Supplement_D, May 2023, Pages D312–D322, https://doi.org/10.1093/eurheartjsupp/suad100
Close - Share Icon Share
Abstract
Patients suffering from acute coronary syndrome (ACS) present a high risk of recurrence and new adverse cardiovascular events after hospital discharge. Elevated plasma LDL-cholesterol (LDL-C) levels have been shown to be a causal factor for the development of coronary heart disease, and robust clinical evidence has documented that LDL-C levels decrease linearly correlates with a reduction in cardiovascular events. Recent studies have also demonstrated the safety and efficacy of an early and significant reduction in LDL-C levels in patients with ACS. In this position paper, Italian Association of Hospital Cardiologists proposes a decision algorithm on early adoption of lipid-lowering strategies at hospital discharge and short-term follow-up of patients with ACS, in the light of the multiple evidence generated in recent years on the treatment of hypercholesterolaemia and the available therapeutic options, considering current reimbursement criteria.
Introduction
Six years after the publication of the position paper of the Italian Association of Hospital Cardiologists (ANMCO) on the management of hypercholesterolaemia after acute coronary syndrome (ACS)1 and the inter-society consensus document on the diagnostic–therapeutic pathway on hypercholesterolaemia and cardiovascular risk in Italy,2 ANMCO decided to publish this position paper in the light of the multiple and solid evidence generated in recent years on the treatment of hypercholesterolaemia and the new therapeutic options available.
Patients suffering from an ACS have a high risk of new short- and long-term adverse cardiovascular events after hospital discharge.3,4 Elevated plasma levels of LDL-cholesterol (LDL-C) have unequivocally been shown to be a causative factor in the development of coronary artery disease (CAD), and strong clinical evidence has documented that decreasing LDL-C levels linearly correlates with a reduction in cardiovascular (CV) events.5,6 A meta-analysis involving 26 trials and ∼170 000 patients (most of whom had documented CAD and a history of ACS) showed that each 1.0-mmol/L (∼39 mg/dL) reduction in LDL-C was associated with a 20% relative reduction per year of adverse events, including coronary death, non-fatal myocardial infarction, coronary revascularization, and ischaemic stroke.6 This observation is further supported by Mendelian randomization studies which showed that subjects with genetic mutations associated with a reduction in LDL-C had a substantially reduced development of CV disease.7,8 Recent studies have also suggested that there is no threshold beyond which LDL-C reduction is not associated with clinical benefits. In fact, approval trials of monoclonal proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor antibodies have demonstrated a reduction in adverse atherothrombotic events even at LDL-C levels never reached (<40 mg/dL), with a favourable safety profile.9
LDL-cholesterol in patients with acute coronary syndrome
The systemic inflammatory response that is associated with ACS induces significant spontaneous changes in LDL-C levels, even in the absence of any cholesterol-lowering treatment. Indeed, clinical studies carried out in ACS before the widespread use of revascularization showed a spontaneous reduction in LDL-C values of around 30%.10 Subsequent investigations in patients with ACS who underwent pharmacological or mechanical reperfusion procedures revealed minor changes, with an average reduction in LDL-C of ∼10%.11,12 Typically, LDL-C values are significantly reduced within the first 24 h after admission and reach their minimums ∼7 days after clinical onset.11,12 Therefore, it is appropriate to assess LDL-C levels as early as possible in the course of hospitalization as a reference value and support for the choice of cholesterol-lowering therapy. In this regard, it is important to point out that average LDL-C values during hospitalization for ACS in patients not previously treated with statins (statin naive) range between 120 and 130 mg/dL.5,13 Significantly lower levels (90–110 mg/dL) are to be found in patients admitted to hospital for ACS despite ongoing treatment with statins.5,13
Clinical benefits of early initiation of oral cholesterol-lowering agents in acute coronary syndrome
Several observational studies and small trials with mechanistic endpoints have evaluated the benefit of early administration of statins in patients with ACS.14,15 The large Global Registry of Acute Coronary Events study recruited ∼20 000 patients with ACS and compared CV events among patients who had not started a statin treatment during index hospitalization with those who had started but stopped it or with those who had continued it during hospitalization.16 The latter group was found to have a significantly lower risk of death and stroke (odd ratio (OR) 0.66; 95% confident intervals (CI) 0.56–0.77). Furthermore, patients who started statin treatment during hospitalization but discontinued it for whatever reason had a similar risk of CV events as those who had not started any statin treatment (OR 1.02; 95% CI 0.74–1.41).16 An analysis of the Euro Heart Survey on ACS17 subsequently showed in 8197 patients that initiation of statin therapy within 24 h of hospital admission was associated with an improvement in CV outcome and all-cause mortality at 7 and 30 days even after adjustment for multiple variables (hazard ratio (HR) 0.90; 95% CI 0.60–1.3). More recently, an analysis of the SWEDEHEART registry conducted on more than 40 500 patients with ACS followed for about four years showed that a 50% reduction in LDL-C compared with baseline, achieved with high-intensity statins prescribed during hospitalization, was associated with a significant reduction in composite CV endpoints such as mortality, myocardial infarction and stroke, lower all-cause mortality, and a reduced incidence of hospitalization for stroke, heart failure, and coronary revascularization.18 Several randomized clinical trials have also demonstrated the benefit of early initiation of statin therapy in patients with ACS19–29 (Table 1). It is worth mentioning among others the Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial, in which 3086 patients with ACS without ST-segment elevation were randomized within four days after the acute event to atorvastatin 80 mg or placebo for 16 weeks.21 The primary endpoint, a composite of resuscitated cardiac arrest death and hospitalization for recurrent symptomatic myocardial ischaemia, was significantly reduced by early use of atorvastatin (HR 0.84, 95% CI 0.70–1.00, P = 0.048). Subsequently, the PROVE IT study (The Pravastatin or Atorvastatin with Aggressive Cholesterol Lowering)5 demonstrated in 4162 patients with ACS < 10 days, an additional benefit of high-intensity cholesterol-lowering therapy using atorvastatin 80 mg compared with conventional therapy with pravastatin 40 mg. Indeed, at 2 years the composite endpoint consisting of mortality, myocardial infarction, and hospitalization for unstable angina or revascularization was significantly reduced by the use of the high-intensity statin (HR 0.84, 95% CI 0.74–0.95, P = 0.005).
