-
PDF
- Split View
-
Views
-
Cite
Cite
Giancarlo Casolo, Michele Massimo Gulizia, Daniela Aschieri, Alessandra Chinaglia, Marco Corda, Daniele Nassiacos, Salvatore Ivan Caico, Cristina Chimenti, Marzia Giaccardi, Enrico Gotti, Stefano Maffé, Roberta Magnano, Gianluca Solarino, Domenico Gabrielli, Fabrizio Oliva, Furio Colivicchi, ANMCO position paper: guide to the appropriate use of the wearable cardioverter defibrillator in clinical practice for patients at high transient risk of sudden cardiac death, European Heart Journal Supplements, Volume 25, Issue Supplement_D, May 2023, Pages D294–D311, https://doi.org/10.1093/eurheartjsupp/suad101
Close - Share Icon Share
Abstract
Extended risk stratification and optimal management of patients with a permanently increased risk of sudden cardiac death (SCD) are becoming increasingly important. There are several clinical conditions where the risk of arrhythmic death is present albeit only transient. As an example, patients with depressed left ventricular function have a high risk of SCD that may be only transient if there will be a significant recovery of function. It is important to protect the patients while receiving and titrating to the optimal dose the recommended drugs that may lead to an improved left ventricular function. In several other conditions, a transient risk of SCD can be observed even if the left ventricular function is not compromised. Examples are patients with acute myocarditis, during the diagnostic work-up of some arrhythmic conditions or after extraction of infected catheters while eradicating the associated infection. In all these conditions, it is important to offer a protection to these patients. The wearable cardioverter defibrillator (WCD) is of particular importance as a temporary non-invasive technology for both arrhythmia monitoring and therapy in patients with increased risk of SCD. Previous studies have shown the WCD to be an effective and safe therapy for the prevention of SCD caused by ventricular tachycardia/fibrillation. The aim of this ANMCO position paper is to provide a recommendation for clinical utilization of the WCD in Italy, based upon current data and international guidelines. In this document, we will review the WCD functionality, indications, clinical evidence, and guideline recommendations. Finally, a recommendation for the utilization of the WCD in routine clinical practice will be presented, in order to provide physicians with a practical guidance for SCD risk stratification in patients who may benefit from this device.
Objectives of the document
Sudden cardiac death (SCD) is a dramatic event for both the patient and any bystander, usually caused by a high-rate ventricular tachyarrhythmia (VT) or ventricular fibrillation (VF).
The chances of survival in affected individuals are extremely low in the absence of a prompt recognition of the clinical picture and immediate use of cardiopulmonary resuscitation manoeuvres. The most effective early treatment of arrhythmia is electrical defibrillation, which, if successful, results in the rapid restoration of mechanical activity of the heart and circulation. Early intervention in cases of arrhythmic cardiac arrest is an essential factor in helping restore rhythm and circulation, and attempts to ensure a rapid access to early defibrillation are one of the most important health challenges in counteracting arrhythmic death.
The clinical conditions predisposing to arrhythmic death are well known, and for many years, the availability of the implantable cardioverter defibrillator (ICD) has made its successful prevention possible in patients with a recognized and irreversible risk. In some conditions, however, the arrhythmic risk may be transient and implantation of the defibrillator potentially avoidable, depending on the evolution of the clinical picture. This can occur, for example, in the first 40 days after a myocardial infarction, or during the titration phase of the drugs used in patients with new-onset heart failure with systolic dysfunction (usually over a period of 3–6 months), as well as many other conditions. These include patients who have to wait for a new implantation after an ICD catheter extraction.
Since 2005, a therapeutic solution has been available that can mitigate the transient risk of arrhythmic death pending the permanent protection or definitive assessment of the need for an ICD. The wearable cardioverter defibrillator (WCD) is an instrument that is now widely used in clinical practice for this purpose and has proved to be safe and reliable in this indication.
While the most recent European Society of Cardiology (ESC) guidelines list the WCD with a Class IIb indication, it is also true that the use of this instrument is constantly increasing in Italy and worldwide, and new studies and evidence are accumulating on its efficacy and safety.
The aim of this ANMCO position paper is to provide a summary of the most recent recommendations for the use of the WCD, how to carry out the screening/selection of patients, and also to suggest indications on the decision process and management of patients during the period of WCD use. The technological evolution of this tool, critical issues, and limitations in its use will be also briefly discussed.
Definition and epidemiology of sudden cardiac death today
Sudden death is defined as an unexpected death, occurring within 1 h of the onset of acute symptomatology, in healthy individuals or patients in whom the underlying disease did not predict such a rapid outcome. The prevention of SCD is still a priority objective for any healthcare system. Despite intensive research and elaborate prevention campaigns, cardiovascular disease is still the leading cause of death, accounting for almost 35% (222 448 out of 645 620) of deaths in Italy. The incidence of SCD has been stable for years at around 70 000 patients per year.1,2 Depending on the age of the patient, arrhythmic death has different causes and is mainly attributable to ischaemic and non-ischaemic cardiomyopathies, myocarditis, hereditary cardiomyopathies, or channelopathies.
In order to prevent SCD, much has been done with the implementation of automated external defibrillators in public areas. However, recent studies have shown that the final outcome of patients resuscitated outside the hospital is still far from satisfactory. Current survival rates are in fact described as <8%.3,4 One of the main problems is that most cases of SCD occur without witnesses, often at home and during sleep. For this reason, it is often not possible to provide sufficiently rapid help.5
Where a risk of SCD can be identified, patients can be protected with an ICD. In the secondary prevention, after ruling out a reversible cause and possible contraindications, it is recommended to implant an ICD without waiting any longer. For primary prevention, on the other hand, a less aggressive attitude is recommended. Indeed, we know that a significant number of patients can improve their cardiac function expressed as left ventricular ejection fraction (LVEF) with appropriate therapeutic measures (drug therapy for heart failure, coronary revascularization, and other causative therapeutic approaches for other conditions).6 Furthermore, studies such as DINAMIT and IRIS or DANISH have shown that early or non-discriminatory ICD implantation does not confer any benefit on mortality.7–9 Based on these findings, all major scientific societies recommend that implantation should not take place before 40 days in cases of acute myocardial infarction (AMI) and before 3 months in most other cases of newly diagnosed heart failure in ischaemic cardiomyopathy (ICM) and non-ischaemic cardiomyopathy (NICM). However, these recommendations are at odds with the observation that the risk of SCD is highest in the first 3 months after an acute event and after the initial diagnosis of LVEF impairment. In the case of a myocardial infarction, the risk of impending death is 2.5%, which is almost 10 times higher than 1 year after the infarction.10,11 Similar observations were found for patients with a first diagnosis of ICM or NICM in the PROLONG I and II studies, WEARIT-II, WEARIT-II-Europe, and the Röger study.12–16 It is obvious that drug therapy alone does not provide sufficient protection against potentially life-threatening arrhythmias, especially at the beginning of patient care.17
Therefore, the correct and early identification of patients at increased risk of SCD and the identification of the cause and appropriate treatment are of crucial importance. As this risk may be only transient but may also persist in time, both a short- and long-term prevention of SCD must be considered. As an effective tool for temporary protection, the WCD has been available in many parts of Europe since 2005. The WCD is capable of effectively detecting ventricular arrhythmias, delivering an external shock and interrupting a life-threatening arrhythmia. Therefore, in the initial period after the diagnosis of high risk of SCD, the WCD provides temporary protection, e.g. during optimization of pharmacological therapy and during the phase of improvement of systolic function.
Brief description of the device and its operation
The WCD is currently manufactured by a single company, ZOLL, and is currently the only commercially available device of this type in Europe. It provides non-invasive protection from VF and VT for as long as it is worn, potentially 24 h a day. The WCD received Food and Drug Administration approval in 2001 and has been available in Italy with the current delivery model since 2015. The WCD system consists of a wearable vest on which are integrated four electrocardiogram (ECG) detection electrodes and three ‘pads’ for delivering the defibrillation therapy, i.e. the electric shock. The defibrillation pads and electrodes are connected to a battery-powered monitor. The WCD is supplied with a battery charger and a hot spot for transmitting the recorded data. The vest is available in different sizes that can be adapted to the patient.