Main characteristics of randomized clinical trials that compared early statin use vs. placebo or usual care in patients with acute coronary syndrome
| Trial . | Statin . | Control . | No. of patient . | Statin administration (average, days) . |
|---|---|---|---|---|
| LAMIL19 | Pravastatin | Placebo | 69 | 2 |
| PAIS20 | Pravastatin | Placebo | 99 | 2 |
| MIRACL21 | Atorvastatin | Placebo | 3086 | 3 |
| PTT22 | Pravastatin | Trattamento attivo | 164 | 1 |
| LIPS23 | Pravastatin | Placebo | 824 | 2 |
| PACT24 | Pravastatina | Placebo | 3408 | 1 |
| ESTABLISH25 | Atorvastatin | Active treatment | 70 | 1 |
| Macin et al.26 | Atorvastatin | Placebo | 90 | 2 |
| Ren et al.27 | Simvastatin | Placebo | 86 | <3 |
| FACS28 | Fluvastatin | Placebo | 156 | 1 |
| ARMYDA-ACS29 | Atorvastatin | Placebo | 171 | 11 |
| Trial . | Statin . | Control . | No. of patient . | Statin administration (average, days) . |
|---|---|---|---|---|
| LAMIL19 | Pravastatin | Placebo | 69 | 2 |
| PAIS20 | Pravastatin | Placebo | 99 | 2 |
| MIRACL21 | Atorvastatin | Placebo | 3086 | 3 |
| PTT22 | Pravastatin | Trattamento attivo | 164 | 1 |
| LIPS23 | Pravastatin | Placebo | 824 | 2 |
| PACT24 | Pravastatina | Placebo | 3408 | 1 |
| ESTABLISH25 | Atorvastatin | Active treatment | 70 | 1 |
| Macin et al.26 | Atorvastatin | Placebo | 90 | 2 |
| Ren et al.27 | Simvastatin | Placebo | 86 | <3 |
| FACS28 | Fluvastatin | Placebo | 156 | 1 |
| ARMYDA-ACS29 | Atorvastatin | Placebo | 171 | 11 |
In all studies, the statin was administered within three days after symptom onset.
ARMYDA, Atorvastatin for Reduction of MYocardial Damage During Angioplasty; LAMIL, Lipid Acute Myocardial Infarction Lowering; PAIS, Pravastatin in Acute Ischaemic Syndromes; PTT, Pravastatin Turkish Trial; PACT, Pravastatin in Acute Coronary Treatment; LIPS, Lescol Intervention Prevention Study; MIRACL, Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering; FACS, Fluvastatin in the first-line therapy of Acute Coronary Syndrome.