The ECG is derived from two channels via the four sensing electrodes placed on the antero-posteral and latero-lateral chest surface in order to derive bipolar ECG traces. The ECG recordings are stored in the monitor unit. The monitor unit is equipped with a touch screen (LCD screen) for patient interaction, battery, and two response buttons. Two batteries are supplied, each with a 24-h capacity to ensure continuous anti-arrhythmic control, as it is possible to charge one of the batteries while the other is in use.
From the monitor, it is possible to programme, as with ICD’s, a VT zone and a VF zone and frequency intervals for the detection of ventricular arrhythmias with (120–250 b/min), it is possible to set the delay between arrhythmia detection and shock delivery (60–180 s in the VT zone and 25–55 s in the VF zone), and finally the energy of the shock itself (75–150 J) can be programmed. If the patient’s heart rate exceeds the limit set for VT, algorithms are used to improve detection and discriminate between ventricular and supraventricular arrhythmias. If a ventricular arrhythmia is detected (detection time 10–15 s), the patient is alerted with increasing alarms (vibration, red light, siren, and voice messages) so that he/she can manually deactivate the shock delivery if conscious. The alarms are cyclically interrupted by voice messages urging bystanders not to intervene and to call for help.
If the patient is unable to react by pressing the response buttons due to loss of consciousness, the shock sequence is not suppressed, and a therapeutic shock is delivered after the set reaction time, this after the shock electrodes have released a blue conductive gel automatically to prevent burns between the skin and the surface of the electrode. Response buttons, which allow the patient’s state of consciousness to be checked, are a unique feature of the WCD. They largely avoid inappropriate and unnecessary shocks due to self-limiting VT or atrial arrhythmias. This helps to increase the sensitivity and specificity of the device and to enhance its safety.
Up to five shocks can be delivered for a single arrhythmia episode, and multiple episodes can be recognized. After the delivery of electrical therapy, the ECG of the arrhythmia is transmitted to a password-protected server (ZOLL Patient Management Network—ZPM), which the referring physician has access to in order to be able to analyse the recordings. The doctor can also be automatically notified by e-mail, fax, or SMS and print out the corresponding reports. After the delivery of a shock, the patient management service of the manufacturer provides a device change, and an immediate notification to the treating physician occurs. This 24/7 service is an essential part of the system.
It is also possible to record an ECG trace manually at the patient’s initiative on a perceived symptom, as is the case with event recorders. The corresponding data are then transmitted to the ZPM. Thanks to this application, otherwise undetected atrial tachyarrhythmias can be detected and appropriately treated. The presence of an implanted pacemaker does not constitute a contraindication for the use of the WCD, provided it has been programmed for bipolar pacing.
Patient compliance and training
A crucial factor for the effectiveness of the WCD, as with any therapy, is adequate patient compliance. Detailed training, provided by the manufacturer to the patient at the time of delivery of the device, is mandatory and can be repeated if the patient has special needs. Training of relatives or caregivers may also be helpful. Due to the simplicity of the device, even elderly patients are generally able to take in the information provided and handle the device correctly.
Remote monitoring, as described above, can be used to check the wearing time and thus the patient’s compliance. It is possible to inform the practitioner if the patient’s WCD wearing time falls below a minimum level so that prompt corrective action can be taken by calling the patient and encouraging them to pay more attention to their wearing time.
In the European registers, a consistently high median compliance of >21 h/day was demonstrated.18–21 Only in the randomized controlled trial VEST was compliance lower, with a median of 18 h. It has to be considered that the study protocol did not allow the use of the telephone helpline system (available 24/7) nor the use of the remote monitoring system ZPM, which may have contributed to the lower daily utilization time compared with other registries. However, despite this suboptimal compliance, a significant reduction in all-cause mortality was achieved in all three analyses conducted.11,22
In the case of insufficient compliance, a follow-up training and discussion with the patient are recommended, in order to better understand the possible reasons for the poor compliance and to remedy it. If even with these actions no improvement is achieved, the prescribing doctor may consider discontinuing the therapy, bearing in mind that the patient is no longer protected against the risk of SCD.
Patients who are candidates for wearable defibrillator use with a transient risk of sudden cardiac death
In the following sections, the conditions suitable for appropriate indications to the WCD in accordance with the latest guidelines are briefly summarized. It is the scope of this document to aid in the correct selection of patients who may benefit from this diagnostic and therapeutic tool. In the following paragraphs, we will go into detail to describe the indications for different aetiologic groups.
Patients with ischaemic cardiomyopathy
Patients with recent acute myocardial infarction and reduced ejection fraction (≤35%)
Coronary artery disease is the leading cause of death in Western countries; in particular, it is the leading cause of death in Italy, being responsible for 35.8% of all deaths. Coronary atherosclerosis results in obstruction of varying degrees of the vessel lumen and can lead to the development of myocardial ischaemia when the oxygen supply to the myocardium is inadequate with respect to the requirements. Even though chest pain may be absent or not predominant in some manifestations of coronary artery disease (silent ischaemia, arrhythmias, sudden death, heart failure, and diabetics), the most typical clinical manifestation of myocardial ischaemia is angina, usually described as severe chest oppression or constriction and/or difficulty breathing, often radiating to the neck or arm. Acute myocardial infarction [either ST elevation myocardial infarction (STEMI) or non-ST elevation myocardial infarction (NSTEMI)] may develop following a coronary occlusion leading to severe ischaemia or irreversible myocardial necrosis. When the jeopardized myocardium is sufficiently large, left ventricular dysfunction develops, and LVEF may be severely reduced. This may happen even after a prompt restoration of the coronary flow by coronary angioplasty [percutaneous coronary intervention (PCI)] or coronary artery bypass graft (CABG). Several studies have shown that the risk of SCD is significantly increased in these patients, particularly in the first 30 days after the infarction.23 Patients with coronary artery disease and left ventricular dysfunction are at risk of SCD even after this time interval. In the STICH study, the risk of SCD in the first 3 months was 1.2%.24 Furthermore, it has been shown in various studies (including the WEARIT-II register series,16 Garcia et al.19 and Röger et al.14) that ventricular function can improve substantially in the first months after an acute event in 40–50% of cases, making a long-term protection with an ICD no longer necessary.
Various retrospective and prospective studies have evaluated the benefit of the WCD in patients after AMI. One of the largest post-infarction studies reported was that by Epstein et al.,25 which enrolled 8453 patients with recent myocardial infarction (within 3 months) and LVEF ≤ 35%. Of these, 133 patients (1.6%) received 309 appropriate shocks. The risk of SCD was highest in the first month of WCD use (median 16 days), and in treated patients, 75% received treatment in the first month and 96% within the first 3 months of use.
The VEST trial11,22 confirmed a high risk of total mortality of 4.9% within the first 3 months.
The risk of arrhythmic mortality was 2.4%.
We therefore have a population at transient risk of arrhythmic death that is reasonable to protect for the period in which the risk is greatest while waiting for a possible spontaneous or therapy-induced contractile recovery. As already reported, in the DINAMIT and IRIS trials, the survival benefit of an ICD at this early stage could not be demonstrated.7,8 On the contrary, in the VEST trial, all types of mortality, including the non-arrhythmic mortality, were reduced in those assigned to the WCD use. Total mortality was significantly reduced in all three types of analysis (Table 1). Arrhythmic and non-arrhythmic mortality were significantly reduced in both the ‘as-treated’ and ‘per-protocol’ analyses.11,22
| . | Total mortality . | Arrhythmic mortality . | Non-arrhythmic mortality . |
|---|---|---|---|
| Intention to treat | RR 0.64 (CI 95% 0.43–0.98) P = 0.04 | RR 0.67 (CI 95% 0.37–1.21) P = 0.18 | RR 0.63 (CI 95% 0.33–1.19) P = 0.15 |
| As treated | RR 0.26 (CI 95% 0.14–0.48) P < 0.001 | RR 0.43 (CI 95% 0.21–0.91) P = 0.03 | RR 0.09 (CI 95% 0.02–0.35) P = 0.001 |
| Per protocol | HR 0.25 (CI 95% 0.13–0.48) P < 0.001 | HR 0.38 (CI 95% 0.17–0.86) P = 0.02 | HR 0.09 (CI 95% 0.02–0.39) P = 0.001 |
| . | Total mortality . | Arrhythmic mortality . | Non-arrhythmic mortality . |
|---|---|---|---|
| Intention to treat | RR 0.64 (CI 95% 0.43–0.98) P = 0.04 | RR 0.67 (CI 95% 0.37–1.21) P = 0.18 | RR 0.63 (CI 95% 0.33–1.19) P = 0.15 |
| As treated | RR 0.26 (CI 95% 0.14–0.48) P < 0.001 | RR 0.43 (CI 95% 0.21–0.91) P = 0.03 | RR 0.09 (CI 95% 0.02–0.35) P = 0.001 |
| Per protocol | HR 0.25 (CI 95% 0.13–0.48) P < 0.001 | HR 0.38 (CI 95% 0.17–0.86) P = 0.02 | HR 0.09 (CI 95% 0.02–0.39) P = 0.001 |
HR, hazard ratio; CI, confidence interval; RR, relative risk.