Main characteristics of randomized clinical trials that compared early statin use vs. placebo or usual care in patients with acute coronary syndrome
| Trial . | Statin . | Control . | No. of patient . | Statin administration (average, days) . |
|---|---|---|---|---|
| LAMIL19 | Pravastatin | Placebo | 69 | 2 |
| PAIS20 | Pravastatin | Placebo | 99 | 2 |
| MIRACL21 | Atorvastatin | Placebo | 3086 | 3 |
| PTT22 | Pravastatin | Trattamento attivo | 164 | 1 |
| LIPS23 | Pravastatin | Placebo | 824 | 2 |
| PACT24 | Pravastatina | Placebo | 3408 | 1 |
| ESTABLISH25 | Atorvastatin | Active treatment | 70 | 1 |
| Macin et al.26 | Atorvastatin | Placebo | 90 | 2 |
| Ren et al.27 | Simvastatin | Placebo | 86 | <3 |
| FACS28 | Fluvastatin | Placebo | 156 | 1 |
| ARMYDA-ACS29 | Atorvastatin | Placebo | 171 | 11 |
| Trial . | Statin . | Control . | No. of patient . | Statin administration (average, days) . |
|---|---|---|---|---|
| LAMIL19 | Pravastatin | Placebo | 69 | 2 |
| PAIS20 | Pravastatin | Placebo | 99 | 2 |
| MIRACL21 | Atorvastatin | Placebo | 3086 | 3 |
| PTT22 | Pravastatin | Trattamento attivo | 164 | 1 |
| LIPS23 | Pravastatin | Placebo | 824 | 2 |
| PACT24 | Pravastatina | Placebo | 3408 | 1 |
| ESTABLISH25 | Atorvastatin | Active treatment | 70 | 1 |
| Macin et al.26 | Atorvastatin | Placebo | 90 | 2 |
| Ren et al.27 | Simvastatin | Placebo | 86 | <3 |
| FACS28 | Fluvastatin | Placebo | 156 | 1 |
| ARMYDA-ACS29 | Atorvastatin | Placebo | 171 | 11 |
In all studies, the statin was administered within three days after symptom onset.
ARMYDA, Atorvastatin for Reduction of MYocardial Damage During Angioplasty; LAMIL, Lipid Acute Myocardial Infarction Lowering; PAIS, Pravastatin in Acute Ischaemic Syndromes; PTT, Pravastatin Turkish Trial; PACT, Pravastatin in Acute Coronary Treatment; LIPS, Lescol Intervention Prevention Study; MIRACL, Myocardial Ischaemia Reduction with Aggressive Cholesterol Lowering; FACS, Fluvastatin in the first-line therapy of Acute Coronary Syndrome.
However, it seems important to emphasize that the benefit of early statin therapy in ACS appears to be dependent on the patient's residual clinical risk and baseline LDL-C levels. In a sub-analysis of the PROVE IT-TIMI 22 trial (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22), the benefit of intensive cholesterol-lowering treatment with atorvastatin 80 mg over pravastatin 40 mg was progressively reduced as baseline LDL-C levels decreased in statin-naïve ACS patients.30
Ezetimibe is a potent inhibitor of intestinal absorption of free cholesterol31 (Figure 1). In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) study, the combination of ezetimibe with the maximal dose of simvastatin led to a reduction in the risk of CV death, major coronary events, and non-fatal stroke in ∼18 000 patients with recent ACS (<10 days after admission).32 These benefits were particularly relevant in higher risk ACS populations such as those with diabetes mellitus or previous surgical revascularization.33,34 It is important to consider that the benefit of adding ezetimibe to the statin in ACS also appears to be relative to the LDL-C concentration at the time of admission. In a recent post-hoc analysis of the Heart Institute of Japan-PRoper level of lipid lOwering with Pitavastatin and Ezetimibe in acute coRonary syndrome (HIJ-PROPER) study,35 a multicentre prospective randomized open-label study comparing two cholesterol-lowering treatments in ∼1730 patients with ACS in 19 Japanese hospitals, the addition of ezetimibe to pivastatin produced greater CV benefits when LDL-C levels at hospital admission were above 130 mg/dL.36
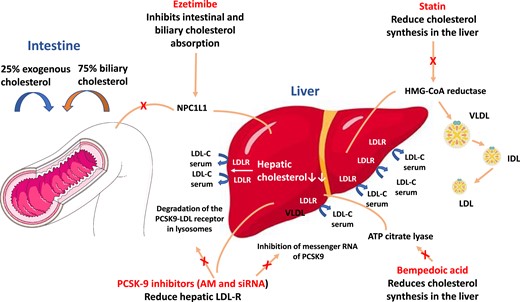
Main agents available for LDL-cholesterolreduction. AM, monoclonal antibodies; HMG-CoA hydroxymethylglutaryl-CoA; IDL, intermediate density lipoprotein; LDL, low-density lipoprotein; LDL-R, LDL receptor; NPC1L1, Niemann-Pick C1-Like 1; siRNA, small interfering RNA; VLDL, very low-density lipoprotein.
Bempedoic acid is a once-daily orally administered propharmaceutical that is rapidly converted in the liver to its active metabolite by a synthetase present only in hepatocytes and not in skeletal muscle, thus being associated with a lower risk of potential muscle-related adverse events (e.g. myalgia and myopathy) than statins.37 Bempedoic acid acts by inhibiting ATP citrate lyase, a cytosolic enzyme located within the enzymatic cascade leading to cholesterol synthesis (Figure 1). The LDL-C-lowering benefits of bempedoic acid were evaluated in the extensive CLEAR [Cholesterol Lowering via Bempedoic Acid, an adenosinetriphosphate citrate lyase (ACL)-Inhibiting Regimen] study programme. These studies have shown that a dosage of 180 mg bempedoic acid in addition to statin therapy at the maximum tolerated dose resulted in an additional reduction in LDL-C levels of 15–20%.38–40 The impact of bempedoic acid on CV events is being evaluated in the CLEAR OUTCOMES trial, which recently completed the enrolment of 14 014 patients intolerant to statins and high CV risk or known CV disease. The results were reported to be positive in terms of reduction of adverse CV events. However, to date, no specific data are available on the impact of bempedolic acid on LDL-C reduction and related clinical effects after a recent ACS, and trials conducted so far have excluded this type of patients.