Statistically significant results are shown in bold.
The secondary endpoint (reduction in all-cause mortality) showed a significant reduction in all intention-to-treated, as-treated and per-protocol analyses, while arrhythmic mortality was significantly reduced in all but the intention-to-treat analysis. It is thus clear that the effectiveness of the wearable cardioverter defibrillator is closely dependent on treatment adherence.
| . | Total mortality . | Arrhythmic mortality . | Non-arrhythmic mortality . |
|---|---|---|---|
| Intention to treat | RR 0.64 (CI 95% 0.43–0.98) P = 0.04 | RR 0.67 (CI 95% 0.37–1.21) P = 0.18 | RR 0.63 (CI 95% 0.33–1.19) P = 0.15 |
| As treated | RR 0.26 (CI 95% 0.14–0.48) P < 0.001 | RR 0.43 (CI 95% 0.21–0.91) P = 0.03 | RR 0.09 (CI 95% 0.02–0.35) P = 0.001 |
| Per protocol | HR 0.25 (CI 95% 0.13–0.48) P < 0.001 | HR 0.38 (CI 95% 0.17–0.86) P = 0.02 | HR 0.09 (CI 95% 0.02–0.39) P = 0.001 |
| . | Total mortality . | Arrhythmic mortality . | Non-arrhythmic mortality . |
|---|---|---|---|
| Intention to treat | RR 0.64 (CI 95% 0.43–0.98) P = 0.04 | RR 0.67 (CI 95% 0.37–1.21) P = 0.18 | RR 0.63 (CI 95% 0.33–1.19) P = 0.15 |
| As treated | RR 0.26 (CI 95% 0.14–0.48) P < 0.001 | RR 0.43 (CI 95% 0.21–0.91) P = 0.03 | RR 0.09 (CI 95% 0.02–0.35) P = 0.001 |
| Per protocol | HR 0.25 (CI 95% 0.13–0.48) P < 0.001 | HR 0.38 (CI 95% 0.17–0.86) P = 0.02 | HR 0.09 (CI 95% 0.02–0.39) P = 0.001 |
HR, hazard ratio; CI, confidence interval; RR, relative risk.
Statistically significant results are shown in bold.
The secondary endpoint (reduction in all-cause mortality) showed a significant reduction in all intention-to-treated, as-treated and per-protocol analyses, while arrhythmic mortality was significantly reduced in all but the intention-to-treat analysis. It is thus clear that the effectiveness of the wearable cardioverter defibrillator is closely dependent on treatment adherence.
Considering these data, it seems reasonable and in accordance with the recommendations of international scientific societies26–31 to use a WCD in post-MI patients treated conservatively who have a severely reduced ejection fraction (≤35%) for at least 40 days, 2–3 months in those who received a PCI, and 3–4 months in those treated by CABG. In all the cases, a re-evaluation of the left ventricular function to detect a possible recovery of function is mandatory. For patients treated conservatively, it is important to record also the occurrence of any worrisome ventricular arrhythmic event during the programmed time interval of surveillance in order to decide if an ICD is still necessary.
Patients with coronary artery disease and reduced ejection fraction (≤35%) without recent myocardial infarction undergoing revascularization (coronary angioplasty or coronary artery bypass)
In the first few hours after coronary revascularization, changes in pH and electrolytes can cause an effect known as ‘reperfusion injury’. This can cause life-threatening arrhythmias in the subsequent remodelling phase.32,33 Therefore, even patients without myocardial infarction are at significant risk of SCD after revascularization.
Current guidelines do not recommend implantation of an ICD before 3 months after revascularization as the left ventricular function improvement is unpredictable.12,13,31 The largest study focusing on this group of patients was that of Zishiri et al.34: mortality at 3 months after CABG was 3% in the patients with WCD and 7% in the cohort of patients without WCD (P = 0.03) and after PCI was 2% vs. 10% (P < 0.0001) in the WCD and non-WCD group, respectively. The use of WCD thus resulted in a 39% reduction in the total mortality rate (P < 0.0001) with an interventional incidence of 1.3%.
In this condition, it therefore seems reasonable, and in accordance with the guidelines of the ESC/European Heart Rhythm Association (EHRA) and the American Heart Association/American College of Cardiology (AHA/ACC),26–31 to use the WCD for 2–3 months after PCI and 3–4 months after CABG also in patients with chronic ischaemic heart disease and severe systolic dysfunction, thus allowing an adequate period of time to verify a possible contractile recovery.
Patients with initial diagnosis of heart failure with reduced ejection fraction (≤35%) of ischaemic aetiology
Clinical studies on SCD have consistently shown that the highest risk of SCD is among patients with severely depressed left ventricular function (LVEF ≤ 35%). Therefore, guidelines on primary prevention of SCD recommend implanting an ICD in patients with severely depressed LVEF ≤ 35%) with symptomatic heart failure [New York Heart Association (NYHA) Class II/III] of ischaemic aetiology (Class I recommendation). However, several studies have shown that at least 50% of these patients show a partial or complete recovery of LVEF at 3 months after the prescription of the guideline-directed medications (GMDT) for heart failure. Therefore, the current guidelines recommend a waiting period sufficient to allow the potential improvement in left ventricular function with GMDT. The time lapse required to assess the indication for an ICD implantation depends on the aetiology of the left ventricular dysfunction and ranges from approximately from 3 to 4 months.29 During this period of time (from the initial diagnosis of reduced left ventricular function until the re-evaluation after GDMT), patients are not protected from SCD, and as shown in the PROLONG I and II studies and in several other retrospective and prospective registries, there is a high risk for arrhythmic death.12,13,35 Most SCD events occur in the first 30 days and are markedly reduced after 3 months in patients with significant recovery of ventricular function.
Therefore, in patients with heart failure with reduced ejection fraction (HFrEF) and ischaemic aetiology, it appears appropriate to use a WCD for approximately 3 months in accordance with the ESC/EHRA and AHA/ACC guidelines.26–31 This observation period will allow the clinician time to better stratify the patient type and make the most appropriate long-term treatment decisions.
Patients with non-ischaemic cardiomyopathy
Patients with initial diagnosis of heart failure with reduced ejection fraction (≤35%) of non-ischaemic aetiology
Dilated cardiomyopathy of non-ischaemic aetiology is generally characterized by dilatation and dysfunction of the left ventricle. At the initial diagnosis of dilated cardiomyopathy with LVEF ≤ 35%, the guidelines of the cardiological societies recommend optimizing the drug therapy for heart failure for at least 3 months and subsequently reassess a possible indication for ICD.17,30
The PROLONG I and II studies, as well as several other retrospective and prospective registries, have shown that there is an increased risk of SCD in this phase, which, however, is only temporary in most cases.12,13,35 The incidence of arrhythmias varies considerably, from 2% to 15%.13,15 Approximately 50% of the newly diagnosed patients with heart failure of non-ischaemic origin achieve a 10% improvement in LVEF with GDMT.29,30,36 The time lapse required to assess the indication for implantation of an ICD depends on the aetiology of the ventricular dysfunction and the specific disease context of the patient and may vary from ∼3 to 4 months for patients with heart failure of non-ischaemic aetiology.29 Currently there is no scientific evidence to support an early ICD implantation in these patients, and a significant LVEF improvement has been observed after the implementation of GMDT. This improvement may remain stable for the subsequent 10–15 years.37
Therefore, even in this condition, it is important to be able to offer sufficient time to observe the trajectory of the patient’s systolic function safely. For this purpose, the WCD should be used for a duration of 3 months, again in accordance with the ESC/EHRA and AHA/ACC26–31 recommendations.
Patients with myocarditis
Myocarditis is an inflammatory-based myocardial disease, usually the result of viral infections or autoimmune mechanisms. Myocarditis is characterized by a high heterogeneity of clinical presentations ranging from no symptom to a rapid deterioration of cardiac function with irreversible heart failure and/or major arrhythmic event and death.38,39 Left ventricular dysfunction is present in ∼60% of cases. It is usually transient but may persist for many months39,40 or persist chronically over time.
Myocarditis is the most common cause of non-ischaemic heart failure.41 Several studies have shown that even chronic forms of inflammation-based cardiomyopathy can regress with complete recovery of left ventricular function through the use of targeted therapies.42,43
In this context, the use of the WCD may be indicated while waiting for the ongoing inflammatory process to fade. Also important is the evaluation of the myocardium with cardiac magnetic resonance imaging (MRI), where the presence and extent of the post-contrast myocardial late gadolinium enhancement (LGE) have a strong predictive value for the occurrence of VT (independent from LVEF).44,45
Consequently, in addition to a detailed diagnosis with ECG, echocardiography, and cardiac MRI and when necessary endomyocardial biopsy, the protection with a WCD for a period of 3–6 months is considered useful. Thereafter, a new clinical–instrumental assessment should be performed and re-evaluate the need for an ICD.26–30
Other non-ischaemic cardiomyopathies
Among the cardiomyopathies and due to their relative frequency, it is worth mentioning the peripartum cardiomyopathy and takotsubo cardiomyopathy. These may manifest as acute and sometimes severe forms of left ventricular dysfunction with a generally good chance of functional recovery. Both in the acute phase may exhibit life-threatening arrhythmias.
In these contexts, the use of WCD could be used in the presence of an LVEF ≤ 35% until the final assessment for an ICD indication.26,27,29,30,36
The indication for ICD implantation for the prevention of sudden death in the context of peripartum cardiomyopathy is very challenging for various reasons. Although about a quarter of all deaths in the first 6 months after the diagnosis are caused by malignant VTs, especially in the presence of severe ventricular impairment, as reported by Duncker et al.,12 the young age of the patients and the possibility of functional recovery in 50% of the cases must lead to caution in the indication for an ICD.12,46 There is no scientific evidence to support the early implantation of an ICD in these patients.
Patients with toxic-based systolic dysfunction (induced by drugs and toxic substances)
Patients exposed to potentially toxic agents, such as chemotherapeutics frequently used in combination or sequentially for oncological diseases, including anthracycline derivatives, taxol, 5- fluorouracil derivatives, and other compounds, may develop severe, transient, or permanent drug-induced left ventricular dysfunction.
The risk of occurrence of malignant arrhythmias/SCD is similar to that observed in patients with new-onset heart failure. In a study on the use of WCD in patients with anthracycline cardiotoxicity, an arrhythmic risk of 7% over a period of 3 months was found, which is significantly higher than in the general population with heart failure.47
Therefore, it is reasonable to prescribe a WCD for as long as necessary to assess the evolution of the initially depressed systolic function. At the same time, after introducing a cardioprotective drug therapy, chemotherapy should be suspended temporarily, when possible and resumed only after adequate functional recovery or replaced with a less toxic one.30
In alcoholic cardiomyopathy, the left ventricular dysfunction, even if severe, is potentially reversible by discontinuing alcoholic beverages with a time interval of 3 to 6–8 months. Arrhythmic risk correlates with the severity of ventricular dysfunction and the presence of left bundle branch block on the ECG, as Guzzo-Merello et al.48 showed in their cohort study of 98 patients with alcoholic cardiomyopathy, in which 18% of these had episodes of malignant ventricular arrhythmias with no major arrhythmic events in those who showed a functional recovery with LVEF > 40%.
Even in this condition, WCD may be an advantageous option to protect the patient while assessing the development of ventricular function and possible arrhythmias.
Patients with genetic cardiomyopathies
We include in this category patients with suspected or established genetic-based cardiomyopathy at risk of SCD.
Brugada syndrome
In the case of rhythm disturbances and/or suspected Brugada syndrome, WCD is a possible option in specific cases until the completion of the diagnostic process and prognostic stratification.
Long QT syndrome
Even in suspected long QT syndrome, it is reasonable to use the WCD until the diagnosis is completed or, in the case of drug-induced long QT, until the ECG is normalized in accordance with the EHRA and ESC guidelines.26,27
Systemic inflammatory disease
Sarcoidosis
About 25% of patients with sarcoidosis have a cardiac involvement. Approximately 30–65% of deaths in patients with sarcoidosis are due to SCD from VTs.38,49 According to the consensus document of the DGK (German Society of Cardiology) and the DGP (German Respiratory Society), steroid therapy should be started and patients re-evaluated in 3–6 months before deciding on a possible ICD implantation in primary prevention.50 Sarcoidosis received specific attention in the ESC 2022 guidelines. The ICD is indicated in primary prevention in those with an LVEF < 35% and should be considered in those with an LVEF > 35% and extensive myocardial LGE on cardiac MRI. During the acute phase of the disease, the use of WCD may be indicated during appropriate risk stratification once corticosteroid therapy has been instituted.50
Patients awaiting cardiac transplantation or with a left ventricular assist device
If hospital discharge is possible, the International Heart and Lung Transplantation recommends the prescription of a WCD because of the significantly increased risk of SCD (Class I). The same recommendation applies to patients with an implanted left ventricular assist device. In both cases, ICD implantation leads to an increased complication rate.51 Moreover, the new ESC 2022 guidelines on ventricular arrhythmias also recommend the prescription of WCDs for patients awaiting cardiac transplantation.31
Patients with an indication for cardiac defibrillator implantation that temporarily cannot be used
Patients undergoing extraction of cardiac defibrillator that cannot be reimplanted immediately
Peri- or post-operative infections, as well as other complications, are not uncommon in patients with a recently implanted ICD (7.6%).52,53 This often requires a complete explant of the device with additional risk of complications. In these patients, it may be assumed an unchanged high risk of SCD due to the already established indication for the ICD. Patients with an ICD indication have been included in cohort studies on WCD. In this population, the rate of ventricular events ranged from 5.2% at 2 months to 8% at 3 months.54,55
WCD is recommended as a bridge until the ICD can be reimplanted. This bridge frequently prolongs until a complete eradication of the infection has been achieved. The level of this recommendation varies between Class I and Class IIa/B.30,31,56
In the investigation by Wan et al. and Ellenbogen et al.,57,58 the authors recommend a waiting period of at least 2 months, as a significant benefit was demonstrated in a longer infection-free interval. Furthermore, they recommend a risk reassessment prior to reimplantation, since 8% of the study patients no longer needed an ICD after the explant.
Patient with transient contraindications to cardiac defibrillator implantation
In some cases, even if there is an indication for an ICD, this cannot be implanted due to transient contraindications, such as acute infections, endocarditis, or peripheral ulcers in patients with obliterative artery disease, abscesses, concomitant radiotherapy treatments, intracardiac thrombi, or intervening comorbidities requiring urgent surgical treatment.
In all these cases, it reasonable to prescribe a WCD until the intervening contraindication has been resolved. Follow-up examinations should be performed at 4-week intervals.
If the contraindication is no longer present, the ICD should be implanted as soon as possible.31
Evidence on the benefits of use and recommendations of international guidelines
Current evidence concerning the use of WCD is based on retrospective and prospective registers of >30 000 patients and a single randomized trial (VEST trial).
Retrospective studies and registers
Since its approval and introduction into clinical practice, WCD has been studied in numerous retrospective and prospective registers.