Role of circulating proprotein convertase subtilisin/kexin type 9 in patients with acute coronary syndrome
Proprotein convertase subtilisin/kexin type 9 is a protein involved in the regulation of LDL receptor degradation present on hepatocyte cell membranes. Recent data suggest that circulating levels of PCSK9 are increased within hours from ACS onset and correlate with increased antiplatelet aggregation, vulnerability of coronary plaque, elevated inflammatory markers, and increased long-term CV events.41 Different evidence suggest that PCSK9 exerts deleterious effects on coronary plaques through different mechanisms including oxidation of LDL-C and direct modification of plaque composition42,43 through the production of cytokines and pro-inflammatory molecules.44–47 Conversely, in coronary plaque, inhibition of PCSK9 reduces the amount of macrophages and necrotic core content and promotes apoptosis.48 In addition, cardiac ischaemia activates upregulation of PCSK9 synthesis and causes dynamic changes in enzyme levels.49 In an animal study, the plasma concentration of PCSK9 was increased at 12–96 h after acute myocardial infarction with a peak at 48 h. In addition, hepatic messenger RNA (mRNA) expression of PCSK9 was increased ∼2.2-fold at 12 h and 4.1-fold at 24 h.49 These observations provide a rationale for considering PCSK9 inhibition at an early stage of ACS as it may not only produce a more rapid reduction in LDL-C levels but may also exert an early effect on the stabilization of activated coronary plaques.41
Clinical studies on anti-proprotein convertase subtilisin/kexin type 9 agents
The monoclonal antibodies alirocumab and evolocumab, administered subcutaneously 1 or 2 times a month, selectively inhibit the PCSK9 protein, ensuring increased LDL receptor efficiency and drastically reducing circulating LDL-C levels (Figure 1). They have been evaluated in numerous randomized Phase 2 and 3 clinical trials in the ODYSSEY and PROFICIO (Programme to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations) research programmes, respectively.50 The largest randomized Phase 3 trials, FOURIER and ODYSSEY OUTCOMES, included patients with baseline LDL-C > 70 mg/dL despite optimized cholesterol-lowering therapy, and evaluated the effects of evolocumab in high-risk patients with known CAD and alirocumab in patients with recent ACS, respectively.51,52 Both drugs resulted in a significant reduction in the primary composite endpoint that included death from CV causes, myocardial infarction, stroke, and coronary revascularization.51,52 A recent meta-analysis that included 39 randomized clinical trials and ∼66 500 patients treated with alirocumab or evolocumab and followed up for ∼2 years confirmed that PCSK9 inhibition is associated with a significant reduction in myocardial infarction, ischaemic stroke, and coronary revascularization compared with placebo, although a significant reduction in death from all causes and CV mortality was not found.50 However, a prolonged open-label follow-up analysis in patients treated with evolocumab (FOURIER-OLE) evaluated at a mean of 5 years showed a significant benefit of PCSK9 inhibition not only in terms of major CV events but also in CV mortality in the absence of major side effects.53 Importantly, a pre-specified analysis of the FOURIER trial, which included 5711 patients with recent myocardial infarction (<12 months prior to randomization), showed that these patients may benefit from a greater absolute risk reduction with PCSK9 inhibition than patients with previous myocardial infarction >12 months after randomization.54 In the ODYSSEY OUTCOMES trial, the use of alirocumab on top of optimized cholesterol-lowering therapy was associated with a 22% improvement in survival among patients with at least 3 years of follow-up available.55,56 This benefit was most pronounced in patients with baseline LDL-C levels ≥100 mg/dL, who had a 29% reduction in relative mortality risk.55 Furthermore, the use of alirocumab was associated with an ∼25% reduction in lipoprotein(a) levels in patients with ACS, consistent with previous evidence obtained with evolocumab in patients with known CAD.57,58 This is particularly relevant as in post-ACS the reduction in lipoprotein(a) achieved with PCSK9 inhibitors is independently associated with a reduction in CV events, which is particularly evident with high basal lipoprotein(a) levels.57,58
Another way to reduce PCSK9 levels is to inhibit its gene expression by neutralizing its mRNA through short interfering molecules (small interfering RNA) (Figure 1). In this sense, inclisiran is a double-stranded ribonucleic acid, administered subcutaneously once every 6 months, which exerts a sustained effect of reducing PCSK9 synthesis in hepatocytes.59,60 In the recent ORION-10 (Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-Density Lipoprotein Cholesterol) and ORION-11 (Inclisiran for Subjects With ACSVD or ACSVD-Risk Equivalents and Elevated Low-Density Lipoprotein Cholesterol) studies, inclisiran was compared with placebo on top of optimal cholesterol-lowering therapy in patients with known CAD or risk factor equivalents and elevated LDL-C levels (≥70 or ≥100 mg/dL, respectively).61 LDL-C levels were halved with inclisiran compared with placebo, with no significant differences in safety apart from minor skin reactions at the injection site. The ongoing ORION-4 (Inclisiran on Clinical Outcomes Among People With Cardiovascular Disease) study will evaluate the impact of inclisiran on ∼15 000 patients with known CAD, including those with previous stroke, although ACS with symptom onset <4 weeks will be excluded from randomization.62 The VICTORION-INCEPTION study is evaluating the efficacy of early administration of inclisiran in ∼380 patients with recent ACS (within 5 weeks) and LDL-C > 70 mg/dL in terms of LDL-C percentage reduction and achievement of lipid targets at 1-year follow-up compared with standard therapy.