The registries of Zishiri et al.34 Chung et al.55 and Epstein et al.25 published in 2010 and 2013, examined the efficacy of WCD, patient compliance, and long-term survival in almost 17 000 patients with ischaemic heart disease. The registry of Chung et al.55 evaluated the data of 3569 patients discharged with WCD for various indications (ICD explant, post-infarction, and newly diagnosed left ventricular dysfunction): arrhythmic events (VT/VF) occurred in 59 patients (1.7%), with a survival rate of 89%. Survival rates were comparable to those of an ICD.34 The median daily time on WCD was 21.7 h, the average of 19.9 h.55
The registry of revascularized patients at the Cleveland Clinic compared with those in the US National WCD database34 analysed patients undergoing surgical or percutaneous revascularization with LVEF < 35%: early mortality was higher in 4149 patients discharged without WCD than in 809 patients discharged with WCD (90-day mortality after CABG 7% vs. 3%, P = 0.03; post-PCI 10% vs. 2%, P < 0.0001).
The registry published by Epstein et al.25 explored data on 8453 patients discharged with a WCD in the first 3 months after a myocardial infarction with LVEF < 35%. The WCD successfully treated arrhythmic events detected in 133 patients (1.6%) who received a total of 309 appropriate shocks. Among the treated arrhythmic events, 96% occurred within the first 3 months after enrolment. The survival of patients with arrhythmic events was 84% in non-revascularized patients and 95% in revascularized patients. The rate of inappropriate shocks was low (0.006 shocks per patient per month of use), while the efficacy in treating ventricular arrhythmias correctly was very high (92% events and 91% patients).
The Austrian registry included 448 patients enrolled in 48 centres: 11 of these (2.5%) had an arrhythmic event treated. A total of 22 shocks were delivered for 19 episodes of VT or VF. Eighteen of the 19 arrhythmic episodes were effectively treated by the WCD (95%). Inappropriate shocks were 0.4%. Fifty-five per cent of the patients subsequently underwent ICD implantation, and an improvement in ejection fraction was observed in 33%, pre-implantation.20 All patients had received structured training by specially trained nursing staff, and reinforcement and support contacts were provided by them. The median wearing time was 23.5 h/day, which is among the highest of those described in the literature.
Similar results were recently confirmed by Kuehn et al.59 in a large registry of 1168 patients with LVEF ≤ 35% undergoing cardiac surgery. Ventricular arrhythmias occurred in 9.1% of the patients, effective defibrillation was administered in 18 patients (1.5%), inappropriate shocks occurred in 0.8%, and only 37% of the patients were subsequently referred for ICD implantation because improvement in left ventricular function >35% was observed in the remaining patients (excluding only patients with ICD explant due to infection). Ninety-three per cent of arrhythmic episodes occurred in the first 3 months. The median wearing time was 23.4 h/day.59
Registries are also available for patients with diagnosis of NICM and LVEF ≤ 35%. The PROLONG I study showed an improvement in LVEF within the first 3 months in 56% of 156 patients with an initial diagnosis of LVEF ≤ 35% in NICM. Twelve appropriate shocks by WCD were observed in 11 patients (7%).12 In 56% of the patients after the first 3 months and in 62% of the treated patients who waited up to 6 months, ICD implantation was no longer necessary after the WCD treatment period due to improvement in LVEF. The improvement was mainly observed in the first 3 months (66 patients), but 26 improved in the following months. The average wearing time was 21.7 ± 4 h/day. The authors of the study reiterate both the importance of waiting even longer than 3 months in optimal medical therapy before proceeding to ICD implantation and that during this period, the risk of potentially fatal arrhythmias is significant and justifies the use of the WCD.12
The PROLONG II study analysed the long-term survival (2.8 ± 1.5 years) of 353 patients with recent left ventricular dysfunction treated with WCD at the Hannover Medical School.13 Seventy-five per cent of the patients wore the WCD for 3 months, 25% for >3 months. The average wearing time was 22 ± 4 h/day. Fourteen patients (4%) received an appropriate shock from the WCD. The average wearing time was 22 ± 4 h/day. Two patients (0.6%) died during the WCD period from non-cardiac causes. Most of the patients included were patients with non-ischaemic left ventricular dysfunction (64% with NICM and 36% with ICM). With the optimized medical therapy, 53% of the patients improved LVEF to >35% and dropped out of the ICD indication. The incidence of tachyarrhythmias and sudden death in patients who had received a life-saving shock from the WCD was analysed: 91% of the patients survived during the follow-up period, and the delivery of the WCD shock was not a predictor of mortality. Patients without a recommendation for ICD implantation at the end of the WCD treatment period did not experience sudden death at follow-up. Nine per cent of patients who received an ICD at 90 days and 3% of patients who received an ICD >3 months after WCD received an appropriate shock from the ICD. In conclusion, the data support the correct identification of patients before implantation of ICD, and WCD allows temporary protection, with good survival even in patients who have received a shock.13
WCD has also been used in patients who survived takotsubo syndrome. Two per cent of 102 patients with takotsubo cardiomyopathy treated with WCD for a mean time of 44 days developed ventricular arrhythmia that required defibrillation. Only six patients subsequently had an ICD implanted.60,61
Another area of use of the WCD concerns patients who have to undergo defibrillator explant and who do not require pacing. These cases are at high risk of sudden death, and in some cases ICD reimplantation must be postponed for a long time. These are patients in whom an improvement in LVEF is unlikely. From the data of the US National Registry, 8058 patients wore a WCD after removal of the ICD, which was reimplanted after a median time of 50 days (interquartile range 24–83): an arrhythmic event rate of 4% (334 patients) with a 93% probability of survival in the first 24 h after WCD shock was found. The cumulative event rate was 10% at 12 months. Implantable cardioverter defibrillator reimplantation was no longer necessary in 8% of patients due to an unexpected improvement in LVEF. The WCD demonstrated high efficacy in protecting against sudden death, and the authors recommend its use in cases where ICD reimplantation should be postponed or avoided.58
A large registry published in 2016 included 6043 patients with ischaemic and non-ischaemic heart disease treated with WCD in 404 centres in Germany: the appropriate shock rate was 1.6%, corresponding to 8.4 events/100 patient-years (dilated cardiomyopathy 9.7/100 patient-years, ICM 8.5/100 patient-years, and ICD removal 19.3/100 patient-years). Compliance was 22.1 h/day. A longer time of use was associated with a higher daily time of use. The rate of inappropriate shocks was 0.4%.18
In conclusion, retrospective studies show a WCD appropriate shock rate ranging from a minimum of 1.5% to a maximum of 7% in the different indications, with a high probability of survival and an incidence of inappropriate shocks of <1%. Adherence to treatment was above 20 h on average in all observational studies. The rate of ICD implantation at the end of WCD treatment was variable with 40–60% of patients without ICD indication due to improved LVEF.
Prospective studies and registers
As early as 1998 and 2003, efficacy studies by Auricchio et al.62 and Reek et al.63 showed that WCD was able to detect and interrupt VTs in all cases. In addition to data from retrospective registries, data from prospective registries on >2000 patients have been available since 2015. Particularly worth mentioning are the WEARIT-II, WEARIT-II-Europe, and the study by Röger et al.14–16,64,65 In these studies, compliance in WCD use, event rates, and the final need for an ICD were analysed.
Röger et al.14 published a prospective study in which they followed 105 patients with ICM and NICM during the time of WCD use. Changes in LVEF after implantation of an ICD were also observed. The WCD was used for a median time of 68.8 ± 50.4 days, with an average daily use of 21.5 ± 3.5 h. Five patients (4.8%) received an appropriate shock. At the end of WCD treatment, the ICD was implanted in only 51% of the patients (51% of NICM patients and 44% of ICM patients) for improvement of LVEF > 35%. During the 18-month follow-up following discontinuation of the WCD, no VT/VF events requiring treatment or SCD were documented in patients who did not undergo ICD implantation. The authors concluded that the WCD safely bridges to ICD implantation or improvement of left ventricular function.14
The results of the WEARIT-II Registry series were largely equivalent to the results of the retrospective analyses: out of 2000 patients with ICM (40%), NICM (46%), or congenital heart disease (13%), 2% (41 patients) experienced VT/VF, which required treatment by WCD in 54% of the cases.16 An ICD was implanted in only 42% of the patients. Compliance averaged 22.5 h/day. The inappropriate shock rate was 0.5%. No deaths from VT/VF were documented while using the WCD (3 deaths from asystole occurred).16 The 1-year follow-up showed a mortality of 4%. In patients who had previously had a VT/VF that required treatment by WCD, the mortality was 10%.64 A further analysis of the WEARIT-II Registry examined 1732 patients divided into two age groups (65 years and <65 years). Patients aged 65 years old had more arrhythmic events treated with WCD than younger ones (6.9 vs. 2.37/100 patient-years) and had a higher adherence to therapy (median wearing time 22.8 vs. 22.3 h/day).65 The authors conclude that WCD also has a role in protecting older patients.
The recently published WEARIT-II-Europe registry reported data on 781 patients with dilative cardiomyopathy and LVEF < 35% treated with WCD for an average of 75 ± 47.7 days, with a daily wearing time of 20.3 ± 4.6 h. The WCD interrupted 13 VT/VF events in 10 patients (1.3%). At the end of the treatment, the ICD was implanted in only 289 patients (37%) for a significant improvement in LVEF during the observation period in the majority of patients in whom WCD was used.15
The VEST study
In 2018, the only randomized controlled trial on WCD was published involving 2302 patients who survived an IMA with LVEF ≤ 35%, randomized to WCD and medical therapy or to medical therapy alone.11 The primary endpoint was defined as sudden death and death due to ventricular tachycardia at 90 days. Secondary endpoints were defined as all-cause mortality and non-arrhythmic death. Implantable cardioverter defibrillator implantation (excluding secondary prevention indications) and crossover were not permitted. The study design included an intention-to-treat (ITT) analysis. The National Death Database was consulted to determine mortality.
A total of 2302 patients were randomized, of whom 1524 received a WCD and 778 were assigned to the control group (drug therapy alone). Arrhythmic death was observed in 25 patients in the WCD group (1.6%) and in 19 (2.4%) in the control group [relative risk (RR) 0.67; 95% confidence interval (CI) 0.37–1.21; P = 0.18]. The reduction in the primary endpoint was not significant in the ITT analysis.22 In contrast, all-cause mortality was significantly lower in the WCD group (3.1%) than in the control group (4.9%) (RR 0.64; CI 95% 0.43–0.43).
A total of 20 patients (1.3%) received an appropriate shock. Another appropriate shock occurred in one of the patients initially allocated to the control group who then received WCD (in the control group 2.6% of the patients then received WCD against protocol). Among the patients in the group randomized to WCD, 2.8% refused treatment after randomization. Inappropriate shocks occurred in 0.6% of the patients. It is important to note that patients in the WCD group wore the device for a median of only 18 h/day. Of the 48 patients who died in the WCD group, only 12 were wearing the device at the time of death. Of the 25 patients who died of arrhythmia in the WCD group, only nine were wearing the WCD at the time of death. This finding and the lower mean time of adherence to therapy than in the observational studies suggest that the WCD could have led to better results under conditions of adequate compliance. In conclusion, the WCD did not lead to a significant reduction in arrhythmic mortality compared with the control group, whereas total mortality was significantly lower.11
To evaluate the real-world practical application of WCD use, the investigators in 2020 published an ‘as-treated’ and ‘per-protocol’ analysis of the trial data. The ‘per-protocol’ analysis showed that WCD use resulted in a significant reduction in total death (P < 0.001) and arrhythmic death (P = 0.02) compared with the control group (Table 1).22
No differences were observed in re-hospitalizations. In terms of adverse events, skin reactions and pruritus were observed more frequently in the WCD group (P < 0.001). In contrast, dyspnoea occurred more frequently in the control group (P = 0.004).11
The authors also performed an analysis to identify predictors of good compliance with WCD use. Factors associated with greater compliance with WCD included being married, having had a cardiac arrest in the acute phase of myocardial infarction, having high creatinine values, and LVEF ≤ 25%. Being of Asian ethnicity, being divorced, having diabetes, or a previous diagnosis of heart failure were associated with an early cessation of WCD use.22
Meta-analysis
Currently, two meta-analyses of data from observational studies are available.
The meta-analysis published by Nguyen et al.66 analysed 11 of 411 studies conducted between 2008 and 2017 with a total of 19 882 patients. Seven studies evaluated WCD in a spectrum of different indications, while the remaining studies covered a single indication. Most of the included studies were retrospective and multicentre. The average time the patient wore the device ranged from 17 to 24 h/day. The meta-analysis showed that 2.6% of the WCD patients had experienced VT/VF episodes and that 1.7% of the patients had received at least one appropriate shock, corresponding to a discharge incidence of 9.1 patients/100 person-years. The WCD successfully interrupted the arrhythmia in 95.5% of the cases. All-cause mortality and mortality due to VT/VF episodes were 1.4% and 0.2%, respectively. The rate of inappropriate shocks was 0.9%.
Another meta-analysis67 published in 2019 included 28 studies of which 27 were observational and the VEST study. The incidence of appropriate therapy by WCD was 5/100 people in 3 months while the incidence of inappropriate shocks was 2/100 people in 3 months. The incidence of appropriate shocks was lower in the VEST study (1/100 persons in 3 months) than in the observational studies (11/100 persons in 3 months).
Indications and recommendations
Since 2015, the WCD has been part of the guideline recommendations of all European and international societies.
In Europe, the 2015 ESC guidelines for the prevention of sudden death26 place WCD in Class IIb on a Type C level of evidence for adult patients with left ventricular dysfunction at risk of sudden death for limited periods, where implantation of an ICD is not indicated, and in Class IIa on a Type C level of evidence for patients with myocarditis with severe left ventricular dysfunction or electrical instability until recovery or ICD implantation. The use of the WCD is included in the ‘gaps in evidence’ as an attractive treatment option in selected patients but requires larger randomized trials to clearly define the indications.26
To complement the guidelines, an EHRA report28 was published in 2016 with an update on the technology, indications, and cost-effectiveness of WCD.
Recent updates of the ESC guidelines (ESC Guidelines 2021 on heart failure and ESC Guidelines 2022 on ventricular arrhythmias) have also evolved the approach to the assessment of WCD. Recommendations for patients at high risk of WCD with various indications, but who do not yet have an indication for defibrillator implantation, have been included/added/enhanced. The WCD now has a recommendation IIb/B in the ESC heart failure guidelines for ischaemic and non-ischaemic patients. Patients after myocardial infarction have a recommendation IIb/B in the guidelines on ventricular arrhythmias. In secondary prevention, WCD is now recommended with a Class IIa/B recommendation.30,31
In 2016, the AHA Science Advisory68 summarizes the indications for WCD in Class IIb, except in cases of temporary contraindication to the ICD (e.g. due to infection), which recognizes a Class IIa. Similarly, a Class IIa indication for WCD is recognized in patients awaiting cardiac transplantation.
In 2017, the AHA/ACC guidelines29 indicate WCD in Class IIb for patients in the first 40 days post-infarction with severe left ventricular dysfunction and in patients with a recurrent (<3 months) diagnosis of non-ischaemic dilated cardiomyopathy to allow optimization of therapy and improvement of left ventricular function. On the other hand, WCD is indicated in Class IIa on a Type B basis in patients in whom ICD removal is necessary, e.g. due to infectious causes.
In 2019, a DGK statement was published,56 which proposes a Class I indications in the case of the need for ICD removal and peripartum heart disease, Class II in the remaining clinical situations. The Japanese Society of Cardiology (JCS/JHRS [Japanese Society]) guidelines36 were renewed in 2019 following the publication of the VEST trial with a Class IIa recommendation in cases of post-infarction left ventricular dysfunction in the first 40 days, newly diagnosed NICM, patients awaiting cardiac transplantation, and in cases of ICD removal due to explant or temporary contraindication to implantation. The indication for WCD is placed in Class IIb in patients undergoing secondary prevention of sudden death in whom a follow-up period is considered a priority in order to await response to medical treatment before proceeding to ICD implantation.