Studies on the early administration of proprotein convertase subtilisin/kexin type 9 inhibitors in acute coronary syndrome
In the EVOPACS (Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes) study, the efficacy and safety of evolocumab were assessed during the acute phase of ACS in 308 patients.63 These patients were randomized to receive placebo or evolocumab 420 mg in addition to high-intensity statin therapy. Evolocumab was associated with a 40% reduction in LDL-C levels and enabled 90% of the treated patients to reach the LDL-C target value of 55 mg/dL at 1 month compared with 11% of those who received statin alone (P 0.001). In the Evolocumab in Acute Coronary Syndrome (EVACS) study, patients with ACS without ST-segment elevation were enroled and treated with evolocumab SQ 420 mg or placebo within 24 h after the acute event.64 At hospital discharge, 65% of patients treated with evolocumab reached the recommended LDL-C target (<55 mg/dL), compared with 24% of patients randomized to statin treatment alone. Finally, the recently published EPIC-STEMI study confirmed that alirocumab also significantly reduced LDL-C levels at 6 weeks compared with control when administered acutely during primary angioplasty for acute ST-segment elevation myocardial infarction, in the absence of side effects.65
Effects of cholesterol-lowering therapies on volume and plaque characteristics in acute coronary syndrome
A large atheroma extension measured by intravascular ultrasonography (IVUS), a large lipid extension measured by near-infrared spectroscopy (NIRS), and the presence of a fibrous cap of thin plaque assessed by optical coherence tomography (OCT) have been associated with an increased risk of CV events.66 The investigation of the so-called vulnerable plaques appears to be of particular importance in patients with ACS since wide natural history studies of ACS have shown that after coronary angioplasty the recurrence of CV events is equally related to culprit lesions treated during the acute phase and to lesions on non-culprit coronary arteries at the index hospitalization.66 In this context, the identification of thin-cap fibroatheroma diagnosed by IVUS with virtual histology was found to be an independent predictor of late CV events in non-culprit lesions.66 Several studies have shown that the early administration of cholesterol-lowering therapies in ACS activates a plaque-modifying, anti-platelet process and results in improved endothelial function. High-intensity statin therapy has been shown in several experimental studies to prevent the evolution of coronary atheroma extension and to positively influence plaque composition by reducing the lipid content, increasing the thickness of the fibrous cap and thus reducing plaque vulnerability in patients with ACS.67–69 More recently, the GLAGOV (GLobal Assessment of Plaque ReGression with a PCSK9 AntibOdy as Measured by IntraVascular Ultrasound) trial documented that the addition of evolocumab to statin treatment in patients with CAD reduced the progression of coronary atherosclerosis as measured by IVUS at 76 weeks.70 These benefits in terms of reduction and stabilization of atherosclerotic plaque were subsequently confirmed in the HUYGENS (High-Resolution Assessment of Coronary Plaques in a Global Evolocumab Randomized Study) study, which evaluated the effects of evolocumab in a population of patients with ACS without ST-segment elevation by OCT.71 In the randomized, multicentre, double-blind ODYSSEY J-IVUS trial, the use of alirocumab vs. placebo for 14 weeks stabilized atherosclerotic plaque in high CV risk and statin intolerant patients with a concomitant reduction in parietal inflammation assessed by PET/CT.72 Recently, the PACMAN-AMI trial (Effects of the PCSK9 Antibody Alirocumab on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction) enroled 300 patients undergoing coronary angioplasty for ACS and evaluated the effects of early administration of 150 mg alirocumab on coronary atherosclerosis assessed by multimodality intracoronary imaging (IVUS, NIRS, and OCT) of non-culprit lesions compared with placebo.73 At 52 weeks, the coronary plaque volume was significantly reduced in the alirocumab group compared with placebo (between-group difference, −1.21%; 95% CI, −1.78% to −0.65%; P < 0.001). Favourable changes were also observed in secondary endpoints that included a greater reduction in lipid burden assessed by NIRS and a greater increase in fibrotic cap plaque assessed by OCT. All these qualitative and quantitative changes in coronary plaque characteristics were directly correlated with LDL-C levels.73
Guidelines’ recommendations on LDL-cholesterol targets