The pathway of the patient candidate for the use of the wearable cardioverter defibrillator
Screening, selection, and follow-up of patients
Prevention of SCD can be either primary or secondary. Primary prevention is aimed at avoiding a SCD in a high-risk patient who has never had any potentially dangerous arrhythmic episode. Secondary prevention is aimed at protecting the patient who has already suffered a life-threatening tachyarrhythmia. In secondary prevention, implantation of an ICD is generally indicated unless the condition that caused the malignant arrhythmia is clearly identifiable and removable, or it is certainly transient. A patient with an ICD that has to be removed, e.g. due to an infection of the device, is also considered to be in secondary prevention.
Although great efforts have been made to identify predictive risk markers that are sufficiently robust to identify those who are at increased risk of SCD, the most common one still is a severely reduced LVEF.26,29,69 The pool of patients who are candidates for ICDs on the basis of reduced ejection fraction is therefore very large.70 Some patients develop left ventricular dysfunction as a result of a more or less extensive myocardial infarction, others are patients with cardiomyopathies, outcomes of inflammatory diseases or as a result of specific therapies. In many cases, the dysfunction is transient and may result in recovery of the ejection fraction spontaneously or after drug therapy for an appropriate period.37 In any case, only when the drug therapy has been optimized and the guideline-recommended waiting periods are over, it can be assumed that the risk is persistent and the ICD implantation is granted.
According to current European and American guidelines, it is necessary to wait between 40 and 90 days in order to more accurately assess the arrhythmic risk of a patient with an initial diagnosis of systolic dysfunction.
After an infarction, it is necessary to wait at least 6–12 weeks to assess whether LVEF has recovered. On the other hand, if the patient undergoes percutaneous revascularization or CABG, it is necessary to wait 90 days after the event, under optimal medical therapy. These indications are derived from studies conducted in ischaemic patients undergoing early ICD implantation, in whom this strategy did not show a significant benefit. For example, in the DINAMIT study and the IRIS study, the lower number of arrhythmic deaths achieved by an early ICD implantation was offset by the non-arrhythmic deaths at the end of follow-up.71 Furthermore, early ICD implantation, i.e. not evidence-based, has been linked to a higher incidence of adverse events, including death, peri-procedural complications, and re-hospitalization at 90 days and at 1 year.72
Similarly, in patients with non-ischaemic systolic dysfunction, it is necessary to wait at least 90 days, under optimal medical therapy, to assess the indication for ICD implantation.
In spite of the unquestionable benefits of the new heart failure medical therapies, death from arrhythmic causes albeit reduced still are present in 2–3% in the various case series in the first months after discharge, both in ischaemic patients (2.3%)71 and in those revascularized after CABG (7%)73 and in non-ischaemic patients (1.8%).74 In addition, sudden death continues to be a largely unpredictable event without clear predisposing factors, and the absence of cardiovascular events during follow-up may represent a false reassurance that the patient’s arrhythmic risk has decreased.75
For this reason, the use of the WCD can be a valid option for patients who are considered to be at risk of arrhythmic events but who do not yet have a definite indication for definitive ICD implantation, as the risk may significantly decrease as LVEF improves.
It would be particularly useful to have more sophisticated risk markers than LVEF alone, but at present, the guidelines are still anchored to this parameter.
An early identification of those patients who will not recover their left ventricular function with respect to those who will would allow an early discrimination among those suitable for an ICD or to a WCD. To date, there is growing evidence and indications for the use of other markers, especially (but not only) the presence and extent of areas of LGE at magnetic resonance imaging.35,76–78 However, at present, there is not sufficient evidence to support a change from the currently recommended indications from the international guidelines.
In clinical practice, once the presence of a significant systolic dysfunction has been identified, and once the diagnostic procedure necessary to identify the aetiology has been completed, the patient must be included in a surveillance programme, which should include the use of the WCD in cases where contractile recovery is considered possible. It is obviously necessary to plan a structured follow-up to the patient allowing the progressive implementation of the needed therapies (as an example with the four classes of drugs recommended and their titration for HFrEF) while the WCD is worn and to check the systolic function after 1, 3, and 6 months.30
During the follow-up, a significant proportion of patients regain adequate systolic function to the extent that they no longer have a high risk of SCD or require an ICD. This occurs with the patient protected by the WCD. Therefore, having the WCD makes it possible to comply with international recommendations, keep the patient safe, and also increase the probability of avoiding an unnecessary ICD, with its associated costs and possible complications.
Figure 1 presents a flow chart with the main aspects and considerations for the different indications of WCD use.

Flow chart showing the main conditions, indications for the use of the wearable cardioverter defibrillator, and subsequent clinical pathway (A) in patients with newly found 35% ejection fraction and (B) in patients at risk of sudden cardiac death. CAD, coronary artery disease; CMP, cardiomyopathy; LVEF, left ventricular ejection fraction; GDMT, guideline-guided medical therapy; ICD, implantable cardioverter defibrillator; AMI, acute myocardial infarction; LVAD, left ventricular assist device; OMT, optimal medical therapy; ACS, acute coronary syndrome; EPS, electrophysiological study; SVT, sustained ventricular tachycardia. *Suspected hereditary channelopathy or CMP judged to be high risk by history and symptoms pending diagnostic and therapeutic definition in selected cases. #ICD temporarily contraindicated: endocarditis, radiotherapy/chemotherapy, awaiting other treatment (e.g. surgery), and transient comorbidity (e.g. sepsis, anaemia, and hypokalaemia).
Future potential
Currently only one WCD (ZOLL, LifeVest) is available on the European market. This model is equipped with several telemetry features. The recorded parameters are accessible to the treating physician via a secure account server, the ZPM. The device is able to record arrhythmic events, heart rate, physical activity, body position during the day and during night rest (Figure 2), and last but not least the time of wearing (Figure 3). With the numerous data transmitted by the WCD, it is therefore possible to monitor the patient’s progress at a distance and the response to medical therapy.
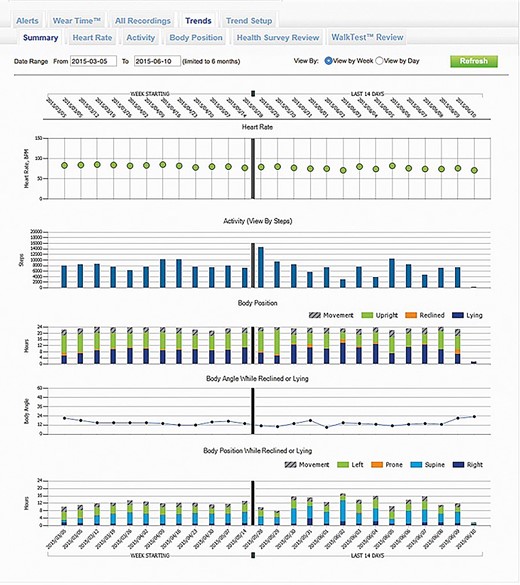
Screenshot of the wearable cardioverter defibrillator remote monitoring website where trends in heart rate, activity, body position, and body angle are visible when the patient is lying down.
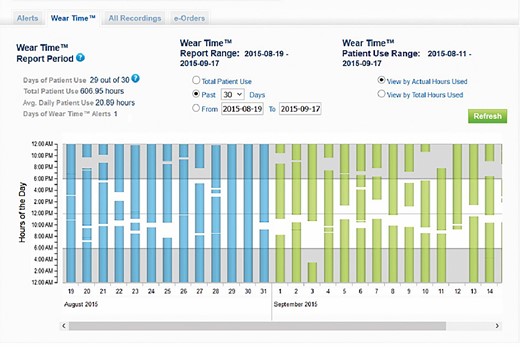
Screenshot of the remote monitoring site of the wearable cardioverter defibrillator where the wearing time of the device can be checked.
In addition, arrhythmia monitoring allows early intervention in the event of a shock, increased ventricular arrhythmic burden, or the onset of supraventricular arrhythmias such as atrial fibrillation, a frequent cause of early re-hospitalization for heart failure (Figure 4).