Guidelines for the treatment of hypercholesterolaemia have been updated in recent years by both North American and European cardiology societies and both have further intensified the approach to LDL-C reduction for secondary prevention.74,75 However, the different guidelines differ in the LDL-C levels to be achieved (≤70 mg/dL in the US vs. ≤55 mg/dL in Europe).74,75 Both guidelines recommend in patients with ACS the assessment of target reached within 4 weeks and the achievement of this target in a stepwise approach by first recommending the use of high-intensity statins and subsequently the addition of ezetimibe and PCSK9 inhibitors as second or third line based on several factors (baseline LDL-C, statin naive, recurrent events, etc.). In the American guidelines, the first approach to LDL-C reduction is the statin at the maximum tolerated dose (Class I) and, if the target is not reached, the addition of ezetimibe or the PCSK9 inhibitor (Class IIa) is recommended.74 European guidelines recommend starting the statin at the highest intensity or highest tolerated dosage as soon as possible regardless of LDL-C values in order to reduce levels by ≥50% from baseline with a target of ≤55 mg/dL (Class I, Level A).75 If the target (baseline LDL-C halving and ≤55 mg/dL in secondary prevention or ≤40 mg/dL in patients with recurrent events in the last 2 years) has not been reached at a 4–6-week assessment, the combination with ezetimibe (Class I, Level B) and, after a further 4–6 weeks, the addition of a PCSK9 inhibitor (Class I, Level A) as a third-line therapy is suggested.75 For patients, on the other hand, who are hospitalized for ACS and are not on target for LDL-C levels despite treatment with statins at the highest tolerated dose and ezetimibe or for patients with recurrences of ACS in the last 2 years (extreme risk), the addition of the PCSK9 inhibitor can be considered already during hospitalization for the acute event (Class II, Level C).75
Achieving lipid targets in the real world
Despite the clear demonstrated benefits in terms of reduction of CV events of cholesterol-lowering therapies in patients with ACS and the increased number of available drugs,76 there remains an important real-world gap from the LDL-C targets recommended by international guidelines, due to underuse of LDL-C-lowering drugs. The reasons for this underuse of lipid-lowering drugs are many and include the side effects and perceived risk of statins, geographic and gender disparities, different health care protocols and systems, costs, and therapeutic inertia. In the DA VINCI study, which evaluated the use of lipid-lowering therapies in several European countries, the majority of patients with atherosclerotic CV disease were receiving a moderate-intensity statin monotherapy (44%) or a high-intensity statin monotherapy (38%).77 Only 9% of patients were treated with a combination of statin and ezetimibe and 1% with a drug combination including a PCSK9 inhibitor.77 Consequently, the LDL-C target recommended by international guidelines was achieved in a low percentage of cases. Other European survey data78 and drug prescription data from Germany or Poland also suggested that ∼20% of very high-risk patients achieved the previous guideline recommended target (≤70 mg/dL).79,80 Even from analysis of Italian registries on ACS or very high-risk patients, where more than 85% of patients were discharged on statin therapy alone, attainment of the LDL-C target of <70 mg/dL was achieved in approximately half of patients.81–83
Proposed algorithm and intervention strategies
The patient with ACS requires a personalized therapeutic intervention, aimed at achieving the lipid targets clearly outlined in the guidelines. Indeed, LDL-C must be halved from baseline and brought below the threshold of 55 mg/dL.75 In clinical practice, the physician must take into account all elements that may cause side effects and adverse reactions, remembering that most undesirable events occur when using high doses of statins, such as, for example, patients with hypothyroidism, chronic kidney disease, or vitamin D deficiency.84 With the premise that any therapeutic strategy appears reasonable for the purpose of reducing LDL-C and that the early approach is safe and leads to a faster attainment of the target,85 an intensive approach of cholesterol-lowering strategies is proposed, in compliance with the recommendations of the European guidelines and the criteria for drugs reimbursement. In this regard, the June 2022 update of the AIFA monitoring registers for PCSK9-inhibiting monoclonal antibodies allows these drugs to be used in secondary prevention for patients aged ≤80 years with LDL-C levels ≥ 70 mg/dL after a single LDL-C detection in case of recent AMI (last 12 months) or multiple CV events or with demonstrated intolerance to statins and/or ezetimibe. It is also important to consider that studies conducted over the past decades have made it possible to accurately quantify the estimated mean LDL-C reduction for each drug used. This makes possible to reliably predict the LDL-C levels that can be achieved with different therapeutic strategies by generating a simplified treatment algorithm for patients with ACS (Figure 2). In the proposed algorithm, statin therapy in ACS statin-naive patients should start during hospitalization as early as possible and should include the use of high-intensity statins, which provide at least a 50% reduction in LDL-C, in combination with ezetimibe86,87 (Figure 3). In ACS patients on statin therapy, the drug and dosage in use prior to the event should first be considered. In patients with ACS with a history of established intolerance to statin treatment, it is reasonable to prescribe ezetimibe 10 mg/day and a PCSK9 inhibitor (Table 2); in addition, if the patient has high baseline LDL-C values (>150 mg/dL), it seems reasonable to immediately consider a combination with bempedoic acid (Figure 3). In patients with ACS and a history of chronic renal disease, associated with a glomerular filtration rate < 30 mL/min, it seems appropriate to consider the use of the combination of ezetimibe 10 mg with a moderate-intensity statin (Table 3) in order to reduce the risk of possible side effects or adverse reactions. In patients with at least one ACS event in the year prior to the index admission for ACS, it is reasonable, in subjects <80 years of age and especially if baseline LDL-C is > 150 mg/dL, to start with the triple therapy consisting of a high-intensity statin at the maximum tolerated dosage, ezetimibe and a PCSK9 inhibitor.87 In patients over 80 years of age, it is possible to replace the PCSK9 inhibitor with bempedoic acid, bearing in mind that the average age of patients enroled in clinical trials of bempedoic acid to date is 65 years, so particular care is suggested in the use of this drug in older patients, especially when used in combination with high-dose statins.88 In all cases it is however recommended to start treatment with a high-intensity statin and ezetimibe from discharge (Figure 3). Obviously, in order to improve therapeutic adherence, it is desirable to favour pre-constituted statin and ezetimibe combinations.89 Lipid-lowering therapies should be continued indefinitely after discharge, taking care to verify the achievement and maintenance of the recommended lipid target over time (LDL-C reduced by 50% and <55 mg/dL). A first check of the LDL-C can be carried out 4 weeks after discharge, or according to the ‘diagnostic–therapeutic care pathways’ in force at the individual facility (Figure 4). Subsequent controls must be defined in relation to clinical needs. At each check-up, the patient's adherence to the prescriptions and the possible occurrence of side effects or adverse reactions correlated to lipid-lowering therapies must be checked.1 In patients aged < 80 years who, at the first check-up, despite treatment with the maximum tolerated dose of a high-intensity statin do not reach the target and have LDL-C values > 70 mg/dL, the addition of a PCSK9 inhibitor should be considered (Figure 4). In elderly patients (≥ 80 years) with LDL-C values between 55 and 70 mg/dL, the addition of bempedoic acid88 can be considered (Figure 4). If side effects or adverse reactions develop during statin treatment, a reduction of the statin dosage may be considered. This measure ensures a lower risk of side effects and improved therapeutic adherence. The management of these patients, however, must conform to the suggestions of the ANMCO document on the diagnostic–therapeutic pathway in patients with hypercholesterolaemia and intolerance to statin therapy.90

Estimated reduction of LDL-cholesterol levels with various combinations of the main cholesterol-lowering drugs. *Relative LDL-cholesterol reduction reported in the summary of product characteristics. AB, bempedoic acid; Ato, atorvastatin; Rosu, rosuvastatin; Sinva, sinvastatin.
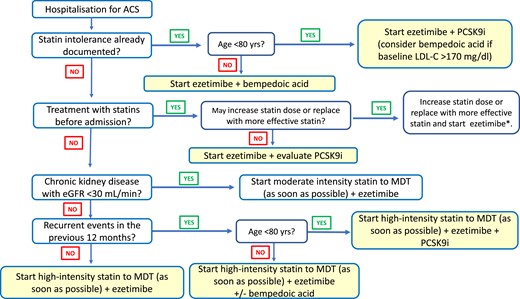
Initiation of cholesterol-lowering therapy during hospitalization in patient with acute coronary syndrome. *Also evaluate PCSK9 inhibitor addition in case of recurrent event. eGFR, estimated glomerular filtration rate; MDT, maximum tolerated dose; PCSK9i, PCSK9 inhibitors.
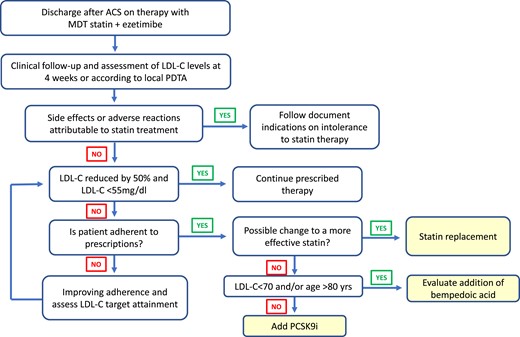
Management of cholesterol-lowering treatment after hospital discharge for acute coronary syndrome in patients already treated with the statin/ezetimibe combination. ACS, acute coronary syndrome; LDL-C, LDL-cholesterol; MDT, maximum tolerated dose; PDTA, diagnostic–therapeutic care pathway.