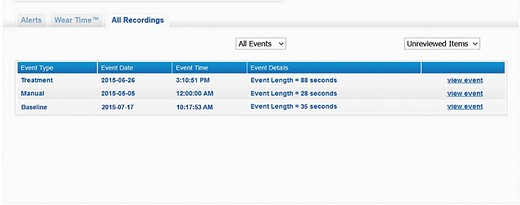
Screenshot of the wearable cardioverter defibrillator remote monitoring website where the electrocardiographic recordings can be viewed, classified according to the following categories: ‘baseline’ (acquisition of the electrocardiographic signal upon delivery of the device), ‘manual’ recording (initiated by patient), ‘automatic’ recording (arrhythmias that did not lead to the delivery of a shock), ‘treatment’ (arrhythmias with shock delivery), and ‘asystole’.
A recent retrospective study confirmed an association between heart rate, recorded by WCD, and mortality. Heart rate monitoring by WCD may help to titrate the use of beta-blockers more effectively.79
Another important aspect is the possibility of remotely monitoring the patient during the 6-min walk test, guided by the device, and thus assessing the patient’s physical endurance and autonomy by means of a calculation of distances covered, symptoms, and heart rate before, during, and after the exercise (Figure 5).
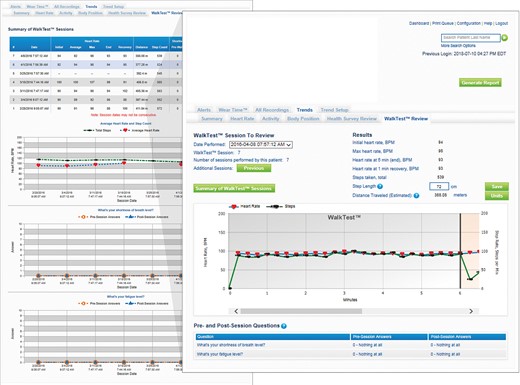
Screenshot of the wearable cardioverter defibrillator’s remote monitoring website showing the results of the walk test in terms of distance travelled and heart rate trend during the individual test (right) and the variations over time between different walk tests performed for the parameters distance travelled and average heart rate (left).
A questionnaire inspired by the Minnesota Quality of Life can be programmed on the device and used to assess health status, symptoms, adherence to treatment, presence of peripheral oedema, etc., by means of simple questions to which the patient can easily answer yes/no or with a numeric scale80 (Figure 6).
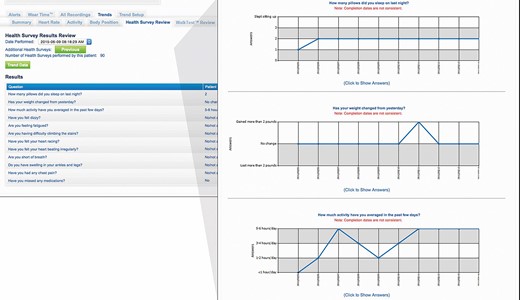
Screenshot of the wearable cardioverter defibrillator remote monitoring website where the patient’s answers to the individual health questionnaire (left) and the trend of answers over time for each question (right) are displayed.
All these features and the amount of remotely transmitted data allow a rigorous management of the patient, giving the physician the possibility to identify the need for a therapeutic adaptation thus potentially avoiding unplanned access to the Hospital for acute decompensation events.81 Potential functions that could be added in the future are the calculation of heart rate variability and the measurement of pulmonary congestion, the latter already included in another device of the same manufacturer.
Limitations of the device and its use
Like any therapy, the WCD only works to its full potential if it is used appropriately. Correct and careful selection and training of patients requiring the device are therefore essential to ensure a good compliance and optimal results. The presence of mental or physical disabilities, which may limit its proper use, should be considered a contraindication. When identifying the candidate patient, it is important that the size of the device is correct, and, to this end, five different sizes are available.
Registries in several European countries show that in the real world with a correct patient selection, wearability and compliance do not seem to be a problem, with daily use times reported as consistently high.18–21,82
In the context of device compliance, due attention must be paid to patient response to alarms. In fact, the WCD emits tactile and acoustic alarms when a shockable rhythm is identified prior to shock delivery, so that, if the patient is conscious, he/she is alerted and presses the response buttons on the monitor to avoid unnecessary defibrillation. Studies in the literature show that even if few patients were annoyed by the alarms, the net compliance was not adversely affected.83 A good device compliance also does not interfere with the quality of life of patients with WCD.22
Finally, anti-bradycardia or anti-tachycardia pacing and ‘post-shock’ pacing are not available on the system. This limitation does not seem to impact the performance of the WCD. Several studies, in particular the VEST trial,22 have shown that asystole plays a negligible role in the prognosis of these patients.
Conclusions
In recent years, the WCD has increasingly gained an established role in the temporary protection of patients who have an increased risk of life-threatening VTs. This protection allows a precise risk stratification and selection of potential candidates to ICD. During the risk assessment process, the WCD detects and treats potentially lethal ventricular rhythms while the patient receives the optimal therapy for the underlying disease or recovers from a toxic or inflammatory condition affecting the heart. By safely protecting during the time necessary for the recovery of left ventricular dysfunction or the ease of the transiently pro-arrhythmic condition, the WCD facilitates a guideline-compliant evaluation phase prior to the implantation of an ICD and can avoid the unnecessary implants.
The efficacy and safety of WCD have been confirmed by numerous retrospective and prospective studies worldwide, including a randomized controlled trial.
Finally, it should be emphasized that WCD is a tool that allows protection from any life-threatening arrhythmic events even in patients who, for various reasons unrelated to LVEF, have a transient risk of SCD.
Summary
Risk stratification of SCD and the optimal management of patients with long-term SCD risk are issues of great clinical relevance. There are several conditions in which the risk of arrhythmic death is only transient. Patients with reduced left ventricular systolic function, for example, have a very high risk, which, however, is greatly reduced in those who undergo a contractile recovery. For many of them, therefore, the arrhythmic risk is only temporary, and it is very important to protect these patients during the recommended time necessary to decide whether an ICD is necessary. During this time lapse, the before necessary therapeutic measures can be safely implemented while the patient is protected.
In other cases, the arrhythmic risk, although established as transient, is not accompanied by a reduced systolic function. Examples are patients suffering from acute myocarditis, during diagnostic investigations of certain arrhythmic conditions, or after the extraction of catheters while awaiting recovery from the infection and prior to a new implantation. Even in these conditions, it is necessary to be able to protect the patient from life-threatening arrhythmias. In this context, the WCD is of particular importance as a temporary, non-invasive tool for monitoring and treating arrhythmias in patients at high risk of SCD. Available studies have shown that the WCD is an effective and safe therapy for the prevention of SCD caused by ventricular tachycardia and fibrillation. The aim of this ANMCO position paper is to provide guidance on the clinical use of the WCD in Italy, based on current data and international guidelines. The paper will review WCD functionality, indications, clinical evidence, and guideline recommendations. Based on these, a practical scheme for identifying patients at risk of SCD who are candidates for WCD use is proposed.
Funding
This paper was published as part of a supplement financially supported by the Italian National Association of Hospital Cardiologists (ANMCO).
Conflict of interest: None declared.
Disclaimer This Position Paper was originally published in the Italian language as ‘Position paper ANMCO: Guida all'uso appropriato del defibrillatore indossabile nella pratica clinica per i pazienti ad elevato rischio transitorio di morte improvvisa', in the official journal of the Italian Federation of Cardiology (IFC) ‘Giornale Italiano di Cardiologia’, published by Il Pensiero Scientifico Editore. This paper was translated by a representative of the Italian Association of Hospital Cardiologists (ANMCO) and reprinted with permission of IFC and Il Pensiero Scientifico Editore.
Data availability
No new data were generated or analysed in support of this research.
References
- cardiac arrhythmia
- ischemia
- ventricular function, left
- left ventricular ejection fraction
- sudden cardiac death
- italy
- recovery of function
- infections
- diagnosis
- guidelines
- wearable automatic external defibrillator
- implantable defibrillator insertion
- stratification
- medical devices
- appropriate use
- prevention