Options of currently and soon to be available cholesterol-lowering strategies in statin-intolerant patients and estimated LDL-cholesterol reduction
| Estimated reduction in LDL-cholesterol levels . | ||
|---|---|---|
| . | >35% . | >60% . |
| Currently available options | Ezetimibe 10 + Alirocumab/Evolocumaba | |
| Soon available options | Bempedoic acid 180 + Ezetimibe 10 | Bempedoic acid 180 + Ezetimibe 10 + Anti-PCSK9a |
| Ezetimibe 10 + Inclisiran | ||
| Estimated reduction in LDL-cholesterol levels . | ||
|---|---|---|
| . | >35% . | >60% . |
| Currently available options | Ezetimibe 10 + Alirocumab/Evolocumaba | |
| Soon available options | Bempedoic acid 180 + Ezetimibe 10 | Bempedoic acid 180 + Ezetimibe 10 + Anti-PCSK9a |
| Ezetimibe 10 + Inclisiran | ||
Monoclonal antibodies (alirocumab or evolocumab) or siRNA (inclisiran).
Options of currently and soon to be available cholesterol-lowering strategies in statin-intolerant patients and estimated LDL-cholesterol reduction
| Estimated reduction in LDL-cholesterol levels . | ||
|---|---|---|
| . | >35% . | >60% . |
| Currently available options | Ezetimibe 10 + Alirocumab/Evolocumaba | |
| Soon available options | Bempedoic acid 180 + Ezetimibe 10 | Bempedoic acid 180 + Ezetimibe 10 + Anti-PCSK9a |
| Ezetimibe 10 + Inclisiran | ||
| Estimated reduction in LDL-cholesterol levels . | ||
|---|---|---|
| . | >35% . | >60% . |
| Currently available options | Ezetimibe 10 + Alirocumab/Evolocumaba | |
| Soon available options | Bempedoic acid 180 + Ezetimibe 10 | Bempedoic acid 180 + Ezetimibe 10 + Anti-PCSK9a |
| Ezetimibe 10 + Inclisiran | ||
Monoclonal antibodies (alirocumab or evolocumab) or siRNA (inclisiran).
| High-intensity statinsa . | Moderate-intensity statinsb . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin 80 mg | |
| Pitavastatin 2–4 mg |
| High-intensity statinsa . | Moderate-intensity statinsb . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin 80 mg | |
| Pitavastatin 2–4 mg |
Daily dose reduces LDL-C by ∼50%.
Daily dose reduces LDL-C by ∼30–49%.
| High-intensity statinsa . | Moderate-intensity statinsb . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin 80 mg | |
| Pitavastatin 2–4 mg |
| High-intensity statinsa . | Moderate-intensity statinsb . |
|---|---|
| Atorvastatin 40–80 mg | Atorvastatin 10–20 mg |
| Rosuvastatin 20–40 mg | Rosuvastatin 5–10 mg |
| Simvastatin 20–40 mg | |
| Pravastatin 40–80 mg | |
| Lovastatin 40 mg | |
| Fluvastatin 80 mg | |
| Pitavastatin 2–4 mg |
Daily dose reduces LDL-C by ∼50%.
Daily dose reduces LDL-C by ∼30–49%.
Extreme risk and elderly patients
There are two groups of patients who have precise indications for lipid-lowering therapy intensification in guidelines and regulatory bodies, namely patients with infarct recurrence (extreme risk) and elderly patients. These two types of patients are increasingly encountered in secondary prevention, thanks also to the improvement in treatment of ACS, which has led to a lengthening of life expectancy with a concomitant worsening of clinical conditions and an increase in comorbidities. Patients with ACS recurrence within 1–2 years after the index event constitute 10–20% of patients admitted for ACS and have an extremely higher short- and medium-term risk of CV events and mortality than patients with no history of ACS events.91 In the EYESHOT POST-MI study which included ∼1600 patients with myocardial infarction in 165 Italian cardiology centres, 13% had a history of 2 and 5% more than three infarct events. The latter had more risk factors and were older than patients at their first ACS event.92 According to registries conducted in cardiology intensive care units, patients aged ≥ 75 years represent ∼30% of patients with an ST-segment elevation ACS and 40% of those without ST-segment elevation ACS.3,93–95 These data underestimate the true number of elderly patients with ACS since more than 17% of these patients are admitted to non-cardiac wards.96 Elderly patients constitute a group of patients at high risk and clinical complexity that requires geriatric and interdisciplinary evaluation in order to pursue a holistic approach and optimize therapeutic strategies on the basis of individual biological vulnerability.96
Funding
This paper was published as part of a supplement financially supported by the Italian National Association of Hospital Cardiologists (ANMCO).
Conflict of interest: None declared.
Disclaimer: This Position Paper was originally published in the Italian language as ‘Position paper ANMCO: Gestione dell'ipercolesterolemia nei pazienti con sindrome coronarica acuta', in the official journal of the Italian Federation of Cardiology (IFC) ‘Giornale Italiano di Cardiologia’, published by Il Pensiero Scientifico Editore. This paper was translated by a representative of the Italian Association of Hospital Cardiologists (ANMCO) and reprinted with permission of IFC and Il Pensiero Scientifico Editore.
Data availability
No new data were generated or analysed in support of this research.



